Abstract
Clinical islet transplantation has demonstrated success in treating type 1 diabetes. A current limitation is the intrahepatic portal vein transplant site, which is prone to mechanical stress and inflammation. Transplantation of pancreatic islets into alternative sites is preferable, but challenging, as it may require a three-dimensional vehicle to confer mechanical protection and to confine islets to a well-defined, retrievable space where islet neovascularization can occur. We have fabricated biostable, macroporous scaffolds from poly(dimethylsiloxane) (PDMS) and investigated islet retention and distribution, metabolic function, and glucose-dependent insulin secretion within these materials. Islets from multiple sources, including rodents, non-human primates, and humans, were tested in vitro. We observed high islet retention and distribution within PDMS scaffolds, with retention of small islets (< 100 µm) improved through the post-loading addition of fibrin gel. Islets loaded within PDMS scaffolds exhibited viability and function comparable to standard culture conditions when incubated under normal oxygen tensions, but displayed improved viability compared to standard two-dimensional culture controls under low oxygen tensions. In vivo efficacy of scaffolds to support islet grafts was evaluated after transplantation in the omental pouch of chemically-induced diabetic syngeneic rats, which promptly achieved normoglycemia. Collectively, these results are promising in that they indicate the potential for transplanting islets into a clinically relevant, extrahepatic site that provides spatial distribution of islets, as well as intra-device vascularization.
Keywords: islet transplantation, scaffold, extrahepatic sites, omentum, poly(dimethylsiloxane) (PDMS), diabetes, human islets, rat islets, nonhuman primate islets
INTRODUCTION
Treatment of patients with brittle type 1 diabetes mellitus (T1DM) via intraportal infusion of islets has demonstrated success in current clinical trials, conferring superior control of blood glucose, increase in C-peptide levels, and insulin independence for several years after treatment (51,62). Following intrahepatic infusion, however, a large number of islets are quickly destroyed by nonspecific inflammation and exhibit dysfunction. This phenomenon has been primarily associated with islet exposure to mechanical stress and inflammatory responses immediately after infusion, as well as high drug and toxin loads in the liver (6,31,33,41,49,52,56,59,69,75). The design of an alternative site that minimizes these factors could provide a superior environment for islet engraftment. The transplantation of islets into alternative sites would benefit from the use of scaffolds or devices that impart mechanical protection, spatial distribution, vascular infiltration, and the safety of retrievability (10,22,61). Over the past decades, several researchers have designed and tested numerous devices for this purpose in multiple forms such as beads, sheets, rods, disks, and intravascular devices (13,14,55,66,67,74). The majority of these devices focused on the achievement of immunoisolation, which has the caveat of preventing islet revascularization (12,15,20,30,39,64), resulting in the significant depletion of nutrients due to the high metabolic demand of the islets. As a result, these avascular devices typically developed necrotic core regions in the grafted tissue (20,64,65). The design of devices that allows for both intra-device vascularization and a means to spatially distribute the cells would provide a more favorable nutrient environment, while permitting for translatable scales for large animal models and clinical use. The use of highly porous materials that permit vascular migration might not only reduce nutritional gradients, but also improve the responsiveness of the entrapped islets to glucose challenges due to intra-islet vascularization.
The ideal matrix to attain the above outlined goals would be one that is highly porous, with deep, interconnected pores to trap the islets throughout the device, while also allowing for host remodeling and vascular infiltration. Previously, Blomeier et al. demonstrated that a highly porous poly(D,L-lactide-co-glycolide) (PLG) scaffold, seeded with islets and transplanted into the intraperitoneal fat pad of a mouse, could be highly efficacious in a syngeneic diabetic mouse model (10). While the degradation of the scaffold over time restricts the ability to remove the implant at a later date, these studies illustrate the benefits of permitting intra-device vascularization on transplant efficacy.
Another important factor to consider for extrahepatic transplantation is the selection of the transplant site. Numerous implantation sites have been studied, with various observed benefits for each site (see (22) for review). For small animal models, islets transplanted under the kidney capsule, spleen, or epididymal fat pad have demonstrated success (29,34,70–73); however, these sites are not clinically relevant (10,22). Moreover, for transplantation in large animal models and humans, the implant must be scaled to account for the larger islet mass required, necessitating a site that can accommodate a larger tissue volume. The omental pouch meets these requirements and is easily accessible, allowing for retrieval of implants if necessary (22). Additionally, this site is drained via the portal system, which allows for a more physiological function of the islets when compared to systemic drainage (18). The intraomental transplantation of free islets into small and large animal models has established the efficacy of this site, although the IEQ requirements at this site tend to be higher when compared to the kidney capsule site (2,3,25,36,37,63,76). While the use of devices or biomaterials to contain islets within the omentum has shown promise in large animal models (7,24,38,66), success rates in rat isografts has been limited, with no published reports of consistently stable graft function (26,40,50).
Herein, we describe the development of a non-absorbable, three dimensional, macroporous scaffold for housing islet grafts within an extrahepatic site, specifically the omental pouch. We fabricated macroporous PDMS scaffolds via the solvent casting and particulate leaching technique (SCPL), selecting PDMS based on its biostability, biocompatibility, oxygen solubility, and ease in surface modification (17,44,48,54,68). Resulting scaffolds exhibited high mechanical stability, even with 90% void space, with large, interconnected pores. We investigated the suitability of these PDMS macroporous scaffolds for housing pancreatic islets, through assessment of islet retention, viability, and function in vitro. The efficacy of these scaffolds to support islet engraftment, vascularization and function was tested within the omental pouch of syngeneic, streptozotocin-induced diabetic rats. The use of this novel PDMS-based platform to house islets within alternative sites is discussed, as well as the ability of this scaffold to serve as a multi-functional platform to improve islet engraftment and long-term function.
MATERIALS AND METHODS
Materials
Poly(dimethylsiloxane) (PDMS) polymer components were purchased from GE Silicone (RTV 615 A&B). Sodium chloride crystals were purchased from Mallinckrodt Baker. Sieves with openings of 250 µm and 425 µm were purchased from W.S. Tyler through VWR. Human plasma fibronectin (FN) was purchased from Gibco. Agarose powder type VII was purchased from Sigma-Aldrich. All culture media was purchased from Mediatech. MTT cell viability kit was purchased from Promega (Madison, WI). LIVE/DEAD Viability/Cytotoxicity Assay Kit was purchased from Invitrogen. Insulin ELISA kits were purchased from Mercodia. All other reagents, unless otherwise indicated, were purchased from Sigma. All tissue culture supplies, unless otherwise indicated, were purchased from VWR.
Scaffold Fabrication
Macroporous PDMS scaffolds were fabricated using the solvent casting and particulate leaching technique (SCPL). PDMS polymer was prepared by mixing PDMS monomer with platinum catalyst per manufacturer’s instructions; with the exception that 4:1 v/v was used. Salt, sieved to contain particles between 250 µm to 425 µm in diameter, was mixed with PDMS polymer at a 90 % v/v salt / PDMS ratio, loaded into prefabricated, silicone molds (10 mm diameter, 2 mm height), and incubated at 37 °C for 48 h in order to completely cross-link the PDMS. The scaffolds were soaked in deionized water for 5 d to leach out the salt, with water changes every 24 h. Scaffolds were then dried at 40 °C for 24 h and steam sterilized in an autoclave. Scanning electron microscopy (SEM) (JEOL, JSM-5600LV, 29 Pa, 20 kV) was employed to visualize the resulting scaffold. To alter the surface of the hydrophobic PDMS, the scaffold surface was coated with fibronectin via incubation in a 250 µg/mL for 24 h.
Cell Isolation and Culture
Islets were obtained from 250 – 280 g male Lewis rats (Harlan Laboratory) via mechanically-enhanced enzymatic digestion followed by density gradient purification, as previously described (58). Non-human primate baboon (NHP) islets were isolated using methods, as described elsewhere (7). NHP islets Human islets were obtained through the NIH/JDRF ICR Consortium and the NIH-IIDP. Human islet donors (n=2) statistics were female, age 42 and male, age 60. Rat and NHP islets were cultured in CMRL 1066-based medium (Mediatech) supplemented with 10% fetal bovine serum (FBS; Sigma), 1% penicillin-streptomycin (Sigma), and 1% L-glutamine (Sigma) for 24 h prior to seeding into scaffolds. Human islets were cultured in MM1 (Mediatech) for 24 h following arrival, prior to loading into scaffolds.
In Vitro Cell Loading and Assessment for Scaffolds, Hydrogels, or Controls
Scaffolds were prepared for islet loading via washing with respective islet culture media. Islet aliquots were based on calculation of islet equivalent (IEQ) (11) and were loaded into the fabricated scaffolds by concentrating islets into 50 µL of media and seeding them onto the scaffolds, where they filtered through the micro-sized pores via gravity. For all in vitro studies, 1500 IEQ per scaffold was used. For a subset of scaffolds, fibrin gel (30 µL) was added to the islet-loaded scaffolds by loading onto the top of the PDMS scaffold. Fibrin gel was fabricated via the addition of fibrinogen (4 mM, Enzyme Research Lab), thrombin (2 U/mL, Sigma Aldrich), aprotinin (85 ug/mL, Roche Applied Science), and CaCl2 (5 mM), as described in (46,47). Islet loaded scaffolds were then incubated at 37 °C for 15 min. Scaffolds were then transferred to 35 mm culture dishes, 4 mL of media was added, and they were cultured in a humidified 37 °C, 5 % CO2 / 95 % air incubator for 24 hrs.
The viability of islets cultured on PDMS scaffolds was compared to that of islets cultured in the micro-porous hydrogel agarose. Cylindrical agarose constructs of identical size (10 mm in diameter, 2 mm final thickness) were fabricated by suspending rat islets in 2 % agarose at 37 °C at a density of 1500 IEQ per construct, and allowing it to gel for 3 min at room temperature. Agarose and PDMS scaffolds were then cultured in 35 mm culture dishes with 4 mL of culture media for 48 hrs in an incubator set at 0.05 mM oxygen (“hypoxic” condition).
Islets within scaffolds or constructs were compared to conventional culture, which was 1500 IEQ freely dispersed in 1.6 mL media in 35 mm culture dishes in a humidified incubator for 24 hrs at either standard (0.2 mM) or hypoxic (0.05 mM) oxygen conditions, depending on the experiment.
In Vitro Assessment of Metabolic Viability and Retention of Islets
MTT cell viability assay was used to evaluate the metabolic activity of islets. Islet-loaded scaffolds (1500 IEQ) were washed once, suspended in 250 µL of media within a 48-well non-tissue culture treated plate, and placed in a humidified incubator for 1 h to recover from manipulation. Following 1 h incubation, MTT dye was added to each well to a final concentration of 0.5 mg/mL and samples were incubated for an additional hour. Afterwards, 185 µL of stop/solubilization solution was added to each well to quench the reaction and the plate was wrapped in parafilm, protected from light, and stored for 24 h to fully solubilize the formazan crystals. The next day, 120 µL samples from each well were dispensed into a 96-well plate and the absorption at 570 nm was measured using a plate reader (Molecular Devices). Changes in optical density due to culture media used were compensated by subtracting media blanks from all wells. Effect of debris was minimized via subtracting results from readings at 650 nm.
For islet retention studies, islet loss from the scaffold following loading with 1500 IEQ was evaluated. After incubating the scaffolds with 0.5 mL of media in a 48-well non-tissue culture treated plate for 4 hr, the scaffold was agitated and then moved into a separate well. MTT assessment was conducted on both the islet-loaded scaffold and the islets remaining in the well following scaffold removal. Retention % was expressed as the percentage of islets retained within the scaffold, via division of absorbance of islets in scaffold by absorbance of all the islets (islets in scaffold + islets in well).
Cell viability was visualized by the LIVE/DEAD Viability/Cytotoxicity Assay Kit and imaged through a confocal microscope (Zeiss LSM510) using methods outlined previously (27). Briefly, cells were rinsed in HBSS and incubated for 45 min in 4 µM calcein AM and 8 µM ethidium homodimer-1 (EthD-1) solution diluted in PBS. Following a second rinse in HBSS, scaffolds were imaged via a fluorescence scanning confocal microscope (LSM 510, Zeiss, Germany). Live cells (green) were detected by excitation with light at 494 nm and emission at 517 nm while dead cells (dead) were detected by excitation at 528 nm and emission at 617 nm. Multi-slice images were collected and merged using the projection function on LSM image browser (Zeiss, Germany) software.
In Vitro Assessment of Glucose Stimulated Insulin Secretion
The functional insulin secretion rate of islets was determined via static glucose-stimulated insulin release (GSIR) assay. Groups were sequentially incubated for 1 hr intervals in glucose solutions Low1→High1→Low2, with low (40 mg/dL) and high glucose (300 mg/dL) concentrations using a column method, as described (23). Briefly, 10 mL Poly-Prep columns (Bio-Rad) were placed in Poly-column rack (Bio-Rad), filled with 400 µL of a slurry of Sepharose G-10 (GE Healthcare)/dPBS (10% w/v), and washed with low glucose (2.2 mM) Krebs-Ringer Bicarbonate Buffer (KRBB) buffer (99mM NaCl2, 5mM KCl, 1.2mM KH2PO4, 1.2mM MgSO4, 2.6mM CaCl2, 26mM NaHCO3, and 0.2% w/v (g/mL) BSA, 25mmol/L HEPES)). Free islets or one-quarter of the islet-containing scaffold (375 IEQ total) were then placed in the column and an additional 600 µL of bead slurry was added. Columns were then flushed with 4 mL of low glucose KRBB. Flow in the columns ceased when the liquid level reached the surface of the beads, keeping the fluid volume in each column constant. Columns were then incubated for 1 hr for pre-incubation, followed by a 4 mL wash with low glucose KRBB, at which time the first step of the glucose challenge (Low1) was initiated. After 1 hr, low glucose KRBB was exchanged with high glucose KRBB to begin step two of the challenge (High1). After this hr, high glucose was exchanged with low glucose KRBB to begin step three of the challenge (Low2). During each exchange, 1 mL of the respective KRBB solution was added and the 1 mL eluate was collected in tubes and stored at − 80 °C for later analysis. Insulin was quantified using the Mercodia Rat or Human Insulin ELISA (Winston Salem, NC), depending on the islet type.
Islet Transplantation and Graft Assessment
All animal studies were reviewed and approved by the University of Miami Institutional Animal Care and Use Committee. All procedures were conducted according to the guidelines of the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources (National Research Council, Washington DC). Animals were housed in Virus Antibody Free rooms into microisolated cages with free access to autoclaved food and water at the Department of Veterinary Resources of the University of Miami. The Preclinical Cell Processing and Translational Models Core at the Diabetes Research Institute assisted with rodent islet isolations, diabetes induction, and animal maintenance. Female Lewis rats, weighing between 175–185 g (Harlan Laboratory; Indianapolis, IN), were used as transplant recipients. Rats were rendered diabetic by intravenous administration of the beta-cell toxin streptozotocin (two intravenous injections of 60 mg/kg 2–3 days apart; Sigma-Aldrich) and were used as recipients of syngeneic islets only if overtly diabetic upon three consecutive readings of non-fasting blood glucose levels > 350 mg/dL, using portable glucose meters (OneTouchUltra2; Lifescan, Milpitas, CA) (58).
Under general anesthesia (isoflurane USP; Baxter, Deerfield, IL), a mid-line incision was made and the omentum was mobilized, exposed, and spread out using sterile saline. A single PDMS scaffold was placed on the exposed omentum. 1800 IEQ were concentrated into 50 µL of saline and loaded onto the scaffold using a pipette. After 30 s, the omentum was then wrapped around the scaffold by folding each side inward. The edges of the omental pouch were subsequently sealed with fibrin gel. The omental pouch was then placed back into the peritoneal cavity and the incision was sutured and stapled. Transplanted controls included islets freely loaded into the omentum or into the subrenal capsule. For freely loaded omentum islets, 1800 IEQ (concentrated into 50 µL of saline) were pipetted onto the exposed omentum. The omentum was then wrapped around the islets and sealed with fibrin gel. For kidney capsule transplants, 1800 IEQ were loaded within the kidney capsule, as previously described.(53)
Graft function was defined as stable non-fasting glycemic levels < 200 mg/dL for 2 consecutive readings. A glucose tolerance test was performed in animals bearing a functional graft for over 3 months after transplantation for metabolic assessment of the grafted islets. After overnight fasting, rats received an intravenous glucose bolus (2 g/kg in saline) and blood glucose was monitored until it read below 200 mg/dL. Glucose clearance was evaluated by calculating the area under the curve (AUC) from the first reading until a reading < 200 mg/dL. To ensure that the function observed in normoglycemic animals was due to the islet transplant, the graft-bearing omentum was explanted and the glycemic levels monitored to ensure the prompt return to a diabetic state.
Explanted grafts were evaluated via histological analysis. Following fixation in 10 % formalin buffer, grafts were paraffin-embedded and sectioned into 10 µm thick sections. Tissue sections were stained with hematoxylin and eosin, as well as Masson tri-chrome staining (Richard Allan Scientific). Immunofluorescence was used to image islets and vascularization. Staining for insulin was performed using polyclonal primary Guinea pig anti-insulin (1:100; DAKO, A0564) and a secondary goat anti-guinea pig AlexaFluor-488 conjugated antibody (Molecular Probes). Blood vessels were detected via rabbit anti-α-smooth muscle actin (SMA) polyclonal antibody (1:50; Abcam, ab5694) with goat anti-rabbit AlexaFluor-568 conjugated antibody (1:200; Invitrogen A-11036). Nuclei were stained using TO-PRO-3 (1:100; Invitrogen, T3605). All results were compared to isotype controls (no primary) to ensure specificity of detection.
Statistical Analysis
For all retention, viability, and insulin secretion experiments, comparisons between groups (e.g. controls to scaffolds) were made only using the same islet preparation. A minimum of three independent replicate measurements were made for each assay. The number of replicates is indicated in the figure legends, and results are expressed as the mean ± SD. With the exception of human islet studies, a minimum of three independent experiments were made for each assay, with graphs summarizing results from a representative experiment. Statistical analyses for retention, viability, and insulin secretion experiments used a one-way AVOVA and Dunnett’s multiple comparison test (if necessary). For transplant studies, results are expressed as the mean ± SD, with the number of animals within each group indicated in the figure legend. For time course animal studies, repeated-measure analysis of variance with Bonferroni-Holm post hoc analysis for within subject effects was used. Differences were considered significant when p < 0.05.
RESULTS
Retention, Viability, and Function of Islets within Macroporous Scaffolds In-Vitro
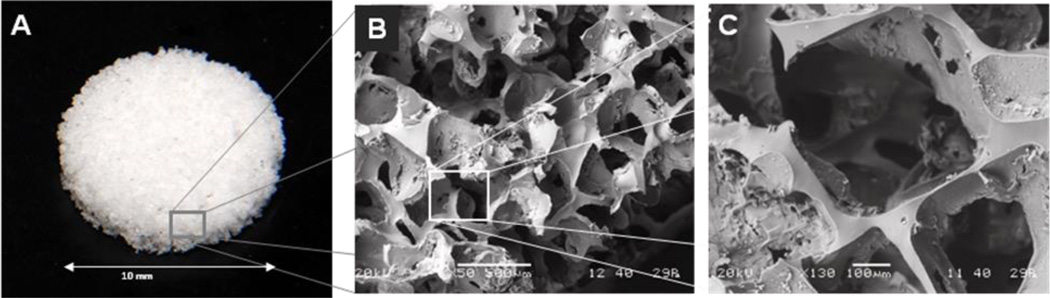
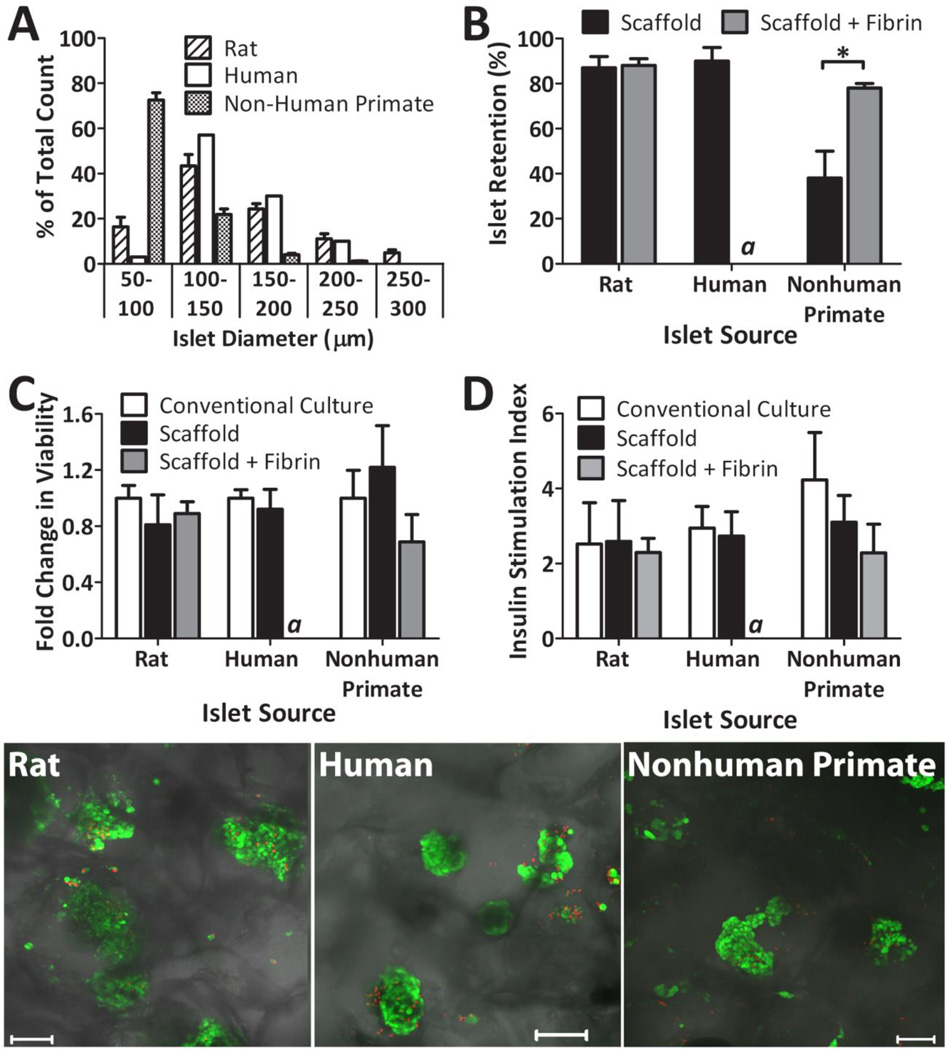
Macroporous PDMS scaffolds were fabricated with 90% porosity and pore sizes ranging from 250–425 µm (see Figure 1). For this study, the final dimension of the scaffold was 10 mm diameter and 2 mm thickness. As shown in Figure 1, PDMS scaffolds were mechanically sound, with open, interconnected pores. The retention of islets within the PDMS scaffold was characterized for rat, non-human primate, and human islets. Initial studies using plain PDMS scaffolds experienced difficulty in islet distribution. Due the hydrophobicity of PDMS, the islet solution did not easily migrate into the scaffold, resulting in pooling of the islets at the top of the scaffold. Adsorption of fibronectin to the surface of the scaffold substantially improved islet distribution, primarily due to the ability of solutions to easily permeate through the now hydrophilic scaffold surface. Fibronectin was selected, in lieu of numerous protein alternatives, due to its ease in adsorption to PDMS, as well as reports indicating positive effects of fibronectin on islet function (19,28). The potential of fibrin gel, filled into the void space of the scaffold after islet loading, to improve islet retention was also evaluated. A summary of retention results, expressed as the percentage of islets retained within the scaffold, for the various islet sources tested is shown in Figure 2B. While rat and human islet sources were found to be highly retained within the scaffold without the need for fibrin gel (~ 90 %), non-human primate (NHP) islets experienced a significant loss. This is likely due to the disparate size distribution of the NHP baboon islets, when compared to the rat and human islets. As shown in Figure 2A, 72.6 % of the NHP baboon islets were less than 100 µm, while 83.6 % of the rat islets and 97 % of the human islets were greater than 100 µm. The addition of fibrin gel to the scaffold after loading increased islet retention of NHP islets to 78 %. These results indicate a correlation between islet retention within these scaffolds and islet size, with increased islet loss for isolations containing a large portion of islets < 100 µm in diameter. To evaluate the effect of the scaffold environment on islet viability and function, the metabolic activity and glucose stimulated insulin release of rat, NHP, and human islets (1500 IEQ / scaffold) loaded within fibronectin coated PDMS scaffolds were evaluated and compared to islets (1500 IEQ) freely floating in petri dishes (labeled as “conventional culture”). Tests were also conducted for rat and NHP islets loaded into scaffolds containing fibrin gel. Assessments were performed after 24 hr culture at 20 % oxygen. As Figure 2C illustrates, the viability of the islets was unaffected by culturing on scaffolds or scaffolds with fibrin gel, regardless of source (p = 0.3278 for rat; p = 0.4214 for human; p = 0.130 for NHP). Static glucose stimulated insulin secretion results are shown in Figure 2D, expressed as insulin stimulation index (insulin release during high glucose over release during low glucose). No statistical difference was observed between culture conditions (conventional culture, scaffold, or scaffold with fibrin gel), regardless of source (p = 0.919 for rat; p = 0.6913 for human; p = 0.1133 for NHP). For all cases, islets displayed well preserved glucose responsiveness, demonstrated as increased insulin output after stimulation with high glucose and a subsequent decrease in output during the second low glucose stimulation. Islet morphology and viability was qualitatively evaluated using multi-slice confocal imaging and live/dead staining. As shown in Figure 2, highly viable islets were found distributed within the scaffold.
Figure 1.
Macroporous PDMS scaffolds as visualized at varying magnifications: photograph (A) and scanning electron microscopy at 50× (B) and 130× (C) magnifications.
Figure 2.
In vitro assessment of islets (1500 IEQ per scaffold or dish) within scaffolds, as compared to conventional culture. (A) Islet distribution for rat, human, and nonhuman primate islets, as tallied using size ranges indicated. (B) Retention of islets within PDMS scaffolds alone or PDMS scaffolds plus fibrin, as quantified via MTT, for rat, human, and nonhuman primate islets. (C) MTT viability, expressed as fold change from control (conventional culture), of rat, nonhuman primate, and human islets in conventional culture dishes, PDMS scaffolds, or PDMS scaffolds with fibrin. No statistically significant change from conventional culture was detected within the same islet source. (D) Insulin secretion index, expressed as the ratio of insulin output under high glucose over insulin output under low glucose, of rat, nonhuman primate, and human islets in conventional culture dishes, PDMS scaffolds, or PDMS scaffolds with fibrin. No statistically significant change from conventional culture was detected within the same islet source. Error bars represent standard deviation (n=3 for A–B; n=4 for C–D). * indicates statistically significant change (p < 0.01). a Experiment not performed. (Bottom Panel) Representative confocal images of LIVE/DEAD (green: viable/ red: dead) staining of rat, human, or nonhuman primate islets within PDMS macroporous scaffolds (no fibrin added). Shadows outline the scaffold PDMS honeycombed architecture.
Evaluation of Islets on Scaffolds under Hypoxic Culture Conditions
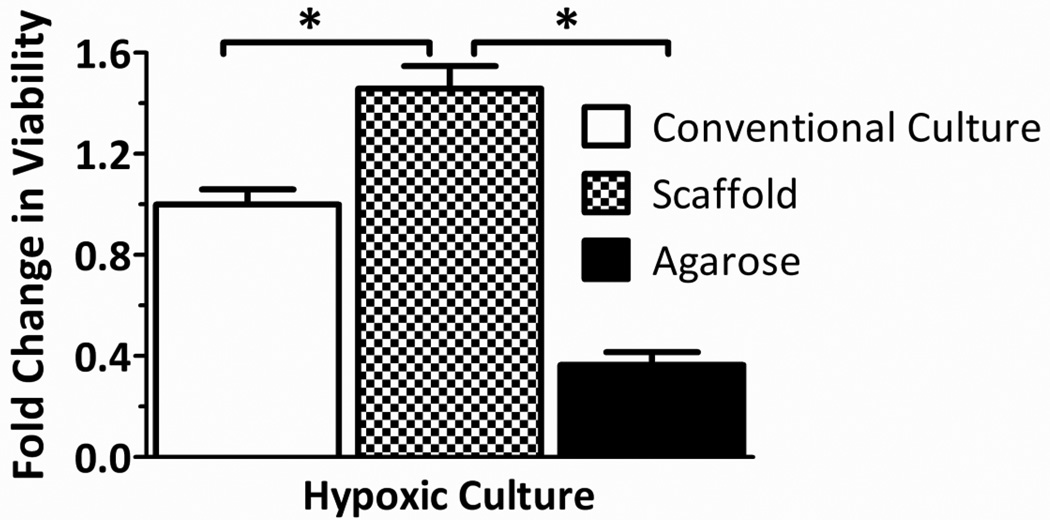
Given that the evaluation of islets within the scaffolds under normoxic conditions likely does not highlight the benefits of a three-dimensional culture system, viability tests were subsequently conducted under conditions more representative of in vivo conditions, where oxygen availability is more restricted. Thus, islets within PDMS macroporous scaffolds were compared to petri dish controls when cultured at hypoxic conditions (0.05 mM oxygen tension). To compare the effects of the scaffold macro-porosity on enhanced viability at low oxygen tensions, results were also compared to islets cultured within microporous agarose hydrogel constructs of identical dimensions. This permitted comparison of the effects of macro- versus micro-porosity, independent of the benefits of three-dimensional distribution. Figure 3 summarizes the changes in islet viability, per MTT, expressed as fold changes from the conventional culture control. Under low oxygen, rat islets on the PDMS scaffold had statistically significant (p = 0.0018) enhancement of viability, when compared to conventional culture controls. This indicates a benefit of macroporous scaffolds when islets are cultured at oxygen tensions typical of that found in vivo. In addition, islets within PDMS macroporous scaffolds were found to exhibit higher viability than islets within agarose microporous constructs, with agarose constructs inferior to even 2-D conventional culture. This indicates that the benefit of the PDMS scaffold is not simply due to the three-dimensional distribution of the islets, but also to the highly macro-porous nature of the scaffold.
Figure 3.
Effects of low oxygen culture on islet viability within scaffolds. Rat islet viability, expressed as fold change from control (conventional culture), of rat islets cultured within conventional standard culture dishes, macroporous PDMS scaffolds, or agarose constructs for 72 hrs at 0.05 mM oxygen. Error bars represent standard deviation (n=3). *indicates statistically significant change (p < 0.01) Agarose constructs were also statistically different from conventional culture group.
Syngeneic Islet Transplantation into Scaffolds
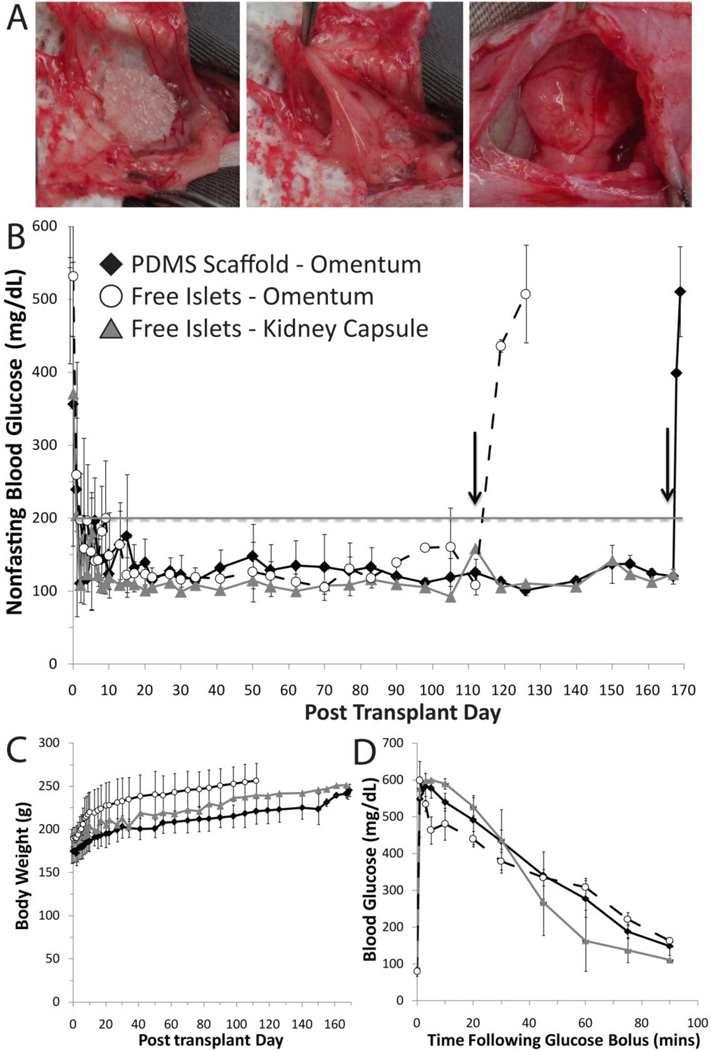
The efficacy of islets, loaded within PDMS macroporous scaffolds, to restore normoglycemia was evaluated in a syngeneic, diabetic rat model. The omentum was selected as the transplant site. Following placement of the scaffold on the omentum, 1800 IEQ were loaded into the scaffold. The scaffold was subsequently wrapped in the omentum, with the tissue edges of the omentum sealed with fibrin gel (Figure 4A). Note that no fibrin gel was used within the scaffold, given that the retention of rat islets within the PDMS scaffold was quite high. For comparison, two control groups were used: free islets in an omental pouch; and free islets in the kidney capsule (1800 IEQ / rat). For all transplants performed, no major adverse events were recorded and all animals survived the surgery.
Figure 4.
Transplants of 1800 IEQ Lewis rat islets into diabetic Lewis rat recipients. (A) Photographs of PDMS scaffold implantation, where a PDMS scaffold was placed onto exposed omentum and loaded with islets (left panel), wrapped in omental tissue (middle panel), sealed with fibrin gel, and placed back into the peritoneum (right panel). (B & C) Nonfasting blood glucose levels (B) and body weight (C) of recipients following transplantation of 1800 IEQ into: PDMS macroporous scaffolds in the omentum (n=5); freely loaded into the omentum (n=5); or freely loaded into the kidney capsule (n=2). Grafts were removed on day 112 for free islets in the omentum and day 168 for islets in PDMS scaffolds (as indicated by arrows), where prompt reversal to diabetic state was observed for all animals. (D) Intravenous glucose tolerance test performed on functional graft recipients at 95 days post transplant. Blood glucose measurements were collected at time points indicated, following injection of bolus glucose, for islets in: PDMS scaffold in omentum (n=4); freely loaded in omentum (n=3); and islets freely loaded into the kidney capsule (n=2). Only functional grafts were used for data analysis shown.
The transplant of islets in PDMS scaffolds in the omentum resulted in a prompt and stable reversal of the diabetic state in 5 of the 6 animals (83.3 %) transplanted, with a mean reversal time of 1.8 ± 1.3 days (1 – 4 days, n = 5). For islets freely loaded into an omental pouch, 5 of the 6 animals (83.3 %) reverted to stable normoglycemia, with a mean reversal time of 5 ± 5.61 days (1 – 15 days, n = 5). Islets in the kidney capsules resulted in reversal from diabetic state (100 %) on day 2 and 6 (n=2). Following reversal, nonfasting blood glucose values for all groups were stable throughout the course of the study. Figure 4B summarizes the average nonfasting blood glucose levels for functional grafts. Body weight of the recipients increased over the time course of the experiment, indicating improved overall metabolic state after transplantation (Figure 4C). Following removal of the omentum, all normoglycemic animals reverted to the diabetic state, confirming that the transplanted grafts were responsible for diabetes reversal, as indicated in Figure 4B.
Evaluation of metabolic control of grafts via a glucose challenge found adequate control for all effective transplants. Glucose tolerance tests on fasted rats (performed at 95 days post-transplant) resulted in prompt clearance of glucose, with restoration to normoglycemic levels (< 200 mg/dL) within 60 mins for all groups, as shown in Figure 4D. Clearance of bolus glucose was statistically identical for all groups, with AUC values at 24, 534 ± 2,700 and 23,401 ± 1,062 for islets in the scaffold and free islets, respectively, while kidney capsule AUC values were 20,881 ± 2,827.
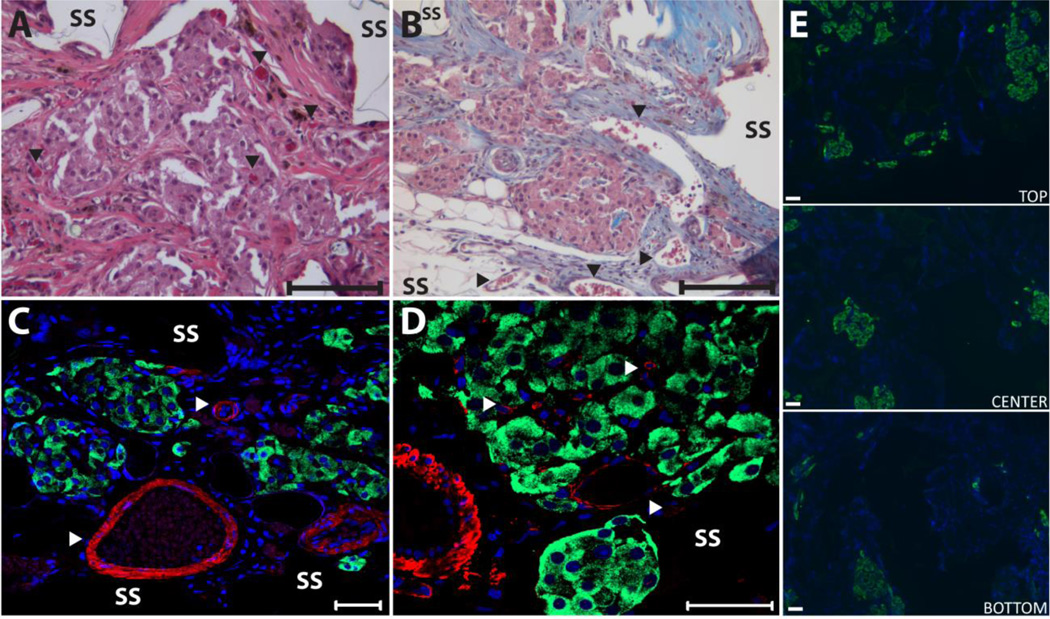
Histological assessment of islets within PDMS scaffolds found robust, vascularized islets expressing high levels of insulin, as shown in Figure 5. H&E and tri-chrome staining of explanted grafts found strong infiltration of host cells and deposition of extracellular matrix into the graft, filling the void space within the scaffold. The scaffold was found to be completely integrated with the surrounding tissue, with no fibrotic capsule detected at the interface with the host and the implant. No observable lymphocytic infiltrate was observed. Islet retained their morphology, with strong insulin expression throughout the islet (Figure 5 C &D; insulin immunofluorescence), illustrating minimal islet fragmentation or central necrosis. As shown in Figure 5 C & D, blood vessels were detected surrounding the embedded islets and within the islets themselves, illustrating intra-scaffold and intra-islet vascularization. Strong α-smooth muscle actin (α-SMA) staining, near and within islets, as well as throughout the scaffold, indicates the presence of mature vessels. Islets were also found distributed throughout the scaffold, as shown in Figure 5E, where insulin immunofluorescence illustrates islets through the entire cross-section of the scaffold. Some asymmetry to the islets loading was found, with a greater portion of the larger islets residing on the top portion, which is assumed to be the loading side of the scaffold.
Figure 5.
Histopathological evaluation of islet-loaded PDMS scaffolds removed on 168 days after implantation in to the omentum. Representative images of H/E (A) and Masson’s Tri-chrome (B) staining of islets within scaffolds; arrows highlight vascular; bar = 100 µm). Representative images of immunofluorescent (C and D) staining of explants for insulin (green) and α-smooth muscle actin (red) with DAPI nuclear counterstain (blue); bar = 50 µm). (E) Representative images of immunofluorescent staining of islets (green=insulin) within explants illustrating islet distribution. Images were collected from top, middle, and lower sections of scaffold (SS=PDMS scaffold).
DISCUSSION
In this study, we sought to evaluate the capacity of a novel macroporous scaffold to serve as a platform for housing islets within a clinically relevant alternative transplant site. Highly porous PDMS scaffolds were fabricated and found to be mechanically sounds with open, interconnected pores. Fibonectin was adsorbed to the PDMS surface, resulting in a hydrophilic surface that may be favorable to the islets. Given that direct comparisons of various proteins coating were not made for this study, we are unable to state the benefits of this approach over other methods to generate a hydrophilic surface (e.g. (4,35,60)). These PDMS macroporous scaffolds were evaluated for their capacity to serve as a matrix for housing islets, both in vitro and in vivo. Our data indicates that islet retention within the scaffold is correlated to islet size. Smaller islets, as those from NHP’s, which in our studies contained the highest proportion of < 100 µm clusters, had lower retention than the rat or human islets tested. With the fabrication method based on particulate leaching, the scaffold pore size can be customized to the desired pore size range in order to enhance islet retention. Nonetheless, the addition of fibrin gel alleviates this loss by improving the retention of small islets within the scaffold following loading.
The viability and functionality of islets under standard culture conditions (0.2 mM oxygen) was not adversely affected by loading within the PDMS scaffold and was similar to controls, which were cultured in petri dishes. Since these experiments were conducted under normal oxygen tension, these results understate the potential of the scaffold for enhancing islet viability in vivo, where oxygen availability is greatly reduced. Decreasing the external oxygen tension to a level more representative of in vivo conditions (0.05 mM oxygen) demonstrated the advantage of three-dimensional islet distribution, with higher cellular viability when compared to two-dimensional cell cultures. Comparisons with micro-porous agarose hydrogels of identical geometry illustrated the benefits of the macro-porosity of the scaffold. While others have entrapped islets within microporous gel matrices in order to prevent islet aggregation, our studies suggest that the use of a macro-porous scaffold may be of greater benefit (5,42).
Islet-loaded scaffolds transplanted into the omental pouch of syngeneic diabetic rats successfully reverted diabetes and sustained long-term normoglycemia. Explanted grafts were found to be well integrated within the host, whereby the macro-porosity and minimal presence of the PDMS material (only 10 % of the implant volume) allowed the infiltrating host cells to largely dictate the environment surrounding the islets. As such, positive extra-cellular matrix deposition was observed, with a presence of blood vessels, both intra- and inter-islet. Given the high metabolism of islets, formation of a well-developed vascular network is a critical component to preservation of islet viability, as well as graft responsiveness.
In vivo islet retention within the scaffold was high, with islets found throughout the implant. Due to the biostability and mechanical integrity of the PDMS scaffold, we did not observe breakage or mechanical disruption of the implant after almost 170 d. While mechanical testing of the PDMS scaffold has yet to be performed, these transplant studies indicate robust mechanical integrity of the scaffold in this animal model.
In terms of clinical applicability, PDMS-based implant have a proven clinical safety profile and prolific use in long-term implants (16). The scale of our device also permits ease in clinical translation. Indeed, by increasing the diameter of the scaffold, islet loading density can be preserved using reasonably sized scaffold(s), while maintaining the ability to accommodate the islet numbers necessary at the clinical scale. The use of the clinically relevant omentum site also provides ease in the translation of these promising studies to larger animal models.
Finally, this study illustrates that the use of a PDMS-based macroporous structure can provide a means to competently house islets in a site alternative to the standard intrahepatic portal system. This study lays the foundation for future work focused on the use of this PDMS platform to further modulate the local environment. Given the material selected, this platform serves as an ideal means for the local and slow delivery of anti-inflammatory drugs and immunomodulatory agents, as well as oxygen delivery (9,21,43,57). Furthermore, given that the fibronectin coated surface of the PDMS scaffold permits cell attachment, this platform can also serve as a means to co-deliver islets with other “helper” cells, such as mesenchymal stem cells or endothelial cells, which have shown great benefit in islet engraftment (1,8,32,45). Future studies are focused on translating this platform to a bioactive scaffold capable of actively modulating the surrounding milieu.
CONCLUSIONS
Given the mechanical, chemical, and inflammatory limitations of the intraportal islet site, finding alternative sites for the transplant of islets is critically important. Housing of islets within a three-dimensional structure, such as the PDMS scaffold presented herein, can provide the following positive attributes: spatial distribution, mechanical integrity, and intra-device vascularization. Biocompatible, highly porous PDMS scaffolds were successfully fabricated and found to be conducive to housing islets of a typical size. We have demonstrated that macroporous PDMS scaffolds have high islet retention, while maintaining islet viability and function. Their biostability and biocompatibility make them appropriate for in vivo implantation. By transplanting them into the omental pouch, we have shown feasibility of both the scaffold and this clinically relevant site. The PDMS scaffold represents a self-contained vehicle for not only transplanting islets, but also for the delivery of drugs, agents, or cells within the micro-niche of the transplant site. While the PDMS scaffold was initially developed for islet transplantation, it can easily be tailored to other applications by modifying its: geometry, porosity, pore size, and protein surface coating. Future studies are focused on scaling up these scaffolds to larger animal studies, as well as evaluating these scaffolds as a multi-functional platform for the modulation of the transplant site.
ACKNOWLEDGEMENTS
This research was supported by the Juvenile Diabetes Research Center for Islet Transplantation at the University of Miami – Diabetes Research Institute (4-2004-361), the Diabetes Research Institute Foundation, the Department of Defense Somatic Cell Processing Facility at the DRI (N00244-07-C-1529), and Converge Biotech, Inc. E.P was supported by an NIH NIDDK Predoctoral Fellowship (1F311EB008970-01A1). We thank the NIDDK- and JDRF-supported Islet Cell Resource Center for providing the human islets used in this study, with funding support by NIH grants DK70460, NIH U42 RR016603, and the City of Hope, Duarte, California. We thank the Diabetes Research Institute Preclinical and Translational Models Core for providing assistance with the rodent studies. We thank Irayme Labrada, Jessica Weaver, and Maria Coronel for their technical assistance in the surgeries. We thank the Joanne C. and Edward A. Dauer Field-Emission Environmental Scanning Electron Microscope Laboratory for use of their instrument, in particular Dr. Edward Dauer for his technical assistance. We thank the Diabetes Research Institute Analytical Imaging Core for use of their facilities, as well as the assistance of George McNamara in image collection and processing. We thank Kevin Johnson from the Diabetes Research Institute Histology Core for his excellent skill in processing the challenging histological samples.
Conflict of interest disclosure. Stockholder (CAF, RDM, NSK, AP, CR and CLS), and scientific advisory board members (CAF, NSK, AP, CR and CLS) of Converge Biotech Inc. (Miami). EP, CAF, NSK, AP, CR and CLS have filed a patent application that includes these PDMS scaffolds.
REFERENCES
- 1.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MHI. Immunomodulation by Mesenchymal Stem Cells. Diabetes. 2008;57(7):1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.al-Abdullah IH, Anil Kumar MS, Kelly-Sullivan D, Abouna GM. Site for unpurified islet transplantation is an important parameter for determination of the outcome of graft survival and function. Cell Transplant. 1995;4(3):297–305. doi: 10.1177/096368979500400308. [DOI] [PubMed] [Google Scholar]
- 3.Ao Z, Matayoshi K, Lakey JR, Rajotte RV, Warnock GL. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation. 1993;56(3):524–529. doi: 10.1097/00007890-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Aucoin L, Griffith CM, Pleizier G, Deslandes Y, Sheardown H. Interactions of corneal epithelial cells and surfaces modified with cell adhesion peptide combinations. J Biomater. Sci. Polym. Ed. 2002;13(4):447–462. doi: 10.1163/156856202320253956. [DOI] [PubMed] [Google Scholar]
- 5.Aung T, Inoue K, Kogire M, Doi R, Kaji H, Tun T, Hayashi H, Echigo Y, Wada M, Imamura M, Fujisata T, Maetani S, Iwata H, Ikada Y. Comparison of various gels for immobilization of islets in bioartificial pancreas using a mesh-reinforced polyvinyl alcohol hydrogel tube. Transplant. Proc. 1995;27(1):619–621. [PubMed] [Google Scholar]
- 6.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups. J. Med. Sci. 2000;105(2):125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 7.Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am. J. Transplant. 2009;9(1):91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'Connor DH, Bartholomew AM, Kenyon NS. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59(10):2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernik DL. Silicon based materials for drug delivery devices and implants. Recent Pat. Nanotechnol. 2007;1(3):186–192. doi: 10.2174/187221007782360402. [DOI] [PubMed] [Google Scholar]
- 10.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald P, Wang X, Khan A, Bernal A, Fraker C, Inverardi L, Ricordi C. Quantitative assessment of islet cell products: estimating the accuracy of the existing protocol and accounting for islet size distribution. Cell Transplant. 2009;18(10):1223–1235. doi: 10.3727/096368909X476968. [DOI] [PubMed] [Google Scholar]
- 12.Calafiore R. Actual perspectives in biohybrid artificial pancreas for the therapy of type 1, insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1998;14(4):315–324. doi: 10.1002/(sici)1099-0895(199812)14:4<315::aid-dmr235>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Calafiore R, Basta G, Falorni A, Jr, Ciabattoni P, Brotzu G, Cortesini R, Brunetti P. Intravascular transplantation of microencapsulated islets in diabetic dogs. Transplant Proc. 1992;24(3):935–936. [PubMed] [Google Scholar]
- 14.Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, Racanicchi L, Mancuso F, Brunetti P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29(1):137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 15.Chaikof EL. Engineering and material considerations in islet cell transplantation. Annu. Rev. Biomed. Eng. 1999;1:103–127. doi: 10.1146/annurev.bioeng.1.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Colas A, Curtis J. Medical Applications of Silicones. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials Science, An Introduction to Materials in Medicine. Burlington: Elsevier; 2004. pp. 697–707. [Google Scholar]
- 17.Cox ME, Dunn B. Oxygen diffusion in poly(dimethyl siloxane) using fluorescence quenching. I. Measurement technique and analysis. J. Poly. Sci. A: Poly. Chem. 1986;24(4):621–636. [Google Scholar]
- 18.Cuthbertson RA, Mandel TE. A comparison of portal versus systemic venous drainage in murine foetal pancreatic islet transplantation. Aust. J. Exp. Biol. Med. Sci. 1986;64(Pt 2):175–184. doi: 10.1038/icb.1986.19. [DOI] [PubMed] [Google Scholar]
- 19.Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 2010;31(7):1676–1682. doi: 10.1016/j.biomaterials.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 20.De Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45(2):159–173. doi: 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- 21.Di Colo G. Controlled drug release from implantable matrices based on hydrophobic polymers. Biomaterials. 1992;13(12):850–856. doi: 10.1016/0142-9612(92)90178-q. [DOI] [PubMed] [Google Scholar]
- 22.Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, Korbutt GS. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9–10):1323–1331. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- 23.Fraker C. Biomedical Engineering. Miami: University of Miami; 2011. The Role of Oxygen During In Vitro Culture and Immunoisolation of Islets of Langerhans; p. 324. [Google Scholar]
- 24.Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL, Jr, Shea LD. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials. 2011;32(36):9677–9684. doi: 10.1016/j.biomaterials.2011.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan J, Zucker PF, Atkison P, Behme MT, Dupre J, Stiller CR. Liver-omental pouch and intrahepatic islet transplants produce portal insulin delivery and prevent hyperinsulinemia in rats. Transplant Proc. 1995;27(6):3236. [PubMed] [Google Scholar]
- 26.Gupta R, Sefton MV. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng. Part A. 2011;17(15–16):2005–2015. doi: 10.1089/ten.tea.2010.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall KK, Gattas-Asfura KM, Stabler CL. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 2011;7(2):614–624. doi: 10.1016/j.actbio.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamamoto Y, Fujimoto S, Inada A, Takehiro M, Nabe K, Shimono D, Kajikawa M, Fujita J, Yamada Y, Seino Y. Beneficial effect of pretreatment of islets with fibronectin on glucose tolerance after islet transplantation. Horm. Metab. Res. 2003;35(8):460–465. doi: 10.1055/s-2003-41802. [DOI] [PubMed] [Google Scholar]
- 29.Hesse UJ, Sutherland DE, Gores PF, Sitges-Serra A, Najarian JS. Comparison of splenic and renal subcapsular islet autografting in dogs. Transplantation. 1986;41(2):271–274. doi: 10.1097/00007890-198602000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Hou QP, Bae YH. Biohybrid artificial pancreas based on macrocapsule device. Adv Drug Deliv Rev. 1999;35(2–3):271–287. doi: 10.1016/s0169-409x(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 31.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgen O, Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 32.Johansson U, Elgue G, Nilsson B, Korsgen O. Composite islet-endothelial cell grafts: a novel approach to counteract innate immunity in islet transplantation. Am. J. Trans. 2005;5:2632–2639. doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 33.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgen O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem. Biophys. Res. Commun. 2003;308:474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman DB, Morel P, Field MJ, Munn SR, Sutherland DE. Purified canine islet autografts. Functional outcome as influenced by islet number and implantation site. Transplantation. 1990;50(3):385–391. [PubMed] [Google Scholar]
- 35.Khorasani MT, Mirzadeh H. Laser surface modification of silicone rubber to reduce platelet adhesion in vitro. J. Biomater. Sci. Polym. Ed. 2004;15(1):59–72. doi: 10.1163/156856204322752237. [DOI] [PubMed] [Google Scholar]
- 36.Kim HI, Yu JE, Park CG, Kim SJ. Comparison of four pancreatic islet implantation sites. J. Korean Med. Sci. 2010;25(2):203–210. doi: 10.3346/jkms.2010.25.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am. J. Transplant. 2003;3(3):281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 38.Kin T, O'Neil JJ, Pawlick R, Korbutt GS, Shapiro AM, Lakey JR. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artif. Organs. 2008;32(12):990–993. doi: 10.1111/j.1525-1594.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, Ko S, Nagao M, Harb G, Nakajima Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006;15(4):359–365. doi: 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- 40.Kriz J, Vilk G, Mazzuca DM, Toleikis PM, Foster PJ, White DJG. A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence. Am. J Surg. 2013;203(16):793–797. doi: 10.1016/j.amjsurg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Kuntz E, Kuntz H-D. Hepatology, Principles and Practice. 2nd ed. Germany: Springer; 2005. [Google Scholar]
- 42.Lacy PE, Hegre OD, Gerasimidi-Vazeou A, Gentile FT, Dionne KE. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254(5039):1782–1784. doi: 10.1126/science.1763328. [DOI] [PubMed] [Google Scholar]
- 43.Langer R. Implantable controlled release systems. Pharmacol. Ther. 1983;21(1):35–51. doi: 10.1016/0163-7258(83)90066-9. [DOI] [PubMed] [Google Scholar]
- 44.Li YX, Li G, Dong WP, Lu DR, Tan JM. Protection of human islets from induction of apoptosis and improved islet function with HO-1 gene transduction. Chin. Med. J. (Engl) 2006;119(19):1639–1645. [PubMed] [Google Scholar]
- 45.Liu M, Han ZC. Stem Cells Review Series: Mesenchymal stem cells: biology and clinical potential in type 1 diabetes therapy. J. Cell. Mol. Med. 2008;12(4):1155–1168. doi: 10.1111/j.1582-4934.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24(12):4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 47.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011;3(100):100ra189. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 48.Mata A, Fleischman A, Roy S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices. 2005;7(4):281–293. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 49.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes. 2004;53(4):948–954. doi: 10.2337/diabetes.53.4.948. [DOI] [PubMed] [Google Scholar]
- 50.McQuilling JP, Arenas-Herrera J, Childers C, Pareta RA, Khanna O, Jiang B, Brey EM, Farney AC, Opara EC. New Alginate Microcapsule System for Angiogenic Protein Delivery and Immunoisolation of Islets for Transplantation in the Rat Omentum Pouch. Transplant. Proc. 2011;43(9):3262–3264. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care. 2009;32(8):1563–1569. doi: 10.2337/dc09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, Ekdahl KN, Korsgen O, Nilsson B. Productions of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 53.Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes. 2001;50(2):270–276. doi: 10.2337/diabetes.50.2.270. [DOI] [PubMed] [Google Scholar]
- 54.Nishikawa M, Yamamoto T, Kojima N, Kikuo K, Fujii T, Sakai Y. Stable immobilization of rat hepatocytes as hemispheroids onto collagen-conjugated poly-dimethylsiloxane (PDMS) surfaces: Importance of direct oxygenation through PDMS for both formation and function. Biotech. Bioeng. 2008;99(6):1472–1481. doi: 10.1002/bit.21690. [DOI] [PubMed] [Google Scholar]
- 55.Ohgawara H, Hirotani S, Miyazaki J, Teraoka S. Membrane immunoisolation of a diffusion chamber for bioartificial pancreas. Artif. Organs. 1998;22(9):788–794. doi: 10.1046/j.1525-1594.1998.06185.x. [DOI] [PubMed] [Google Scholar]
- 56.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–276. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Pedraza E, Coronel M, Fraker C, Ricordi C, Stabler C. Enhancing Beta-Cell Viability via Hydrolytically Activated, Oxygen Generating Biomaterials. Proc. Natl. Acad. Sci USA. 2012;109(11):4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 59.Robertson RP. Intrahepatically transplanted islets--strangers in a strange land. J. Clin. Endocrinol. Metab. 2002;87(12):5416–5417. doi: 10.1210/jc.2002-021612. [DOI] [PubMed] [Google Scholar]
- 60.Roth J, Albrecht V, Nitschke M, Bellmann C, Simon F, Zschoche S, Michel S, Luhmann C, Grundke K, Voit B. Surface Functionalization of Silicone Rubber for Permanent Adhesion Improvement. Langmuir. 2008;24(21):12603–12611. doi: 10.1021/la801970s. [DOI] [PubMed] [Google Scholar]
- 61.Salvay D, Rives C, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr, Shea LD. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Remms JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 63.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Legeay G, Bellocq JP, Pinget M, Kessler L. Induction of angiogenesis in omentum with vascular endothelial growth factor: influence on the viability of encapsulated rat pancreatic islets during transplantation. J. Vasc. Res. 2003;40(4):359–367. doi: 10.1159/000072700. [DOI] [PubMed] [Google Scholar]
- 64.Silva A, de Matos A, Brons I, Mateus M. An overview on the development of a bio-artificial pancreas as a treatment of insulin-dependent diabetes mellitus. Med. Res. Rev. 2006;26(2):181–222. doi: 10.1002/med.20047. [DOI] [PubMed] [Google Scholar]
- 65.Silva A, Mateus M. Development of a polysulfone hollow fiber vascular bio-artificial pancreas device for in vitro studies. J. Biotech. 2009;139(3):236–249. doi: 10.1016/j.jbiotec.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Storrs R, Dorian R, King SR, Lakey J, Rilo H. Preclinical development of the Islet Sheet. Ann. N.Y. Acad. Sci. 2001;944:252–266. doi: 10.1111/j.1749-6632.2001.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 67.Sun AM. Microencapsulation of pancreatic islet cells: a bioartificial endocrine pancreas. Methods Enzymol. 1988;137:575–580. doi: 10.1016/0076-6879(88)37053-9. [DOI] [PubMed] [Google Scholar]
- 68.Toworfe GK, Composto RJ, Adams CS, Shapiro IM, Ducheyne P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. Biomed. Mater. Res. A. 2004;71A(3):449–461. doi: 10.1002/jbm.a.30164. [DOI] [PubMed] [Google Scholar]
- 69.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 70.Warnock GL, Dabbs KD, Evans MG, Cattral MS, Kneteman NM, Rajotte RV. Critical mass of islets that function after implantation in a large mammalian. Horm. Metab. Res. Suppl. 1990;25:156–161. [PubMed] [Google Scholar]
- 71.Warnock GL, Rajotte RV. Pancreatic Islet Cell Transplantation: A new era in transplantation. Can. Fam. Physician. 1992;38:1655–1660. [PMC free article] [PubMed] [Google Scholar]
- 72.White SA, Davies JE, Pollard C, Swift SM, Clayton HA, Sutton CD, Weymss-Holden S, Musto PP, Berry DP, Dennison AR. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann. Surg. 2001;233(3):423–431. doi: 10.1097/00000658-200103000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White SA, London NJ, Johnson PR, Davies JE, Pollard C, Contractor HH, Hughes DP, Robertson GS, Musto PP, Dennison AR. The risks of total pancreatectomy and splenic islet autotransplantation. Cell Transplant. 2000;9(1):19–24. doi: 10.1177/096368970000900103. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Iwata J, Shimizu H, Takagi T, Tsuji T, Ito F. Comparative studies of in vitro and in vivo function of three different shaped bioartificial pancreases made of agarose hydrogel. Biomaterials. 1994;15(2):113–120. doi: 10.1016/0142-9612(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z, Chen M, Carter J, Ellett J, Smith K, Nadler J. Inflammation blockade improves pancreatic islet function. Transplant Proc. 2004;36:2864–2865. doi: 10.1016/j.transproceed.2004.09.083. [DOI] [PubMed] [Google Scholar]
- 76.Yasunami Y, Lacy PE, Finke EH. A new site for islet transplantation--a peritoneal-omental pouch. Transplantation. 1983;36(2):181–182. doi: 10.1097/00007890-198308000-00014. [DOI] [PubMed] [Google Scholar]