Abstract
Purpose
Symptomatic atherosclerotic disease of the popliteal artery presents challenges for endovascular therapy. We evaluated the technical success, complications and midterm outcomes of atherectomy and angioplasty involving the popliteal segment.
Methods
We conducted a retrospective review of outcomes of popliteal artery intervention using atherectomy or angioplasty performed between 2003 and 2008.
Results
A total of 56 patients (36% women, age 72.8±12.2 years, 77% critical limb ischemia) underwent popliteal atherectomy (n=18) or angioplasty (n=38). These patients had similar clinical characteristics, TASC/TASC II classification, mean lesion length, and run-off scores. We observed a trend toward higher rates of technical success defined as <30% residual stenosis after atherectomy compared to angioplasty (94% vs. 71%, p=0.08). While angioplasty was associated with a higher frequency of arterial dissection (23% vs. 0%, p=0.003), atherectomy was associated with a higher rate of thromboembolic events (22% vs 0%, p=0.01). Adjunctive stenting was used more frequently following angioplasty compared to atherectomy (45% vs. 6%, p=0.005). Thrombolysis was used to treat embolization in 4 patients in the atherectomy group. The improvement in the ankle-brachial index was similar between the two treatment groups. Primary patency of the popliteal artery at 3, 6, and 12 months was 94%, 88%, and 75% in the atherectomy group and 89%, 82%, and 73% in the angioplasty group (p=NS). There were no significant differences in limb salvage and freedom from reintervention at 1 year between the atherectomy and angioplasty groups.
Conclusions
Our experience with popliteal artery endovascular therapy indicates a distinct pattern of procedural complications with atherectomy compared to angioplasty but similar midterm patency, limb salvage and freedom from intervention.
Keywords: atherosclerosis, atherectomy, plaque excision, popliteal, angioplasty, stent
Introduction
The last decade has seen a significant increase in the use of minimally invasive techniques to treat vascular disease. Tried and true open surgical operations used to treat infrainguinal arterial occlusive disease are gradually being replaced by newer endovascular procedures such as atherectomy and angioplasty1. Endovascular procedures, unlike many open techniques which bypass diseased segments, direct treatment to specific areas of the arterial tree.
The literature has traditionally combined the popliteal artery with the femoral vessels.2–6 However, the popliteal artery has unique characteristics. Unlike the superficial femoral artery, the popliteal artery has its embryological origin from the sciatic system.7 Anatomically, unlike the femoral and tibial arteries, the popliteal artery is not contained within a muscular compartment.8 Finally, the popliteal artery is subjected to significant biomechanical forces that result from repetitive flexion of the knee and ankle.9,10 Because specific arterial segments have unique properties, different endovascular modalities applied to these arteries may lead to different outcomes.
We evaluated procedural and midterm outcomes of two endovascular procedures on atherosclerotic occlusive disease of the popliteal artery by reviewing our experience with atherectomy and angioplasty.
Methods
A retrospective review was conducted to identify all patients who, between September 2003 and January 2008, underwent endovascular revascularization including treatment of the popliteal artery with atherectomy using the Silverhawk Plaque Excision Catheter (Fox Hollow Technologies, Inc, Redwood City, California) or balloon angioplasty. All patients were initially considered for both open and endovascular procedures. Patients were selected for endovascular revascularization based on presence of comorbid conditions that would increase the risk for open operation, absence of adequate autogenous vein conduit, poor distal target, or physician preference. Patients who had primary stenting or both modalities used in the popliteal artery were excluded. Additionally, any procedure that did not have imaging adequate for analysis was excluded. This study was approved by the Boston Medical Center Institutional Review Board.
After the patients were identified from a prospective database, the inpatient and outpatient records were reviewed. From these records the following data was extracted; demographic data, associated comorbid conditions, medications (antiplatelets, anticoagulants and statins), indications for intervention, procedural details, and complications.
Procedural angiograms were reviewed to extract the following data; extent of disease as classified by the TransAtlantic Inter-Society Consensus (TASC and TASC II) for femoropopliteal disease2,3, lesion length, presence of occlusive lesions, vessels treated, adjunctive procedures, intraoperative complications and run-off. Definitions for comorbid conditions were based on recommended standards for reporting by Rutherford et al.11 Run-off score was calculated based on completion arteriograms done at the time of the procedure.11
Technical success, lesion-specific complications, need for adjunctive procedures, and change in peri-procedural ankle brachial index (ABI) were evaluated. Mid-term outcomes of limb salvage, primary patency, and freedom from reintervention were assessed at 3, 6 and 12 months post-procedure.
All endovascular procedures were performed in a dedicated endovascular suite. Once the target lesion was identified the decision to treat with either atherectomy or angioplasty was at the discretion of the interventionalist. All patients were administered heparin to maintain an activated clotting time (ACT) above 260 seconds. If atherectomy was used, an appropriate Silverhawk device was introduced through a 7 French sheath, advanced over a 0.014 inch guidewire to the proximal aspect of the lesion, and activated. A distal embolic protection device was not used. If angioplasty was chosen, then a 6 French sheath was placed and an appropriately sized balloon was selected. Balloon inflation was executed above the nominal pressure for the duration of one minute. A completion arteriogram was obtained in all patients to assess technical results and evaluate for any complications. Stents were placed only under the condition of flow limiting dissection or inadequate technical result. Only self expanding Nitinol stents were deployed. All patients that had a stent placed were given clopidogrel unless contraindicated. Embolization was treated with suction embolectomy, mechanical thrombolysis using the Angiojet device (Possis Medical, Minneapolis, MN), or chemical thrombolysis with Alteplase (recombinant Tissue Plasminogen Activator, Genentech Inc, San Francisco, CA).
Technical success of the stand-alone procedure (angioplasty or atherectomy) was defined as residual stenosis less than 30% without the use of adjunctive procedures including stents. Vessel-specific complications were defined as thrombosis, dissection, perforation or embolization related to the treated vessel. Any intervention required at the time of the atherectomy or angioplasty to achieve adequate revascularization was considered an adjunctive procedure. This included placement of stents, thrombolysis (chemical or mechanical), or urgent surgical intervention. The change in ABI between groups was calculated based on the difference between pre-procedural and post-procedural ABI values. Post-procedural ABI values were obtained within one month of the procedure.
Any adverse event occurring within 30 days of the procedure was recorded as a complication. Primary patency was defined as loss of patency on duplex or angiogram or date of reintervention. Freedom from reintervention was defined as freedom from any secondary endovascular or surgical intervention during the follow-up period. Limb salvage was defined as avoidance of a major amputation above the ankle.
Statistical analysis was performed using Statistical Analysis Software 9.1 (SAS Institute Inc., Cary, NC). Results for continuous variables were presented as mean±standard deviation unless otherwise noted. Two-group comparisons of continuous variables were assessed by the independent Wilcoxon rank sum test. Group comparisons of categorical variables were assessed by Fisher’s exact test. Multivariate linear regression models were used to assess the influence of the type of procedure on change in ABI while adjusting for potential confounding variables.
To assess the impact of the type of procedure upon survival, limb salvage, primary patency, and freedom from reintervention, the distribution of these outcome variables for the patients undergoing angioplasty and atherectomy were compared. Survival curves were estimated using the Kaplan-Meier method, and compared using the log-rank test. The assumption of proportional hazards was assessed prior to attempting Cox modeling. Multivariate Cox proportional hazard models were used to assess the influence of the type of procedure on patient outcomes while adjusting for confounding variables. The patient outcomes evaluated were survival, limb salvage, primary patency, and freedom from reintervention.
The clinical co-variates included in the multivariate models were selected either because they were distributed differently across the two procedure groups (clopidogrel, aspirin, stent, dissection, thrombosis, residual stenosis, and run-off), significantly associated with outcome (pre-ABI, run-off, lesion length, chronic kidney disease, and occlusive lesions) or were believed to be clinically important based on the current literature (diabetes). Backward selection with exit levels of 0.2 was used to eliminate unimportant variables.
Results
Between September 2003 and January 2008, we identified a total of 60 consecutive patients who underwent endovascular treatment of a popliteal artery lesion. Two of these patients had primary popliteal artery stents placed and were excluded, one was eliminated because imaging was not adequate for analysis, and the remaining patient was eliminated because separate lesions within the popliteal artery were treated with both modalities. In the remaining 56 patients, 56 popliteal arteries were treated. Eighteen patients underwent atherectomy and 38 patients were treated with angioplasty.
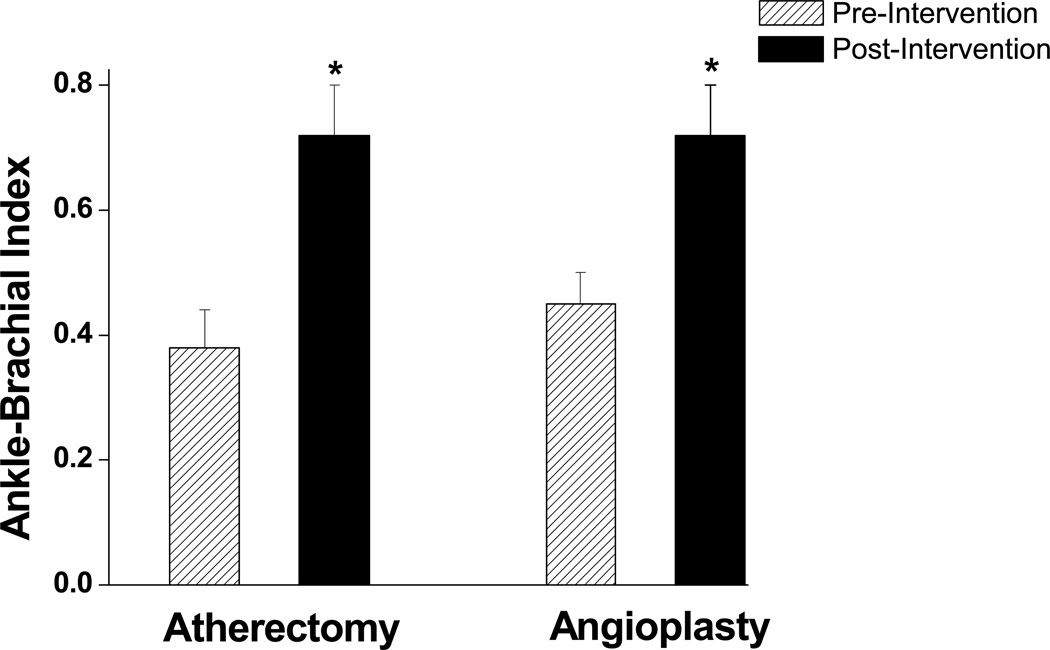
The mean age at presentation was 72.8±12.2 years and 36% of the patients were female. There was no significant difference between the two groups with respect to gender, indication for intervention, associated comorbid conditions, use of statins, aspirin or anticoagulant medications (Table 1). Pre-procedural clopidogrel usage was greater in the atherectomy group. The mean ABI at presentation was not significantly different between the two groups (Figure 1).
Table 1.
Clinical Characteristics by Treatment Group
| Atherectomy (n=18) |
Angioplasty (n=38) |
|
|---|---|---|
| Age, years | 74±11 | 73± 3 |
| Sex, male | 13 (72%) | 23 (61%) |
| Critical limb ischemia | 12(67%) | 31(82%) |
| Smoking | 7 (39%) | 16 (42%) |
| Diabetes | 10 (56%) | 25 (66%) |
| Hypertension | 16 (89%) | 36 (95%) |
| Hyperlipidemia | 11 (61%) | 28 (74%) |
| Chronic kidney disease | 3 (17%) | 11 (29%) |
| Coronary artery disease | 11 (61%) | 28 (74%) |
| Cerebrovascular disease | 8 (44%) | 14 (37%) |
| Medications | ||
| Statins | 10 (56%) | 25 (66%) |
| Clopidogrel* | 13 (72%) | 16 (42%) |
| Aspirin | 13 (72%) | 35 (92%) |
| Coumadin | 6 (33%) | 10 (26%) |
All P=NS except
P=0.047
Figure 1.
Effect of endovascular treatment with angioplasty or atherectomy on ABI. As shown, in both groups ABI was higher after treatment (*P<0.001). There were no differences in baseline ABI (P=0.82) or the magnitude of increase in ABI (P=0.63) following intervention between atherectomy and angioplasty groups.
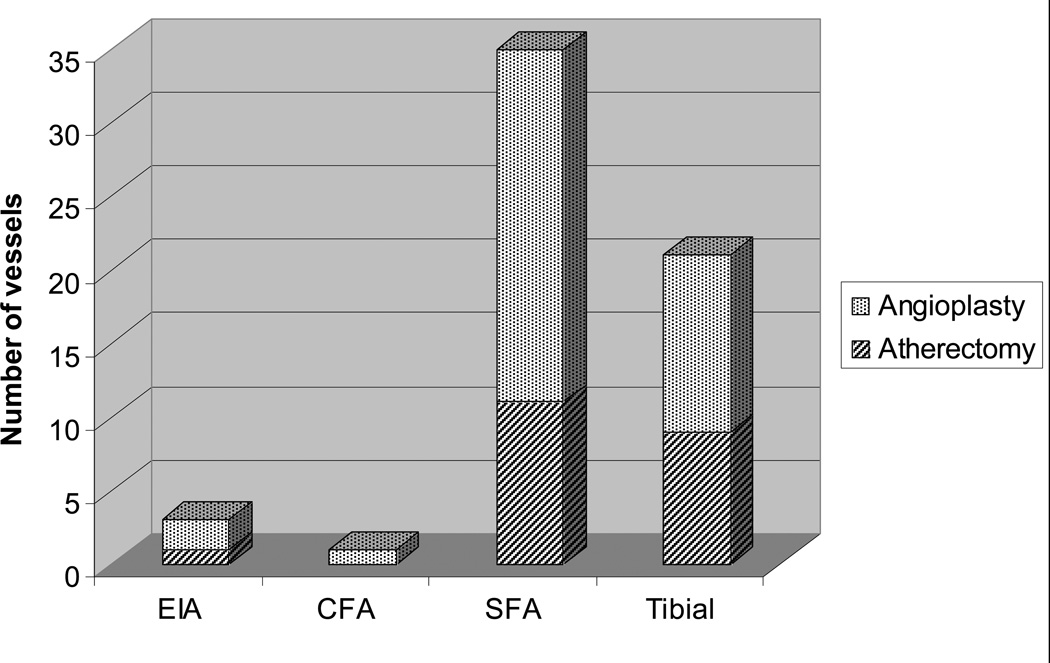
The atherectomy and angioplasty groups did not significantly differ with respect to TASC and TASC II classifications, run-off score, lesion length, number of vessels treated, and the presence of occlusive lesions (Table 2). Overall, 21% of the patients had isolated popliteal lesions. Of the 44 patients that had additional lesions treated, the most common vessel treated was the superficial femoral artery (80%) (Appendix 1). With the exception of one patient in each treatment group, patients with additional lesions were treated with the same respective modality.
Table 2.
Anatomic Description of Lesions
| Atherectomy (n=18) |
Angioplasty (n=38) |
|
|---|---|---|
| TASC (Femoropopliteal) |
||
| A | 3 (17%) | 5 (13%) |
| B | 5 (28%) | 15 (40%) |
| C | 8 (44%) | 8 (21%) |
| D | 2 (11%) | 10 (26%) |
| TASC II (Femoropopliteal) |
||
| A | 0 (0%) | 0 (0%) |
| B | 11 (61%) | 24 (63%) |
| C | 7 (39%) | 12 (32%) |
| D | 0 (0%) | 2 (5%) |
| Run-Off Score | 5.67±2.39 | 6.55±2.88 |
| Lesion length (cm) | 3.44±2.31 | 4.59±3.60 |
| Occlusive lesions | 6 (33%) | 13 (34%) |
| Mean Number vessels treated per limb |
2.17±0.71 | 2.03±0.72 |
All P=NS
Appendix 1. Distribution of Additional Vessels Treated.
Number of additional vessels treated in various arterial segments. EIA, external iliac artery, CFA, common femoral artery, SFA, superficial femoral artery, Tibial, includes tibioperoneal trunk, anterior tibial, peroneal, and posterior tibial arteries.
As shown in Table 3, there was a trend toward higher technical success for the primary popliteal artery procedure with atherectomy as compared to angioplasty. Overall complication rates were similar; however, the specific complications were distinctive to the treatment modality. Dissections occurred in the angioplasty group but not the atherectomy group. A greater rate of thromboembolic events occurred in the atherectomy group with 3 cases of popliteal artery thrombosis, and 1 tibial embolization. Thromboembolic complications were treated with mechanical and chemical thrombolysis and were successful in restoring flow in all but one case in each group. In both of these cases stents were ultimately deployed. There were no vessel perforations or need for urgent open revascularization in either group.
Table 3.
Procedural results and complications
| Atherectomy (n=18) |
Angioplasty (n=38) |
P-value | |
|---|---|---|---|
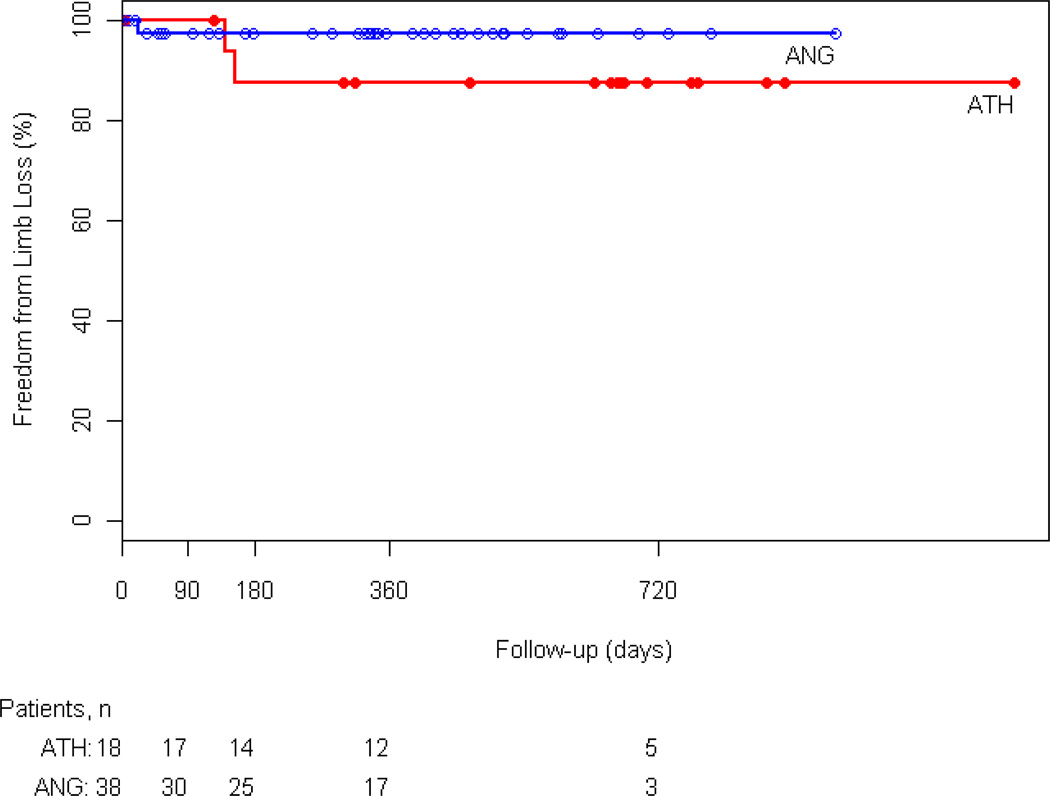
| Technical success | 17 (94%) | 27 (71%) | .079 |
| Lesion-Specific Complications | |||
| Perforation | 0 (0%) | 0 (0%) | - |
| Dissection | 0 (0%) | 10 (26%) | .022 |
| Thromboembolism | 4 (22%) | 0 (0%) | .008 |
| Total | 4 (22%) | 10 (26%) | .999 |
| Adjunctive Interventions | |||
| Thrombolysis | 4 (22%) | 0 (0%) | .008 |
| Stent use | 1 (4%) | 17 (45%) | .005 |
| Flow-limiting dissection | 0 | 8 | |
| Residual stenosis >30% | 0 | 9 | |
| Other | 1 | 0 | |
The use of a stent as an adjunctive procedure was performed more frequently in the angioplasty group (Table 3). The indications for stent placement in the angioplasty group included residual stenosis greater than 30% and flow-limiting dissection. There was only one lesion in the atherectomy group that required stent placement due to acute popliteal artery thrombosis unresponsive to thrombolysis. Stents were deployed in the above knee popliteal artery except for one that was placed in a position crossing the knee joint.
The post-procedural ABI and change in the ABI were similar between the two groups (Figure 1). There were no significant differences in 30 day mortality or general complication rates between the two groups. One patient in the angioplasty group died within 30 days of the procedure due to complications from advanced prostate cancer. In the atherectomy group, there were a total of three access site complications: 2 groin hematomas and 1 pseudoaneurysm. In the angioplasty group there was one retroperitoneal hematoma. One patient from each group required amputation within 30 days of the procedure for progressive gangrene. Finally, one patient in the angioplasty group developed popliteal artery stent thombosis 25 days after the procedure and was treated with a femoral tibial bypass.
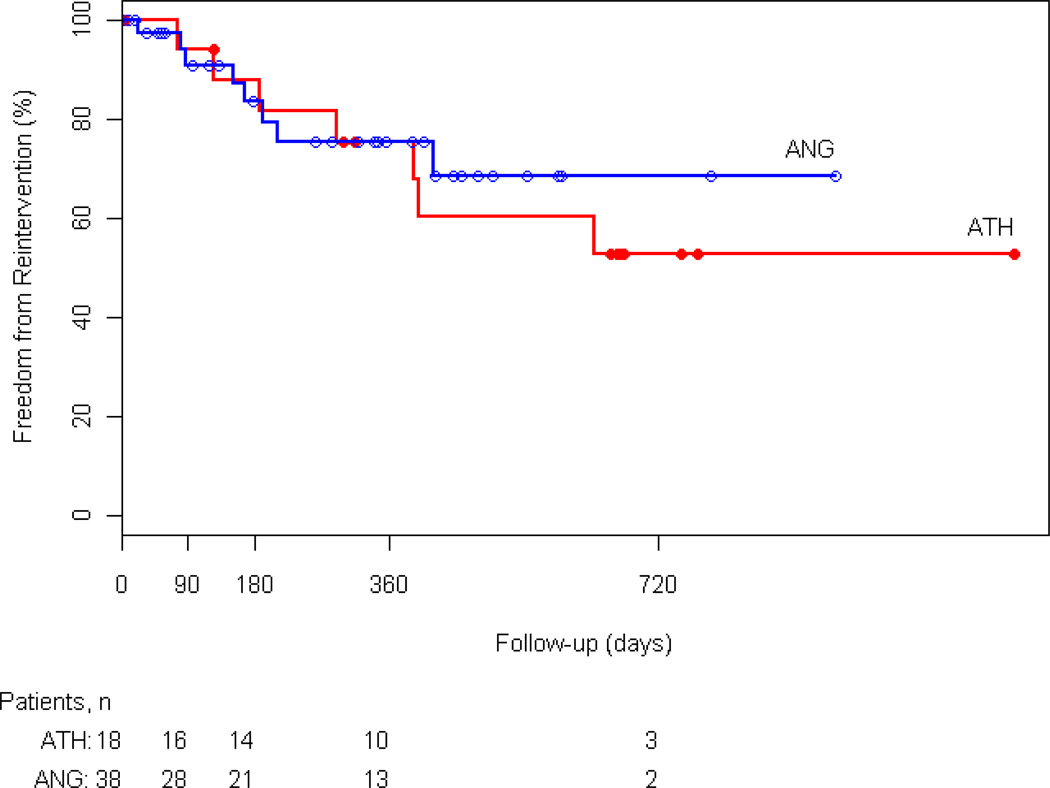
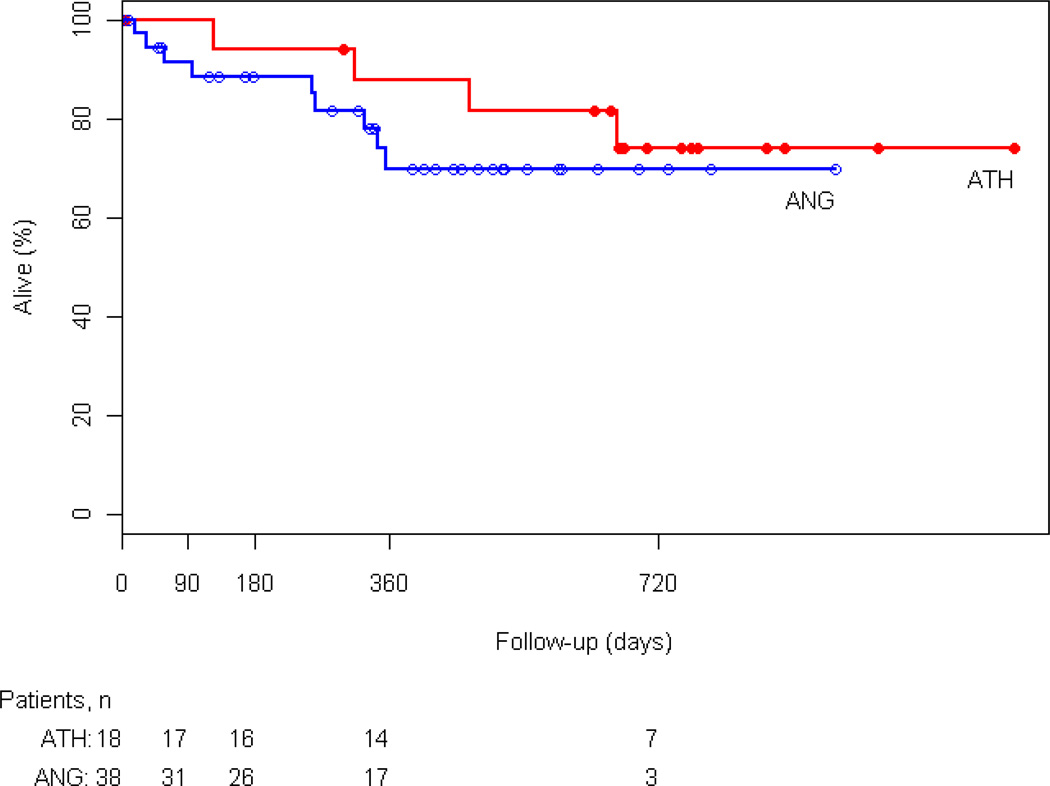
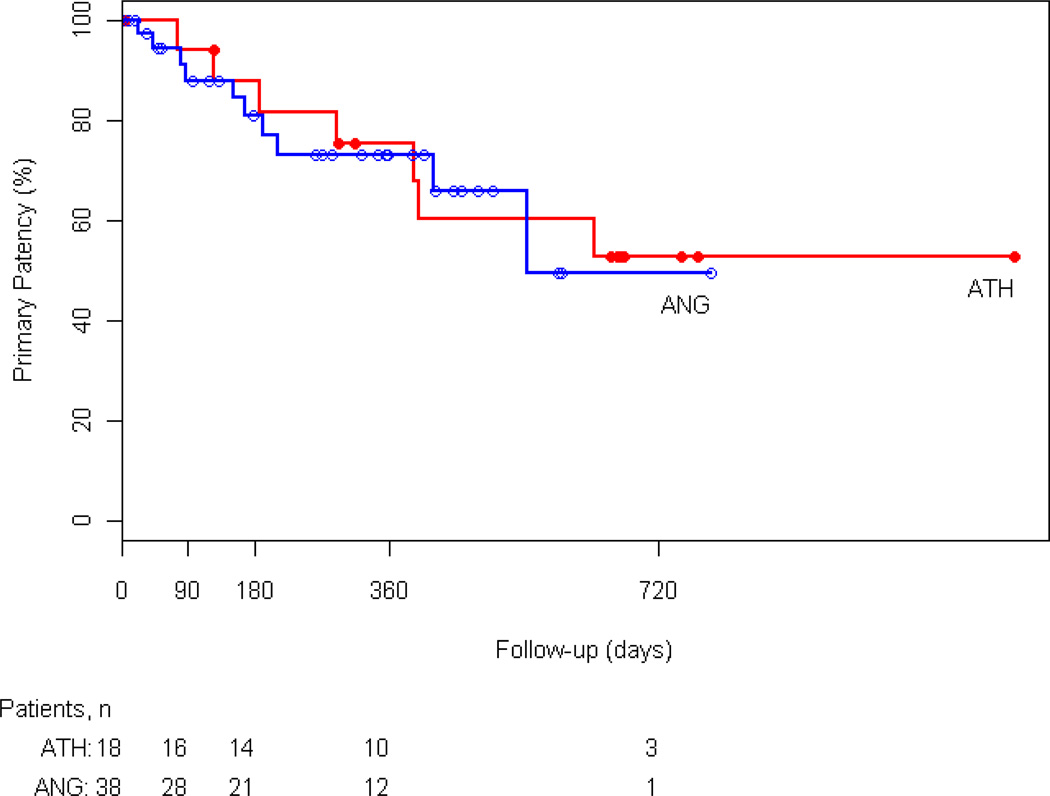
At 3, 6, and 12 months freedom from reintervention was 94%, 88%, and 75% in the atherectomy group and 91%, 84%, and 76% in the angioplasty group, respectively (p=NS) (Figure 2). In the atherectomy group, one patient underwent popliteal to posterior tibial bypass. In the angioplasty group 2 patients had reintervention with femoral to below-knee popliteal artery and femoral tibial bypass. Furthermore, there was no statistically significant difference between the two groups in limb salvage, survival rates, and primary patency. At 12 months 88% of atherectomy and 71% angioplasty patients were alive (Appendix 2). Limb salvage at 1 year was 87% and 97% in the atherectomy and angioplasty groups, respectively (Appendix 3). Primary patency of the popliteal artery at 3, 6, and 12 months was 94%, 88%, and 75% in the atherectomy group and 89%, 82%, and 73% in the angioplasty group (p=NS) (Figure 3).
Figure 2.
Kaplan-Meier analysis of freedom from reintervention for the atherectomy (ATH) and angioplasty (ANG) groups (P=0.592).
Appendix 2.
Kaplan-Meier analysis of Survival for the atherectomy (ATH) and angioplasty (ANG) groups (P=0.759).
Appendix 3.
Kaplan-Meier analysis of limb salvage (LS) for the atherectomy (ATH) and angioplasty (ANG) groups (P=0.141).
Figure 3.
Kaplan-Meier analysis of Primary Patency for the atherectomy (ATH) and angioplasty (ANG) groups (P=0.286).
Multivariate analysis identified higher pre-procedural ABI, run-off score and lack of renal insufficiency as independent predictors of greater improvement in post-procedural ABI. Stent placement was the only significant predictor of an increase in primary patency. There were no significant predictors for rate of limb salvage or freedom from reintervention.
Discussion
In the current study, we evaluated the contemporary endovascular management of popliteal artery atherosclerotic lesions. In comparing two primary therapeutic modalities, we observed a trend toward higher technical success with atherectomy compared to angioplasty alone. Importantly, we report a distinct complication pattern with atherectomy associated with distal embolization and angioplasty with local dissection. In intermediate term follow-up, we found no differences in primary patency or need for repeat procedures at one year. The overall patency rate was high for both modalities with a quarter of patients undergoing reintervention. As has been reported in the femoral artery, the use of a stent in the popliteal artery was associated with increased rate of primary patency. Taken together, our findings indicate that endovascular treatment of popliteal lesions can be performed safely and effectively with both angioplasty and atherectomy.
Endovascular techniques are increasingly used to treat infrainguinal arterial occlusive disease. The most recent international TASC guidelines recommend endovascular modalities to treat select obstructive lesions in the femoral and popliteal segments.3 In general, prior studies investigating the endovascular management of lower extremity atherosclerotic disease have reported combined results for the femoral and popliteal territories. Overall technical success rates in the femoropopliteal region exceed 95% with need for target lesion revascularization reported at 20%.3,12–14 However, there is limited data specifically evaluating the optimal endovascular treatment of popliteal artery lesions.
The popliteal artery possesses distinct anatomic and embryologic characteristics that may influence endovascular treatments. Traditional surgical approaches to infrainguinal disease involve bypass procedures which, by definition, circumvent direct treatment of the femoral and popliteal arteries. As endovascular approaches necessitate artery directed interventions, it is of increasing importance to understand the particular response of individual arteries including the popliteal artery. Compared to the femoral region, the popliteal artery is not housed within a muscular compartment and is subject to torsional forces related to knee flexion. 8–10 Our study extends the prior literature regarding endovascular treatment of infrainguinal disease by providing specific information about the technical success, complication rates, outcomes and revascularization rates in the popliteal artery.
While angioplasty is the most commonly used technique utilized for femoropopliteal lesions, there is growing acceptance of atherectomy. As is the case for angioplasty, previous studies describing infrainguinal atherectomy have combined femoral and popliteal regions.4,5,6,15–17,20,21 Reported technical success rates in treating femoropopliteal disease with atherectomy exceed 80% with one year primary patency rates approaching 50%. 4,6,15 One recent study included 110 popliteal lesions treated with atherectomy but did not provide segment specific outcomes.15 The literature reporting results with angioplasty is more robust 13; however technical success and one year patency rates are similar to those with atherectomy. 13,14,26,31 In a randomized trial of angioplasty and atherectomy in femoropopliteal disease, immediate success rates were similar for both modalities with reduced patency rates at two years in those patients treated with atherectomy.6 In the popliteal segment, we observed a trend toward higher immediate success rates with atherectomy as compared to angioplasty. At one year primary patency rates and re-intervention rates were similar between the two modalities. The apparent discrepancy with prior reports may be explained by the inclusion of popliteal lesions alone, differences in the length of follow-up and exclusion of patients with prior interventions.
Atherectomy and angioplasty utilize distinctive mechanisms to relieve arterial obstruction that may account for the observed differences in complications. During plaque excision the rotating blade which shaves the plaque can result in significant embolization of atheroma.29 The residual surface, devoid of endothelium, may increase the risk of thrombosis. During angioplasty mechanical dilatation of the arterial wall causes plaque fracture that can extend into a significant dissection. In our series, atherectomy was associated with thromboembolic complications whereas angioplasty was associated with dissection. A similar trend was noted in a randomized trial comparing angioplasty and an earlier generation atherectomy device in the superficial femoral and popliteal arteries.6 Others have reported the rate of dissection with angioplasty of femoropopliteal lesions to range between 3% to 43%.26 Although the incidence of clinically or angiographically evident embolization ranges between 7% and 9% 4,5,16,17,21,30 evaluation of this phenomenon using distal protection devices noted rates as high as 100%.29 Further studies are needed to define the clinical significance of both dissection and distal embolization in treating popliteal lesions.
Stents have been used in conjunction with both atherectomy and angioplasty. Up to a third of lesions in the femoral artery treated with angioplasty required placement of stents both to treat flow-limiting dissection and residual stenosis.6,23–27 Use of stents with atherectomy is lower, generally less than 5%. 3–5,16,17,21,30 Our use of stents with atherectomy was similar; in contrast our use of stents with angioplasty was higher. This may relate to the fact that we focused exclusively on the popliteal artery, an artery may be more prone to dissection. Interestingly, as has been seen in the superficial femoral artery 26,27, we observed a higher patency rate in those individuals treated with stenting in the popliteal region.
Several limitations of the current study deserve comment. This study is retrospective and treatment options were not randomized. Despite this we did not observe significant baseline clinical differences between individuals in the angioplasty and atherectomy groups. Lastly, patients included in this study often had multi-level disease which was treated. Although treatment of other lesions may have have influenced outcomes, it unlikely affected local conditions at the popliteal artery site.
Conclusion
Our experience with popliteal artery endovascular therapy indicates a distinct pattern of procedural complications with atherectomy compared to angioplasty but similar midterm patency, limb salvage and freedom from intervention. Stenting of the popliteal artery appears to be well tolerated and may have a beneficial impact on primary patency. Further analysis of popliteal interventions is required to better understand the modality of choice for endovascular revascularization.
Footnotes
The authors have no commercial, proprietary, or financial interest in any product or companies described in this article.
References
- 1.Anderson PL, Gelijns A, Moskowitz A, et al. Understanding trends in inpatient surgical volume: Vascular interventions, 1980–2000. J Vasc Surg. 2004;39:1200–1208. doi: 10.1016/j.jvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 2.TASC. Management of Peripheral Arterial Disease (PAD) TransAtlantic Intersociety Consensus (TASC) J Vasc Surg. 2000;31(1part 2):S1–S287. [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Artery Disease (TASC II) J Vasc Surg. 2007 Jan;45(Suppl S):S1–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Zeller T, Rastan A, Sixt S, et al. Long-term results after directional atherectomy of femoro-popliteal lesions. J Am Coll Cardiol. 2006;48:1573–1578. doi: 10.1016/j.jacc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Zeller T, Rastan A, Schwarzwälder U, et al. Percutaneous peripheral atherectomy of femoropopliteal stenoses using a new-generation device: six-month results from a single-center experience. J Endovasc Ther. 2004;11:676–685. doi: 10.1583/04-1316R.1. [DOI] [PubMed] [Google Scholar]
- 6.Vroegindeweij D, Tielbeek AV, Buth J, et al. Directional atherectomy versus balloon angioplasty in segmental femoropopliteal artery disease: Two-year follow-up with color-flow duplex scanning. J Vasc Surg. 1995;21:255–269. doi: 10.1016/s0741-5214(95)70267-9. [DOI] [PubMed] [Google Scholar]
- 7.Sadler TW. Langman’s Medical Embryology. 10th. Philadelphia, PA: Lippincott Williams & Wilkins; 2006; [Google Scholar]
- 8.Valentine RJ, Wind GG. Anatomic Exposures in Vascular Surgery. 2nd. Philadelphia, PA: Lippincott Williams & Wilkins;; 2003. [Google Scholar]
- 9.Kröger K, Santosa F, Goyen M. Biomechanical Incompatibility of Popliteal Stent Placement. J Endovasc Ther. 2004;11:686–694. doi: 10.1583/04-127.1. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann U, Vetter J, Rainoni L, et al. Popliteal artery compression and force of active plantar flexion in young healthy volunteers. J Vasc Surg. 1997;26:281–287. doi: 10.1016/s0741-5214(97)70190-3. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 12.Baril DT, Marone LK, Kim J, et al. Vascular Outcomes of endovascular interventions for TASC II B and C femoropopliteal lesions. J Vasc Surg. 2008 Sep;48(3):627–633. doi: 10.1016/j.jvs.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Kasapis C, Henke PK, Chetcuti SJ, et al. Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2009 Jan;30(1):44–55. doi: 10.1093/eurheartj/ehn514. [DOI] [PubMed] [Google Scholar]
- 14.Muradin GSR, Bosch JL, Stijnen T, et al. Balloon dilation and stent implantation for treatment of femoropopliteal arterial disease: meta-analysis. Radiology. 2001 Oct;221(1):137–145. doi: 10.1148/radiol.2211010039. [DOI] [PubMed] [Google Scholar]
- 15.McKinsey JF, Goldstein L, Khan HU, et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Ann Surg. 2008 Oct;248(4):519–528. doi: 10.1097/SLA.0b013e318188e1de. [DOI] [PubMed] [Google Scholar]
- 16.Ramaiah V, Gammon R, Kiesz S, et al. Midterm outcomes from the TALON Registry: treating peripherals with SilverHawk: outcomes collection. J Endovasc Ther. 2006 Oct;13(5):592–602. doi: 10.1583/05-1780MR.1. [DOI] [PubMed] [Google Scholar]
- 17.Kandzari DE, Kiesz RS, Allie D, et al. Procedural and clinical outcomes with catheter-based plaque excision in critical limb ischemia. J Endovasc Ther. 2006;13:12–22. doi: 10.1583/05-1634.1. [DOI] [PubMed] [Google Scholar]
- 18.Yancey AE, Minion DJ, Rodriguez C, et al. Peripheral atherectomy in TransAtlantic InterSociety Consensus type C femoropopliteal lesions for limb salvage. J Vasc Surg. 2006;44:503–509. doi: 10.1016/j.jvs.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Porter DH, Rosen MP, Skillman JJ, et al. Mid-term and long-term results with directional atherectomy of vein graft stenosis. J Vasc Surg. 1996;23(4):554–567. doi: 10.1016/s0741-5214(96)80033-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Gianturco LE, Porter DH, et al. Peripheral Directional Atherectomy: 4-year Experience. Radiology. 1992;183:773–778. doi: 10.1148/radiology.183.3.1533945. [DOI] [PubMed] [Google Scholar]
- 21.Keeling WB, Shames ML, Stone PA, et al. Plaque excision with the Silverhawk catheter: early results in patients with claudication or critical limb ischemia. J Vasc Surg. 2007 Jan;45(1):25–31. doi: 10.1016/j.jvs.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Babalik E, Gülbaran M, Gürmen T, et al. Fracture of popliteal artery stents. Circ J. 2003 Jul;67(7):643–645. doi: 10.1253/circj.67.643. [DOI] [PubMed] [Google Scholar]
- 23.Surowiec SM, Davies MG, Eberly SW, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41(2):269–278. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Zorger N, Manke C, Lenhart M, et al. Peripheral Arterial Balloon Angioplasty: Effect of Short versus Long Balloon Inflation Times on the Morphologic Results. J Vasc Interv Radiol. 2002;13(4):355–359. doi: 10.1016/s1051-0443(07)61736-9. [DOI] [PubMed] [Google Scholar]
- 25.Gray BH, Sullivan TM, Childs MB, et al. High incidence of restenosis / reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg. 1997;25(1):74–83. [PubMed] [Google Scholar]
- 26.Schillinger M, Schila S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354(18):1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 27.Sabeti S, Schillinger M, Amighi J, et al. Primary Patency of Femoropopliteal Arteries Treated with Nitinol versus Stainless Steel Self-expanding Stents: Propensity Score-adjusted Analysis. Radiology. 2004;232(2):516–521. doi: 10.1148/radiol.2322031345. [DOI] [PubMed] [Google Scholar]
- 28.Schermerhorn ML, Cronenwett JL, Baldwin JC. Open surgical repair versus endovascular therapy for chronic lower extremity occlusive disease. Annu Rev Med. 2003;54:269–283. doi: 10.1146/annurev.med.54.101601.152509. [DOI] [PubMed] [Google Scholar]
- 29.Suri R, Wholey MH, Postoak D, et al. Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc Interv. 2006;67(3):417–422. doi: 10.1002/ccd.20634. [DOI] [PubMed] [Google Scholar]
- 30.Zeller T, Rastan A, Schwarzwälder U, et al. Midterm results after atherectomy-assisted angioplasty of below-knee arteries with the use of the Silverhawk device. J Vasc Interv Radiol. 2004;15:1391–1397. doi: 10.1097/01.RVI.0000138060.05915.9D. [DOI] [PubMed] [Google Scholar]
- 31.Grimm J, Muller-Hulsbeck S, Jahnke T, et al. Randomized study to compare PTA alone versus PTA with Palmaz stent placement for femoropopliteal lesions. J Vasc Interv Radiol. 2001 Aug;12(8):935–942. doi: 10.1016/s1051-0443(07)61572-3. [DOI] [PubMed] [Google Scholar]