Abstract
We investigated whether adults with healthy vision can move their eyes toward an informative target area that is initially hidden by a gaze-contingent scotoma in the periphery when they are under time pressure. In the experimental task, participants had to perform an object-comparison task requiring a same–different judgment about two silhouettes. One silhouette was visible, whereas the other was hidden under the scotoma. Despite time pressure and the presence of the visible silhouette, most participants were able to move their eyes toward the informative region to reveal the hidden silhouette. Saccades to the hidden stimulus occurred when the visible stimulus was presented directly opposite in either fixed or variable locations and when the visible stimulus was presented at an adjacent location. Older participants were also able to perform this task. First saccades in the direction of the hidden stimulus had longer latencies compared with saccades toward the visible stimulus. This suggests the use of a deliberate, nonreflexive saccade strategy (“stop before you saccade”). A subset of participants occasionally made curved saccades that were aimed first toward the visible stimulus and then toward the hidden stimulus. We discuss the implications of our findings for patients who have a biological scotoma, for example, in macular degeneration.
Keywords: artificial scotoma, visual search, antisaccade, curved saccade, macular degeneration, preferred retinal location, low vision
Introduction
To find visual information, people rely on input from the fovea and from more peripheral retinal locations. How easy is it, then, to find information, particularly if that information is presented at a location in the visual field that is hidden by an artificial scotoma? Here we address this question by studying whether healthy adults can make saccades in the direction of an invisible, artificial, gaze-contingent scotoma that is presented in the periphery.
The motivation for this study is twofold. First, it is motivated by theoretical questions about whether humans direct saccades to locations that will maximize the information gained. Second, it is motivated by a practical need to better understand the challenges that people with central field loss face. We discuss each of those motivations in more detail.
Theoretical motivation: Understanding eye movement strategies
Our study ties in with theoretical investigations about whether humans direct saccades to locations that will maximize the information gained. In particular, when searching for known targets under time pressure, there is a debate about whether eye movements are aimed at salient locations (cf., Itti & Koch, 2000; van Zoest, Donk, & Van der Stigchel, 2012), at more target-like locations (e.g., Beutter, Eckstein, & Stone, 2003; Najemnik & Geisler, 2005, 2009; Zhang & Eckstein, 2010), or at informative locations with high uncertainty about target presence (e.g., Butko & Movellan, 2010; Lee & Yu, 2000; Legge, Hooven, Klitz, Mansfield, & Tjan, 2002; Renninger, Coughlan, Verghese, & Malik, 2005; Renninger, Verghese, & Coughlan, 2007; Verghese, 2012). Evidence in support of all three strategies has been reported. However, saccades seem to go more frequently to target-like locations compared with informative locations, even when the former strategy is highly inefficient (Verghese, 2012). In one study, participants made more eye movements to efficient locations when they received immediate saccadic feedback (Verghese & Ghahghaei, 2013). We investigate this further here.
Our study contributes to this debate by providing an extreme instance of a situation in which eye movements can maximize the information gained. Participants needed to gather information from two peripheral locations to judge whether the stimuli at these locations were the same or different. One of these stimuli was clearly visible in the periphery, whereas the other was hidden, and time was limited to allow only one typical saccade. Did participants tend to look at the hidden (informative) location, or did they tend to look at the location with the already-visible stimulus?
Our experimental paradigm is related to the antisaccade task (e.g., Munoz & Everling, 2004). In the antisaccade task, participants are instructed to saccade to a location opposite a briefly flashed stimulus. This requires suppression of a reflexive eye movement toward the flashed stimulus. However, there are also important differences between our task and the antisaccade task. In our task, participants needed to make a decision about whether the stimuli were the same or different. Therefore, the visible stimulus should not be ignored. Because the time allowed for eye movements was limited to about the duration of a single saccade and one of the stimuli was clearly visible, making a saccade to the hidden stimulus was more informative than making a saccade to the already visible stimulus. We investigated what eye-movement strategies participants used to perform this task. In two of our experiments, hidden and visible stimuli were presented diametrically opposite each other (Experiments 1 and 2). In a third experiment, the two stimuli were presented adjacent to each other (Experiment 3).
Practical motivation: Understanding challenges of central field loss
A practical motivation for our work comes from research on central field loss. Central field loss occurs in macular degeneration and involves areas in and around the fovea. It has been shown that eye movements in individuals with central field loss are less directed and smaller in amplitude (Renninger, Dang, Verghese, & Fletcher, 2008; Van der Stigchel et al., 2013), making it more difficult to compensate for the central scotoma with eye movements. There is therefore a need to make saccades as efficient as possible.
One hypothetical efficient strategy for saccades is to initially saccade in the direction of the binocular scotoma. This strategy acquires information in an area where information is missing due to the scotoma. However, it is not clear how easy it is to make the required directed eye movements to a target that has been rendered invisible due to the scotoma.
We investigated whether healthy adults can direct eye movements in the direction of an artificial, gaze-contingent scotoma in the presence of other clearly visible stimuli. In this way we determined whether directing eye movements into the scotoma is a feasible strategy before testing it on patients.
Artificial scotomas have been used frequently in the literature (e.g., Bertera, 1988; Cornelissen, Bruin, & Kooijman, 2005; Henderson, McClure, Pierce, & Schrock, 1997; Kwon, Nandy, & Tjan, 2013; Lingnau, Albrecht, Schwarzbach, & Vorberg, 2014; McIlreavy, Fiser, & Bex, 2012; Van der Stigchel et al., 2013; Walsh & Liu, 2014). Our study differs from preceding work with artificial scotomas in that we present a scotoma in the periphery, not at central fixation. This allows fixation using the natural fovea (i.e., “foveating” saccades as in Whittaker, Cummings, & Swieson, 1991). The main focus of previous work with fovea-centered artificial scotomas was to understand how patients with central field loss learn to adopt a stable eccentric location, a preferred retinal locus (PRL), which takes over functionality from the fovea. In our study, the development of a PRL is not needed. Instead, we focus on how a PRL, once established, might be used to sample information more efficiently from the environment. With a stable PRL, a participant might have the subjective experience that the scotoma is peripheral compared with the location where they focus attention.
Overview
The remainder of this article is structured as follows. First, we lay out the general methods of our object comparison paradigm. In each experiment one stimulus is initially hidden behind the scotoma (‘hidden target”) while another stimulus is visible from the periphery (“distractor”).1 We then present three experiments that examine the conditions under which people are able to look toward the scotoma to find the hidden target: when the hidden target and distractor are presented directly opposite each other in fixed locations within a block (Experiment 1), when they are opposite each other but in variable locations on every trial (Experiment 2), and when a distractor is presented adjacent to the hidden target (Experiment 3). Experiment 1 was performed on two groups of participants: a general group (Experiment 1A) and an older group (Experiment 1B).
Method
Participants
Six participants (three female, three male) took part in Experiments 1A, 2, and 3. Participants' mean age was 33.2 years (SD = 9.8 years; range = 24–52 years). The authors were two of the participants (1 and 2), two other participants had extensive experience with other vision studies (3 and 4), and two were naïve (5 and 6). All participants had normal or corrected-to-normal vision.
For Experiment 1B, we recruited six participants based on the selection criteria that they (a) were 65 years of age or older, (b) had no known vision problems, and (c) were healthy enough that they could take part in a study that lasted multiple hours. The participants' (five females, one male) mean age was 73.2 years (SD = 6.5 years; range = 65–84 years). One participant (103) had extensive experience in vision studies. Participants gave informed consent to participate in the study as paid volunteers. The protocol for the study was approved by the Institutional Review Board at The Smith-Kettlewell Eye Research Institute and conformed to the Declaration of Helsinki.
Stimuli
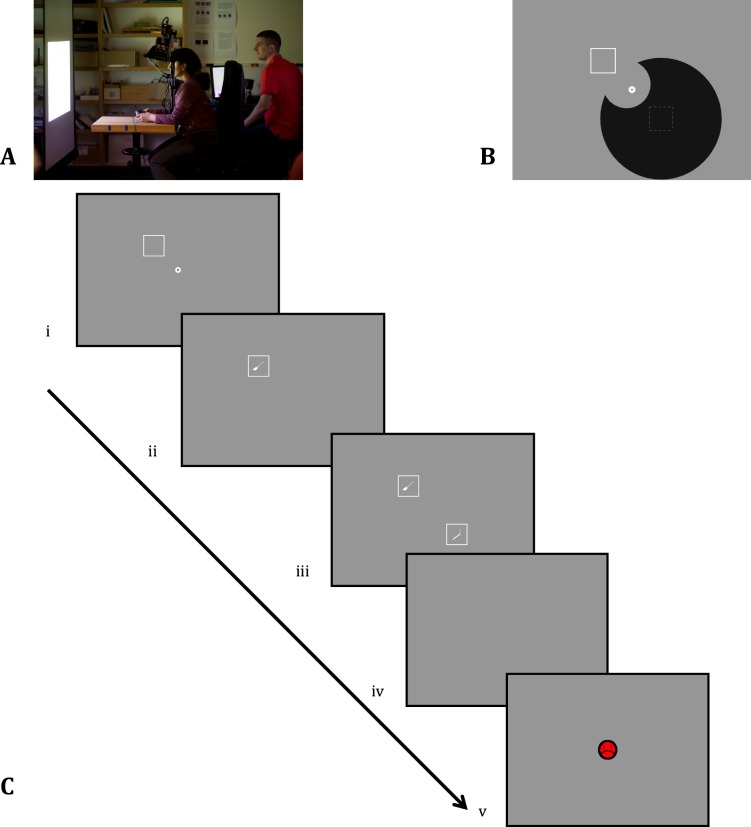
Stimuli were developed in Python using the pylink 2.5 package (SR Research, Ottawa, Ontario, Canada). Stimuli were presented at a distance of 90 cm on a large projection screen (76 × 57 cm, or 40.18° × 32.35°; see Figure 1A).
Figure 1.
(A) Physical set-up. Participants view stimuli on a large screen. (B) Illustration of the artificial scotoma as shown to participants once every 10 trials in Experiment 1. (C) Sequence of events in the experiment: (i) The artificial scotoma hides one of the two stimulus locations and the participant starts the trial by looking at the central fixation circle and pressing a joystick button; (ii) the stimulus is visible in a nonscotoma location; (iii) the hidden stimulus is revealed only when a saccade is made into the scotoma; (iv) the participant indicates whether the stimuli are the same or different; and (v) accuracy feedback is given.
On each trial, participants judged whether two stimuli were the same or different. Stimuli were based on 24 hand-selected images2 in the Snodgrass and Vanderwart (1980) data set. We created a filled white silhouette of each selected image in a gray box (same brightness as the background) with a white border (4.71° × 4.71°).
All silhouettes were elongated objects (e.g., pen, pencil, fork, knife, asparagus; see online supplementary materials for all silhouettes) that were oriented 45° clockwise from vertical. Silhouettes were chosen to look somewhat similar while still being relatively distinguishable when presented peripherally. They were always presented within square bounding boxes. Because the stimuli were superficially similar and because they were all equally likely to be targets, we refer to them as target-like.
In the experiment, two silhouettes were placed at 8.5° from central fixation, as shown in Figure 1B and C. One silhouette (the distractor) was clearly visible from the periphery, whereas the other silhouette (the hidden target) was initially hidden behind an invisible, peripheral, gaze-contingent artificial scotoma. The artificial scotoma was a circular shape with a radius of 12°, centered on the hidden stimulus. A hole (radius 5°) was cut out of the scotoma to avoid overlap with central fixation. Figure 1B illustrates the final shape. Note that in actual trials (Figure 1C) this shape was invisible as it matched the background luminance. In effect, the shape was such that the hidden stimulus was most easily uncovered by a saccade in the direction of the scotoma (e.g., in Figure 1B and C, a saccade to the bottom right).
Design
In this section, we focus on the design for Experiment 1. Changes to the design in Experiments 2 and 3 are highlighted in those sections. Experiment 1 presented the stimulus pairs in fixed locations within blocks of 96 trials. Across blocks, the stimuli were presented at two cardinal and two oblique locations. One hidden stimulus was always paired with a stimulus positioned diametrically opposite. For cardinal locations, the hidden stimulus was positioned at one horizontal location (0° or 180°) and one vertical location (90° or 270°). For the oblique locations, participants experienced two diagonal axes (45° or 225° and 135° or 315°).
Half the participants were presented with cardinal locations during the first two blocks of trials. The other half was first presented with two oblique blocks. Within a block of 96 trials, each silhouette occurred equally often in each 48-trial segment, with half the trials being the same and half the trials being different.
The general group completed all four blocks (Experiment 1A). For the older group (Experiment 1B), we aimed to run two blocks of trials (one cardinal configuration, one oblique configuration) per participant. However, due to differences in endurance and challenges in calibrating the eye tracker in participants with intraocular lenses, one of our participants (participant 104) was able to complete only one block of trials. Our more experienced participant (103) was able to complete four blocks. All other participants completed two blocks.
Procedure
At the start of each block, participants were presented with an overview of all 24 unique silhouettes. The experimenter explained what object each silhouette represented. Participants were encouraged to take their time and familiarize themselves with the silhouettes. This short learning phase was followed by a nine-point eye tracker calibration. This calibration was repeated when needed (e.g., midway through each block of 96 trials or when requested by the participant).
Before the first trial and after every tenth trial, participants saw an outline of the scotoma on the screen, as shown in Figure 1B (however, this information was not shown in Experiment 2). Before the start of the first trial, the experimenter also demonstrated how the scotoma affected what areas of the screen were visible and invisible by having the participant follow the tip of the experimenter's finger as it moved to various parts of the screen.
The procedure on each trial is illustrated in Figure 1C. The participant fixated a white circular annulus (radius = 0.7°) concentric with a red circle. Due to the artificial scotoma, participants could see the outline of one empty square but not both. Participants initiated a trial by pressing a trigger on a joystick. The trial started if the participant's gaze fell within a square of side 2° centered on fixation. At the start of a trial, the inner fixation circle changed from red to the gray background luminance. At this time, the silhouettes appeared with a smooth linear temporal ramp of 250 ms. The central fixation circle disappeared 100 ms after the start of the trial. Saccades that occurred before the offset of the fixation circle were discarded.
Stimuli were visible for at most 2 s after the start of the trial. However, as soon as the participant's gaze went beyond a square window of side 3° centered on fixation, the stimuli remained visible for at most 300 ms (Experiments 1A, 1B, and 2) or 200 ms (Experiment 3). As the maximum display time was 2 s, less time was available if more than 1.7 s (Experiments 1A, 1B, and 2) or 1.8 s (Experiment 3) had already passed since the start of the trial. Given that 300 ms is typically not sufficient to make two saccades and that one stimulus was initially hidden by the artificial scotoma, an efficient saccade should be directed toward the scotoma to uncover the hidden target. However, unlike in an antisaccade, the already-visible stimulus should not be ignored because it contains task-relevant information. Participants were told that the stimulus would turn off 300 ms after saccade initiation. No specific instructions were given about where to make the first saccade because our intention was to study what strategies participants used to complete this task.
After stimulus presentation ended, participants indicated whether the two stimuli were the same or different by pressing the left or right trigger, respectively, on the joystick. Correct responses were followed by the display of a yellow smiley face and the sound of a cash register (“ka-ching”). Incorrect responses were followed by the display of a red grumpy face (as in Figure 1C) and the short sound of a door slamming. Feedback was based on correct response to the same–different judgment and was not based on the exact saccade strategy that participants used.
In cases where a participant tried to start a trial while fixating outside the fixation acceptance square, the trial would not start and the participant had to try again. If a trial start failed on multiple attempts, a recalibration procedure was performed and the trial was restarted.
Each block of 96 trials was followed by a break of typically 5 to 10 min. In Experiments 1A, 2, and 3, participants completed four blocks, and the total procedure lasted between 1.5 and 2 hr for each participant.
In Experiment 1B, the experiment reported here was preceded by vision tests (e.g., MN-Read for reading acuity, the Mars Letter Contrast Sensitivity Test for contrast sensitivity) and an optical coherence tomography - scanning laser ophthalmoscope (OCT-SLO) test to test for any visual deficits. For one participant, potential early signs of retinal change were detected. Because this patient had a good score for binocular acuity and contrast sensitivity (20/20 on MN-Read and 1.76 log contrast sensitivity, with correction), his data were included for analysis. After these initial tests (taking around 1–1.5 hr), we performed Experiment 1B. Experimental blocks were interleaved with participation in a visual search experiment (not reported here).
Eye tracking was more challenging with some individuals in the older group due to intraocular lenses following cataract surgery and to bifocal or progressive lenses. For three participants (102, 103, and 106) from this group we were able to use a nine-point calibration routine. For two participants (104 and 105) we used a five-point calibration routine. For one participant (101) only a three-point calibration routine was possible. The coarser prediction that resulted from this simplified calibration routine may have caused the artificial scotoma to be rendered less accurately; thus, the data of this participant should be interpreted with caution. The total procedure, including vision tests and the other visual search task, took between 3 and 4 hr. Participants received frequent breaks.
Measures
We report four measures of performance and eye-movement strategy: (a) the number of trials in which the first saccade was directed toward the hidden target or the distractor; (b) the latency of the initial saccade, given its direction; (c) the proportion of saccades aimed toward the hidden target across subsets of trials, as an indicator of saccade strategy; and (d) whether saccades were aimed toward the hidden target, toward the distractor, or toward both in turn. We now discuss these measures in more detail.
Eye movements were measured with an Eyelink 1000 eye tracker (SR Research) in a tower-mount set-up (i.e., with a chin and forehead rest) with a sampling frequency of 500 Hz. To determine the direction of the first saccade, we analyzed all first saccade events as identified by the Eyelink software (minimum velocity of 30°/s; minimum acceleration of 8000°/s2). Only saccades that traversed a distance of at least 1.5° relative to central fixation and that occurred at a latency of greater than 100 ms relative to trial start were considered for analysis. The latter criterion was adopted to avoid effects of anticipatory saccades and is similar to criteria used in the literature (Kalesnykas & Hallett, 1987; Kowler, Anderson, Dosher, & Blaser, 1995). The results in Tables 1–4 report what percentage of trials contained such short latency saccades. Some of these short latencies were negative latencies. These were measured because trials could start only if the participant's eye gaze was within a square window of side 2° centered on fixation. Some participants initiated a saccade before a button press but were still within this acceptance window at the time of the button press. This resulted in a negative latency measure.
Table 1.
Percentage of trials in Experiment 1 in which eye gaze was aimed toward the hidden target, the distractor, or both in turn. The right-most column provides the percentage of correct trials. Note that the majority of participants looked solely toward the hidden target.
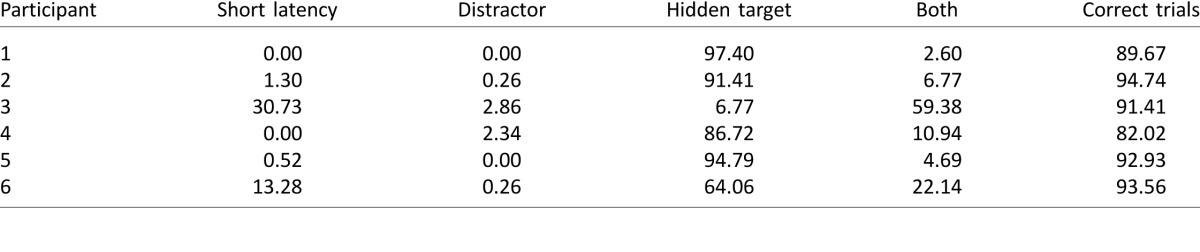
Table 4.
Percentage of trials in Experiment 3 in which eye gaze was aimed toward the hidden target, the distractor, or both in turn. The right-most column provides the percentage of correct trials.
For saccades with latency greater than or equal to 100 ms, we determined the angle of the saccade endpoint relative to central fixation. If this angle fell within 22.5° of the direction of the hidden target, it was classified as a target saccade. If it fell within 22.5° of the direction in which the distractor was presented, it was classified as a distractor saccade. In Experiments 1 and 2, if the angle did not fall in either of these regions, the direction of the saccade was considered as “other” and was not included in further analysis. In Experiment 3, where the target and distractor were 90° apart, we analyzed target and distractor saccades as well as those that were directed in between.
To analyze latency, we calculated the time interval between the start of a trial (button press on joystick) and the initiation of a saccade. To formally test the likelihood that any observed differences in latency between conditions are statistically reliable, we performed a nonparametric permutation test (Efron & Tibshirani, 1993). This analysis was chosen because our latency data violated two common assumptions of standard parametric tests: Our data had an unequal number of observations per condition, and the data were not normally distributed. A permutation test estimates how likely it is that an observed difference between conditions is found by chance only. That is, it determines how likely it is that the observed distributions arise under the null hypothesis.
More specifically, we drew two sets of random samples from the total set of observed data points with the same number of samples as observed in the study for the “toward target” and “toward distractor” saccades, respectively. For each condition, we determined the mean value of each sample set and repeated the process. We then determined the fraction of simulations in which the difference between the simulated means was equal to or greater than the difference between the observed means of the two conditions in the study. If the fraction was less than 0.05, we concluded that there was a real difference between the distributions (i.e., p < 0.05).
We performed two permutation tests per study. In one test we performed the permutation test across participants (i.e., pooling all data of each condition across participants). In this test we ran 100,000 simulations to assess whether there was an effect at the population level. In a second test we performed 10,000 simulations per individual to assess whether there was an effect at the individual level.
Our third analysis focused on what percentage of trials contained a first saccade in the direction of the target. We analyzed saccades per set of 32 trials to investigate the consistency of the participant's saccade strategy.
Our final analysis focused on saccades and gaze data for the entire trial. We investigated what percentage of trials on a block contained saccades in particular areas. We then analyzed whether gaze samples formed an angle with central fixation that was within 22.5° of the angle at which the target or the distractor was presented. This allowed us to determine the percentage of trials on which eye gaze was aimed at the hidden target, the distractor, or both in turn.
Experiment 1: Looking in the direction of an artificial scotoma at a consistent location
Experiment 1 tested what strategies adults with healthy vision used to gain information in our object comparison task. Specifically, we were interested in whether participants tend to look in the direction of the hidden target upon their first saccade when the hidden target was presented at a consistent location throughout a block of trials. In this experiment the distractor was presented directly opposite the hidden stimulus. We ran two versions of this experiment: We tested a general group in Experiment 1A and an older group in Experiment 1B. Because macular degeneration is a disease that develops progressively with age (Friedman et al., 2004), a test with the older group is needed for future comparison with a patient population. We present the results for each group separately and then discuss the results for both groups together.
Results: Experiment 1A
Saccades: Direction and latency
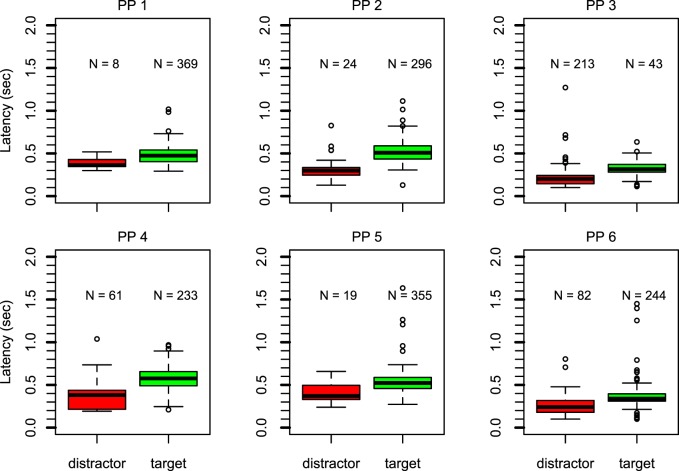
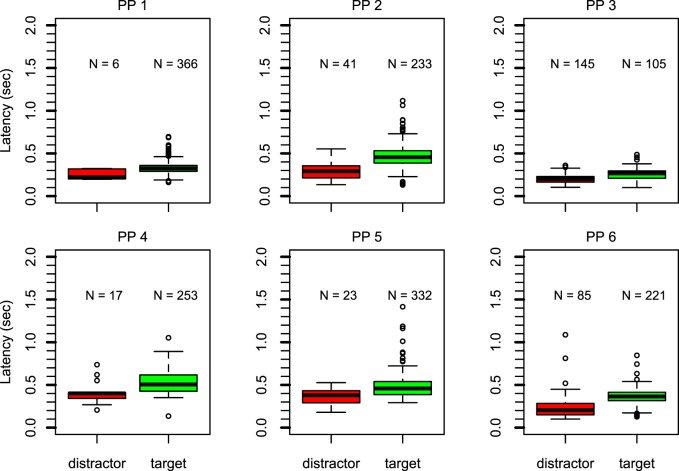
Figure 2 reports latency of the first saccade in box-and-whisker plots. Each panel shows data of one participant. The left (red) boxes show latency of saccades toward the distractor. The right (green) boxes show latency of saccades toward the hidden target. The number of observations per condition is reported above each box (out of 384 trials). For all participants except participant 3, more than 60% of the trials contained a saccade in the direction of the scotoma. For participants 1, 2, and 5, this exceeded 75% of the trials. The pattern is different for participant 3, whose initial saccades were aimed toward the distractor on 55.4% of the trials.
Figure 2.
Box-and-whisker plots for each participant show the latency of the first saccade for saccades toward the distractor (left, red) or the hidden target (right, green). The number above each box represents the number of observations. For five participants, the majority of first saccades went toward the hidden target. These saccades have longer latency.
The latency data (see Figure 2) followed a clear pattern. In general, latency was longer on trials in which the saccade was aimed toward the hidden target. For all participants, the median latency (thick black line) was higher for the trials in which saccades were aimed toward the hidden target compared with those in which saccades were aimed toward the distractor. For all participants there was hardly any overlap between the boxes (which contain 50% of the data per condition) of the two conditions.
In a permutation test at the group level (100,000 simulations), none of the simulated results generated a difference between the simulated means larger than or equal to the observed mean, indicating that the observed difference was highly unlikely to be due to chance, p < 0.00001. At the individual level (with 10,000 simulations), the latency difference for all participants was also highly significant, with p = 0.0096 for participant 1, p = 0.0005 for participant 5, and p < 0.0001 for all other participants.
Figure 3A plots how frequently participants made their initial saccade toward the hidden target and whether this strategy changed with practice. To show possible changes of strategy within a block of 96 trials, we divided each block into three subsets of 32 trials each. Dashed lines separate blocks of trials, each of which had a different target–distractor axis. Only trials in which a saccade satisfied our inclusion criteria (see Method) were included. Participants 1, 2, and 5 quickly learned to saccade consistently into the scotoma region. The saccades of participants 4 and 6 varied between blocks but in general were aimed more frequently toward the target as the block of trials progressed. In contrast, participant 3 had fewer trials in which the first saccade was aimed toward the hidden target. Instead, most of these first saccades were aimed toward the distractor. The Supplementary Appendix contains figures for all participants and all blocks.
Figure 3.
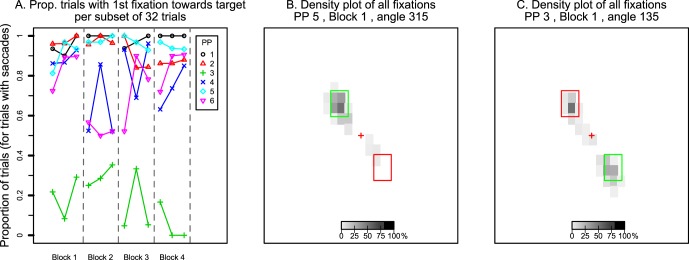
(A) Percentage of trials in which participants made their first saccade toward the hidden target out of all trials with eligible saccades. Data points show values per participant per set of 32 trials. Dashed lines distinguish the four blocks of trials. (B, C) Examples of density plots of where saccades landed throughout the entire trial period. Panel B shows data of a participant who consistently made saccades only toward the target area. Panel C shows data of a participant who made saccades to both the target and the distractor.
Saccade pattern during the entire trial
We next analyzed what the saccade pattern looked like through the entire trial. Specifically, if participants did not saccade toward the hidden target initially, did they look there eventually? Figure 3B and C shows a density plot of where saccades landed during the trial for the first block that participants completed (96 trials). Each figure plots fixation locations in screen coordinates, with fixations clustered in regions of 2° by 2°. Darker regions received fixations on more trials. For four participants, the data looked similar to that of participant 5, as shown in Figure 3B. This participant frequently and consistently made saccades into the target area (highlighted with a green rectangle). Figure 3C shows data of participant 3. This participant (and, to a lesser extent, participant 6) mostly made saccades toward the distractor (highlighted with a red rectangle). However, the participant also made saccades toward the target on some trials. In the next analysis we distinguish whether saccades reached only one of the stimuli or both on individual trials. Figures of all participants in all blocks (and for the other experiments) are included in the online supplementary materials.
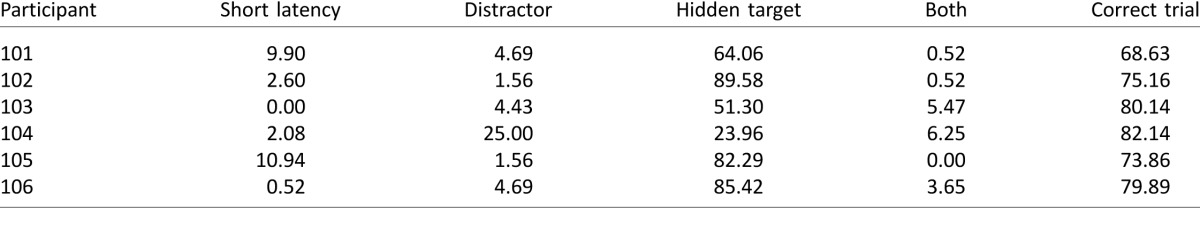
To quantify the strategies that participants used, Table 1 reports the percentage of trials in which eye gaze was directed toward the hidden target, toward the distractor, or toward both. Trials with short-latency saccades (<100 ms) were not included for this analysis and instead are reported in a separate column. Again, for five participants, eye gaze was aimed mostly toward the hidden target on the majority of trials. For one participant (participant 3), eye gaze was aimed toward both the distractor and the target on the majority of trials. For another participant (participant 6), eye gaze was aimed toward both locations on about one-fifth of the trials.
Of the trials in which the participant's gaze reached both targets, we analyzed where initial gaze was aimed. That is, did the first gaze sample fall within 22.5° of the distractor or the hidden target direction? Out of the 409 valid trials, gaze was first aimed at the distractor on 379 trials. Gaze was aimed toward the hidden target on only 27 trials. This suggests that when participants looked at both the distractor and the hidden target, they first looked toward the distractor and then toward the hidden target.
The final column in Table 1 reports the percentage of correct same–different judgments. The percentage is calculated only for valid trials (i.e., where saccade amplitude exceeded the threshold criterion and latency was ≥100 ms). All participants had more than 80% correct. That is, performance was successful when looking only at the target (participants 1, 2, 4, and 5) or when looking at both (participants 3 and 6).
Results: Experiment 1B
Saccades: Direction and latency
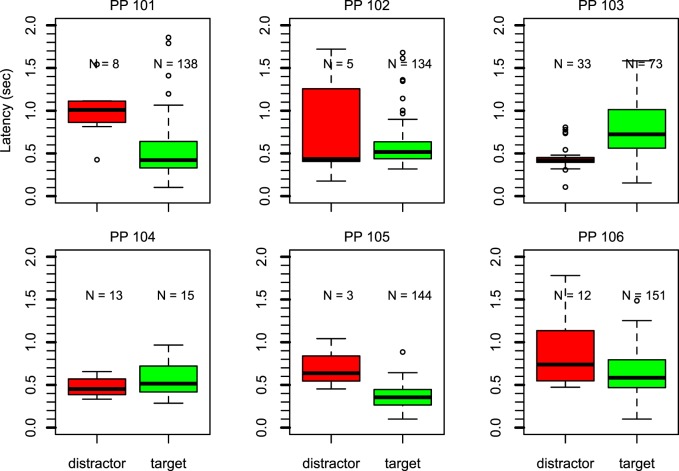
Figure 4 reports saccade latency for the older group as box-and-whisker plots. Similar to results in Experiment 1A, the older adults made their first saccade more frequently toward the hidden target than toward the distractor. The exception is participant 104, although fewer trials (48 trials; valid saccades were made on 28) were observed.
Figure 4.
Box-and-whisker plots for each participant in Experiment 1B show the latency of the first saccade for saccades toward the distractor (left, red) or the hidden target (right, green). The number above each box represents the number of observations. For five participants, the majority of first saccades went toward the hidden target.
When inspecting the latency, the pattern in the data is less clear compared with Experiment 1A. Only for participant 103 is the pattern similar to that observed in Experiment 1A, with longer latencies when saccades were aimed toward the hidden target. The other participants did not seem to conform to this pattern. One explanation might be that due to the smaller number of observations, especially in the “toward distractor” condition, the range of the box area is less representative of the underlying distribution. This limits the ability to detect significant differences. The permutation test at the group level showed no significant difference between the saccade latency toward the target and the distractor (p = 0.199).
Gaze pattern during the entire trial
Table 2 reports where gaze was directed during the trial. For all participants except participant 104, gaze was directed toward the hidden target on the majority of trials. For three of these participants this was true on a high percentage of trials (>80%). Participant 104 looked either toward the distractor or toward the hidden target. In contrast to the findings in Experiment 1A, none of the participants looked consistently at both the distractor and the hidden target in turn.
Table 2.
Percentage of trials in Experiment 1B in which eye gaze was aimed toward the hidden target, the distractor, or both in turn. “Other” saccade strategies bring the saccade totals to 100%. These include trials with blinks, saccades that did not exceed criterion, and saccades that did not land at either target or distractor. The right-most column provides the percentage correct trials in all trials excluding short-latency trials.
The right-most column of Table 2 reports the percentage of correct same–different judgments. Performance was worse than in Experiment 1A. There was no clear correlation between strategy and performance level.
Discussion: Experiment 1
The results demonstrate that participants can adopt strategies that are helpful in maximizing information gain. Specifically, in our setting, participants tended to look in the direction of the artificial scotoma and the hidden target. In both Experiments 1A and 1B, five participants initiated their first saccade in the direction of the hidden target on the majority of the trials (Tables 1 and 2; Figures 2 and 4). In Experiment 1A, two participants tried to look at both the target and the distractor within a single trial. In Experiment 1B, none of the participants adopted such a strategy consistently.
When analyzing saccade latency, we found that participants in Experiment 1A had longer latencies when their first saccade was in the direction of the hidden target compared with when the first saccade was in the direction of the distractor. This suggests that saccades in the direction of the scotoma require a more deliberate, nonreflexive saccade strategy.
In Experiment 1B we did not find support for this effect. However, the latencies for the saccades toward the hidden target were roughly in the same range as those observed in Experiment 1A. One reason why there was no significant difference between conditions might be because there were few observations in the “toward distractor” condition. This makes the observations less reliable as indicators of the underlying distribution of values.
To summarize, we found that most participants in this experiment made informative saccades. Their first saccade was reliably directed toward the target hidden by the scotoma. However, the location of the scotoma was fixed within a block of trials. Might it be that participants' behavior relied on making saccades to a remembered location (Coëffé & O'Regan, 1987) rather than making a conscious eye movement to look at an informative location? To test this, we ran a second experiment in which the location of the scotoma was varied within each block.
Experiment 2: Looking in the direction of an artificial scotoma at a randomized location
In this experiment we tested whether participants still made informative saccades when the angle of the trial was randomized on each trial. Observers who participated in Experiment 1A took part. All materials were kept the same. Participants again completed four blocks of 96 trials each. Within a block, across every set of eight trials, the hidden target (i.e., the scotoma) was presented at eight different angles (0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°, with a distracting stimulus diametrically opposite). Trials were such that the angle was never the same on two consecutive trials. Did participants still look in the direction of the scotoma—the informative region?
Results and discussion: Experiment 2
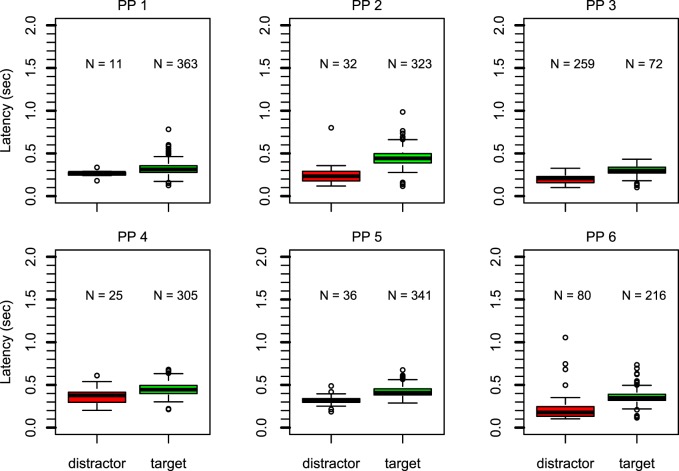
Figure 5 reports box-and-whisker plots of the latency of the first saccades for each participant. The pattern is similar to that in Experiment 1A. All participants except participant 3 aimed their first saccade toward the hidden target on the majority of trials. Latencies were also longer for saccades toward the hidden target compared with saccades toward the distractor. A permutation test at the group level revealed that this observation is highly unlikely to be due to chance, p < 0.00001. At the individual level, this effect also remained highly significant, with p = 0.0204 for participant 1 and p < 0.0001 for all other participants.
Figure 5.
Box-and-whisker plots for each participant in Experiment 2 show the latency of the first saccade for saccades toward the distractor (left, red) or the hidden target (right, green). The number above each box represents the number of observations. For five participants, the majority of first saccades went toward the hidden target. These saccades have longer latency.
Our analysis of eye gaze and performance (see Table 3) is also similar to that reported for Experiment 1A (compare with Table 1). Four participants consistently looked toward the hidden target, one participant (participant 3) consistently looked toward both, and one participant (participant 6) mostly looked toward the hidden target but also looked at both the distractor and the target consecutively on about one-fifth of the trials.
Table 3.
Percentage of trials in Experiment 2 in which eye gaze was aimed toward the hidden target, the distractor, or both in turn. The right-most column provides the percentage of correct trials.
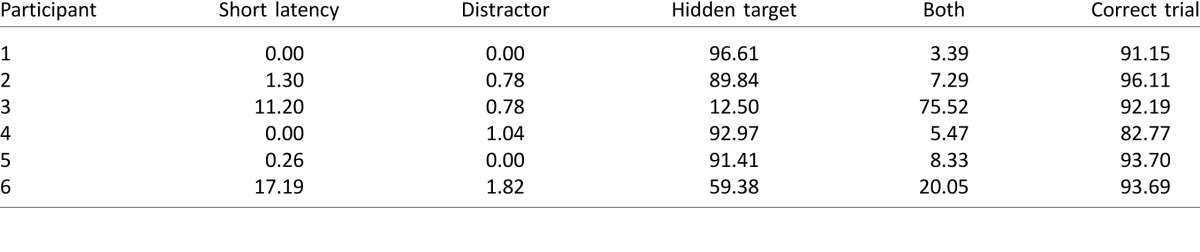
Across participants, on the 461 trials in which gaze covered both stimuli locations (distractor and hidden target), gaze went first to the distractor on the majority of trials (429). Only on 30 trials did gaze first go to the hidden target. Taken together, these results suggests that the ability to look in the direction of a scotoma is not purely due to making saccades to a remembered location (Coëffé & O'Regan, 1987) but is related to a conscious gaze in the direction of the scotoma.
Experiment 3: Looking in the direction of an artificial scotoma with an adjacent distractor
In Experiments 1 and 2, the hidden target and the distractor were presented directly opposite each other relative to central fixation. The configuration of stimuli is reminiscent of an antisaccade set-up. However, in contrast to the antisaccade set-up, participants could not ignore the distractor stimulus because information from this stimulus was needed to make a same–different judgment in the object comparison task.
To generalize our results further and to make an even stronger distinction with the antisaccade paradigm, Experiment 3 tested whether saccade strategy generalized to a situation in which the hidden target and the distractor were not diametrically opposite each other. Instead, in this study, the distractor was presented adjacent to the location of the hidden target (90° clockwise or 90° counterclockwise).
An advantage of this configuration is that it allows for a systematic investigation of the trajectory of eye gaze for participants who try to look at both the distractor and the hidden target on a single trial. Specifically, we analyze the initial and final angle of gaze. Do participants look directly at the target or the distractor? Or do they look somewhere in between? If so, how does such variation in strategy impact performance? Such an analysis was not possible with the preceding experiments because the targets were opposite each other and looking in between the distractor and the target would not have uncovered any part of the hidden target.
Across four blocks of 96 trials each, a hidden target stimulus was presented at four angles: 45°, 135°, 225°, and 315°. Trials were blocked by angle. The order of angles was randomized between blocks, with the constraint that for half the participants the order of the target locations was the inverse order of the order that was experienced by one of the other participants.
For each hidden target, a distractor was presented either 90° clockwise or 90° counterclockwise to it. Half the participants experienced clockwise configurations; half the participants had counterclockwise configurations. Stimuli were presented at 12° distance diagonally from central fixation such that the distance between the center of the target and the center of the distractor was 17° (as in Experiments 1 and 2). The time allowed for eye movements after eye gaze crossed the threshold area was reduced to 200 ms to minimize the opportunity to saccade to both locations.
Measures were as before. For the raw gaze traces, we analyzed not only whether the saccades went toward the target and the distractor but also whether they traversed the region in between. This analysis examined whether an eye movement was curved across the trial. We determined the angle of gaze for two samples of the raw gaze data: (a) the first sample after the participant left the threshold distance and (b) the last sample of the trial. All angles were normalized such that 0° aligned with the angle at which the hidden target was presented and 90° aligned with the angle at which the distractor was presented. We then binned eye movements in sets of 22.5° and analyzed in what direction eye gaze was headed initially and at the end of the trial, whether this implied a curved saccade, and how the saccade path then influenced performance on the same–different judgment task.
Results and discussion: Experiment 3
Saccades: Direction and latency
Figure 6 reports box-and-whisker plots of saccade latency for each participant. Again, all participants except participant 3 made a first saccade in the direction of the hidden target on the majority of trials. Also similar to the preceding experiment, latencies were longer for saccades aimed toward the hidden target compared with saccades aimed toward the distractor. A permutation test at the group level confirmed that it is highly unlikely that the observed difference was due to chance, p < 0.00001. At the individual level, this effect was also highly significant, with p = 0.0071 for participant 1 and p < 0.0001 for all other participants.
Figure 6.
Box-and-whisker plots for each participant in Experiment 3 show the latency of the first saccade for saccades toward the distractor (left, red) or the hidden target (right, green). The number above each represents the number of observations. For five participants, the majority of first saccades went toward the hidden target. These saccades have longer latency.
Saccade pattern during the entire trial
Figure 7 plots data in the same format as Figure 3. Figure 7A shows the percentage of first saccades aimed toward the hidden target. Again, some participants look relatively consistently toward the hidden target (participants 1 and 5 in particular). In contrast, participants 3 and 6 over time had fewer trials in which the first saccade was aimed toward the hidden target.
Figure 7.
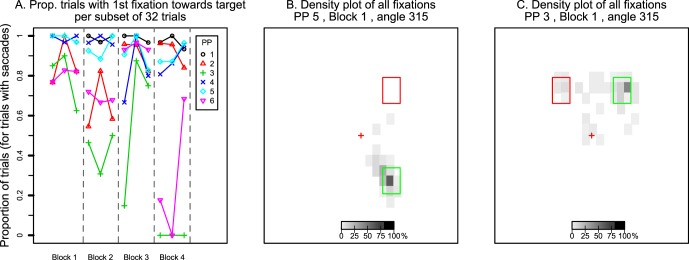
(A) Percentage of trials in which participants made their first saccade toward the hidden target out of all trials with eligible saccades. Data points show values per participant per set of 32 trials. Dashed lines distinguish the four blocks of trials. (B, C) Examples of density plots of where saccades landed throughout the entire trial period. Panel B shows data of a participant who consistently made saccades only toward the target area. Panel C shows data of a participant who made saccades to both the target and the distractor.
Figure 7B and C shows density plots of saccade landing positions within 2° by 2° regions across the screen (for all saccades in a trial, with density calculated across a block of 96 trials). Again, participant 5 (Figure 7B) consistently made saccades toward the hidden target area. In contrast, participant 3's saccades landed in multiple locations across trials: the distractor area; the target area; and various locations within the triangular area between the target, the distractor, and central fixation (Figure 7C).
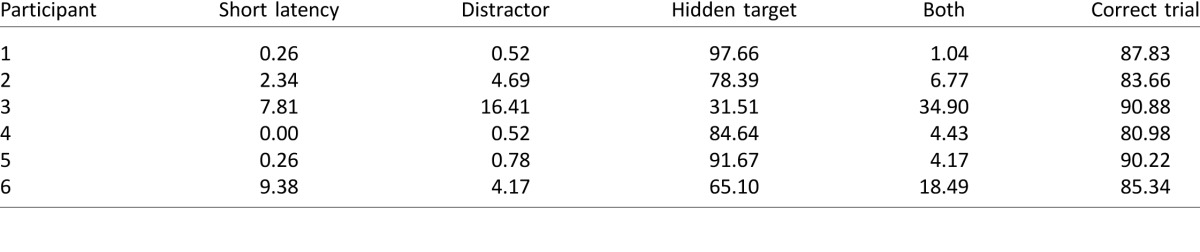
Table 4 reports whether gaze was aimed toward the distractor, the hidden target, or both locations in turn as well as the percentage of correct same–different judgments. Results were again similar to those in the preceding experiments, where five participants mostly looked toward the hidden target only. Participant 3 was now more evenly split in his strategy between looking at the target and looking at both. Performance on the same–different task was a little lower compared with Experiments 1A and 2 but still better than that of the older group in Experiment 1B.
For comparison with Experiments 1 and 2, this analysis considered only saccades that were aimed at targets or distractors. In the next section, we analyze saccades that curved between target and distractor and how this strategy affected performance.
Analysis of curved saccades
To get a more reliable measure of whether participants looked toward both stimuli, we investigated the angle of the saccade for two time stamps: the initial angle and the final angle. The initial angle was defined as the angle of the first gaze sample that passed threshold. The final angle was the angle of the last sample during the trial. All angles were normalized such that 0° aligned with the angle at which the hidden target was presented and 90° aligned with the angle at which the distractor was presented. Angles were binned in subsets of 22.5°.
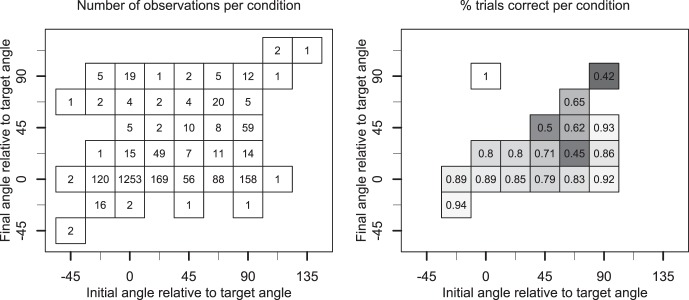
Figure 8A plots the number of trials observed for each combination of initial angle (horizontal axis) and final angle (vertical axis). Saccades that lie on the diagonal (i.e., where the initial angle equals the final angle) did not curve. The majority of trials with direct (noncurved) saccades were directed to the hidden target (1,253), whereas a few trials were directed toward the distractor (12). The off-diagonal entries are curved saccades where the distance between the initial and final saccade indicates the degree of curvature; some saccades curved just enough to end up in an adjacent bin, whereas others curved by as much as 90°. All participants had curved saccades on some trials. Saccades curved by more than 33.75° (all nonadjacent and nonoverlapping bins in Figure 8) in 7, 48, 232, 29, 34, and 85 trials for participants 1 to 6, respectively.
Figure 8.
Results of curvature analysis in Experiment 3. (A) Number of trials binned by the initial angle (horizontal axis) and final angle (vertical axis) of the saccade. Angles were normalized such that 0° aligns with the angle at which the hidden target was presented and 90° aligns with the angle of the distractor. (B) Proportion of correct trials for each cell. Proportion correct is expressed as a number and as a gray level, with lighter gray indicating higher percentages correct. Note that performance is good for strategies that ended up at an angle close to that at which the target was presented (i.e., the horizontal row where final angle is 0). Performance was also good for saccades that ended at the distractor, provided they started at the target (top left). Performance was at chance level if the initial and final angles were not close to the target (top right).
On the curved saccade trials, there were more saccades that initially headed toward the distractor and then went to the target compared with the inverse case. In Figure 8A this can be seen because there are more observations in the bottom right corner compared with the top left corner. In particular, many observations end up near the angle at which the target is presented (the horizontal row of cells near 0° on the vertical axis).
Figure 8B illustrates how the various strategies influenced performance. Here we plot proportion of correct responses in the same–different task as both a number and a gray level, with lighter gray indicating higher percentage correct. Performance is reported only for cells that had at least five observations.
In general, performance is good for all starting directions as long as they end up at the target (i.e., all horizontal cells near 0 on the vertical axis). This is the majority of the observations. Performance was also good for saccades that ended at the distractor, provided they started at the target (i.e., cells in the top left corner). In general, performance is worse, and close to chance level, for strategies that start in the direction of the distractor and that do not come within 45° of the target.
An exception is the performance of a strategy that initially headed to the distractor (90°) and ended up in between the target and the distractor (45°). This result was solely driven by one participant (3), who adopted this strategy on 54 trials and had 98% of those correct. In the online supplementary materials we include an example of the display, showing how much of the target was uncovered when participant 3 used this strategy. These trials occurred within a single block when the hidden target and distractor were at angles of 135° and 225° respectively. The participant moved gaze due left in the 180° direction, thereby uncovering a critical region in the top right of the hidden target. This behavior was not representative of the general participant pool. The other participants employed such a strategy on a total of only five trials and were correct on only two of these. Taken together, these results suggest that the strategy of using curved saccades was useful, particularly if the target area was eventually reached with the curvature.
General discussion
In three experiments we explored the strategies that people use to gather information in an object comparison task under time pressure. Specifically, we investigated whether participants are able to look in the direction of an artificial scotoma, an informative but nonsalient region, even though their attention might be distracted by an initially more salient stimulus that is presented outside of the scotoma. In all experiments, the majority of participants used their first saccade to look in the direction of the artificial scotoma. This was true even for an older population (Experiment 1B), when the position of the scotoma was randomized between trials (Experiment 2), and when a distractor was presented adjacent to, instead of opposite, the scotoma (Experiment 3).
A second finding in our study was that initial saccades that are aimed in the direction of the scotoma had a longer latency compared with those that were aimed toward the distractor. This suggests that looking into a scotoma requires a more deliberate, nonreflexive saccade strategy. This is in line with the literature on antisaccades, curved saccades, and reward-based saccades, as discussed in more detail later. The effect of saccade latency was less pronounced for older adults (Experiment 1B), most likely due to a limited number of observations.
Relation to previous studies
Our study has a theoretical and a practical motivation. The theoretical motivation is to determine whether humans direct saccades to locations that maximize the information gained. Our work provides an extreme setting to test the theory that eye movements are directed toward locations that provide the most information (e.g., Butko & Movellan, 2010; Lee & Yu, 2000; Legge et al., 2002; Renninger et al., 2005, 2007; Verghese, 2012). In our paradigm, a saccade to the hidden target location maximizes the information required for the object comparison task.
We found that participants tended to look toward the informative, hidden target. In contrast, preceding work showed that saccades tend to go more frequently to either salient locations (cf., Itti & Koch, 2000; van Zoest et al., 2012) or more target-like locations (e.g., Beutter et al., 2003; Najemnik & Geisler, 2005, 2009). In particular, in a setting with multiple targets, Verghese (2012) showed that saccades go to more target-like than uncertain locations, even when such a strategy is highly inefficient.
One potential explanation for our finding is that the hidden target was in a fixed location in Experiments 1 and 3. This consistent configuration might have facilitated accurate saccades because the hidden stimulus was in a predictable location within a block of trials (Coëffé & O'Regan, 1987). In Experiment 2, we demonstrated that stimulus predictability cannot fully explain our result because participants were able to direct saccades in the direction opposite the visible, distracting stimulus even when the orientation of the target–distractor pair was randomized.
Other known aspects of our experimental paradigm might have helped direct saccades to informative locations. For example, participants knew that there was always exactly one hidden stimulus and one immediately visible stimulus. The location of the visible stimulus was also a valid cue to the location of the hidden stimulus (either opposite or adjacent). In other work (e.g., Verghese, 2012) such explicit knowledge was not available. These simplifications in our setting might have led to better performance.
Our practical motivation was to better understand a challenge that patients with central vision loss face. They miss information that is obscured by their scotoma. Because the saccades of these patients can have longer latency and be less direct (Renninger et al., 2008; Van der Stigchel et al., 2013), it might take them a long time to uncover information hidden in their scotoma if they direct their gaze only toward visible stimuli. Thus, it is important for them to make efficient saccades in the direction of their scotoma to reveal missing information in a timely fashion.
We demonstrated here that looking in the direction of a peripheral artificial scotoma is a feasible strategy for healthy younger adults. We also found that older adults could do this. This is particularly significant because it demonstrates that this saccade strategy can, in principle, be applied by an age group that is susceptible to macular degeneration (Friedman et al., 2004).
At the moment we are performing tests on patients with macular damage to test the feasibility of our method for patients. An added challenge here is that patients are not always aware of the location of their scotoma (Fletcher, Schuchard, & Renninger, 2012). We are therefore investigating how to train both the efficiency of eye movements as well as awareness of the scotoma location.
Curved saccades
In Experiment 3 we found that participants made curved saccades (McPeek & Keller, 2001) that attempted to go to both target and distractor. A novel finding of our work is that the curved saccades show up in a setting in which initially only one stimulus is visible and where the target is hidden by the scotoma. Previous studies demonstrate curved saccades in various settings that had a visible target and at least one visible distractor: visual search by monkeys (McPeek & Keller, 2001), visually guided reaching by humans (Song & Nakayama, 2008), and moving a mouse cursor toward an object that is being spoken concurrently (Spivey, Grosjean, & Knoblich, 2005). Our results indicate that the competition between saccade goals that is hypothesized in the curved saccade literature (e.g., McPeek, 2003; Port, 2003) extends to the case when one of the stimuli is not visible.
When curved saccades occurred, the behavioral effects were in line with those reported in the literature: Most of these saccades were initially aimed toward the salient distractor and were then either curved toward the target during the saccade or followed by a rapid saccade in the direction of the target. In our experiments curved saccades also occurred within a short interval: Participants had only roughly 200 ms to complete a trial after their saccade started (Experiment 3).
Curved saccades were useful adaptations to the constraints imposed by our task. When the curved saccade reached both the target and the distractor, performance was as good as in trials in which saccades went directly to the target. For trials in which the curve did not reach the target, the success of the strategy depended on how close the gaze came to the target. Many observers performed accurately when they came within 33.75° of the target. One exception was participant 3, who performed almost perfectly even though his saccades came only to within 45° of the target (in one particular configuration). The crux in this strategy was that his gaze moved just far enough to reveal a critical region of the target. Thus, our experiments reveal that humans can come up with creative adaptations to a situation that circumvents theoretical assumptions.
Saccade latency
We found that saccades that were initially aimed toward the target had a longer latency compared with saccades that were initially aimed toward the distractor. This is in line with other lines of research: curved saccades, voluntary saccades to a remembered target, antisaccades (Munoz & Everling, 2004; Smit, Van Gisbergen, & Cools, 1987), and reward-based fixations (Schutz, Trommershauser, & Gegenfurtner, 2012). The longer latency for saccades toward the hidden target suggests that this involves a more deliberate, nonreflexive strategy. Indeed, in the reward-based literature, longer latencies are associated with saccades toward valuable locations, whereas shorter latencies are associated with saccades toward salient locations (Schutz et al., 2012). In contrast to the reward literature, we did not use explicit monetary rewards to guide task performance. In contrast to the antisaccade literature, our stimuli appeared gradually (and were not flashed), and information from both the visible distractor and the hidden target (antisaccade location) was needed to complete the task.
We did not find a consistent effect of saccade direction on latency for our older adults (Experiment 1B). As mentioned in the discussion section of that study, this can be attributed to the relatively smaller number of observations in the “toward distractor” condition. We had more observations of saccades in the direction of the hidden target. The latencies of these saccades had the same range of values as observed in our other studies.
Future work
In our study the location of the visible distractor provided a cue about the location of the scotoma. However, when patients with vision loss search for objects in daily life, such explicit cues are unlikely. Therefore, further studies are needed to determine how well healthy adults are able to direct their eye movements toward the artificial scotoma when they are viewing a cluttered visual scene with no explicit cues about scotoma location. This can be addressed in future studies now that we have established that healthy adults are in principle able to consistently make saccades in the direction of a hidden scotoma.
Conclusions
Adults with healthy vision can aim their first saccade in the direction of an informative target area that is initially hidden by a gaze-contingent scotoma in the periphery when they are under time pressure and when a salient distracting stimulus is presented outside of the scotoma. Eye movements in the direction of the scotoma tend to have longer latencies compared with eye movements in the direction of a distracting stimulus. We suggest that this is because these eye movements require a more deliberate, nonreflexive saccade strategy. These findings inform training methods for patients who suffer from vision loss and who need to learn efficient saccade strategies to compensate for a biological scotoma.
Supplementary Material
Acknowledgments
This research was funded by a Pacific Vision Foundation grant to P. V. and C. P. J., a Rachel C. Atkinson fellowship to C. P. J., and NIH Grant R01 EY0222394 to P. V. We thank Terence Tyson for help with calibrating participants. Part of this work was presented at the 2014 Annual Meeting of the Vision Sciences Society (Janssen & Verghese, 2014).
Commercial relationships: none.
Corresponding author: Christian P Janssen.
Email: c.p.janssen@uu.nl.
Address: Utrecht University, Department of Experimental Psychology, Utrecht, The Netherlands.
Footnotes
Although our paradigm is not a typical visual search task, we refer to the hidden stimulus as target and to the visible stimulus as distractor. We use this terminology because the hidden stimulus, not the visible stimulus, should ideally be the target of eye movements. However, observers need to know the identity of both stimuli for the object comparison task.
The two authors piloted the discriminability of silhouettes by making same–different judgments on all unique combinations of different pairs and an equal number of same pairs (i.e., 1,104 trials). In this pilot, stimulus pairs were flashed for 100 ms at 8.5° left and right from central fixation. The pilot results showed that three silhouettes (chisel, pen, and asparagus) were harder to discriminate from each other and from selected other stimuli. Specifically, the average percentage correct for these stimuli was 88% compared with 97% for the other stimuli. In the final experiment, three of 24 pairs involved these harder stimuli on different trials. The other pairs were easy to discriminate. In effect, this made the same–different task easy, but not trivial, to perform if stimuli were presented without a scotoma.
Contributor Information
Christian P. Janssen, c.p.janssen@uu.nl, www.cpjanssen.nl.
Preeti Verghese, preeti@ski.org, http://www.ski.org/Verghese_Lab/.
References
- Bertera J. H.(1988). The effect of simulated scotomas on visual search in normal subjects. Investigative Ophthalmology and Visual Science, 29, 470–475, http://www.iovs.org/content/29/3/470. [PubMed] [Article] [PubMed] [Google Scholar]
- Beutter B. R.,, Eckstein M. P.,, Stone L. S.(2003). Saccadic and perceptual performance in visual search tasks. I. Contrast detection and discrimination. Journal of the Optical Society of America A, 20, 1341–1355. [DOI] [PubMed] [Google Scholar]
- Butko N. J.,, Movellan J. R.(2010). Infomax control of eye movements. IEEE Transactions on Autonomous Mental Development, 2, 91–107. [Google Scholar]
- Coëffé C.,, O'Regan J. K.(1987). Reducing the influence of non-target stimuli on saccade accuracy: Predictability and latency effects. Vision Research, 27, 227–240, doi:http://dx.doi.org/10.1016/0042-6989(87)90185-4. [DOI] [PubMed] [Google Scholar]
- Cornelissen F. W.,, Bruin K. J.,, Kooijman A. C.(2005). The influence of artificial scotomas on eye movements during visual search. Optometry and Vision Science, 82, 27–35. [PubMed] [Google Scholar]
- Efron B.,, Tibshirani R. J.(1993). An introduction to the bootstrap. New York, NY: Chapman & Hall. [Google Scholar]
- Fletcher D. C.,, Schuchard R. A.,, Renninger L. W.(2012). Patient awareness of binocular central scotoma in age-related macular degeneration. Optometry and Vision Science, 89, 1395–1398. [DOI] [PubMed] [Google Scholar]
- Friedman D. S.,, O'Colmain B. J.,, Munoz B.,, Tomany S. C.,, McCarty C.,, de Jong P., . . . Eye Diseases Prevalence Research Group. (2004). Prevalence of age-related macular degeneration in the United States. Archives of Ophtalmology, 122, 564–573. [DOI] [PubMed] [Google Scholar]
- Henderson J. M.,, McClure K. K.,, Pierce S.,, Schrock G.(1997). Object identification without foveal vision: Evidence from an artificial scotoma paradigm. Perception and Psychophysics, 59, 323–346. [DOI] [PubMed] [Google Scholar]
- Itti L.,, Koch C.(2000). A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research, 40, 1489–1506, doi:http://dx.doi.org/10.1016/S0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Janssen C. P.,, Verghese P.(2014). Stop & think: Looking into a scotoma. Journal of Vision, 14(10): 222, http://www.journalofvision.org/content/14/10/222, doi:10.1167/14.10.222. [Abstract] [Google Scholar]
- Kalesnykas R. P.,, Hallett P. E.(1987). The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Experimental Brain Research, 68, 115–121, doi:http://dx.doi.org/10.1007/BF00255238. [DOI] [PubMed] [Google Scholar]
- Kowler E.,, Anderson E.,, Dosher B.,, Blaser E.(1995). The role of attention in the programming of saccades. Vision Research, 35, 1897–1916. [DOI] [PubMed] [Google Scholar]
- Kwon M. Y.,, Nandy A. S.,, Tjan B. S.(2013). Rapid and persistent adaptability of human oculomotor control in response to simulated central vision loss. Current Biology, 23, 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. S.,, Yu S. X.(2000). An information-theoretic framework for understanding saccadic eye movements. Advances in Neural Information Processing Systems, 12, 834–840. [Google Scholar]
- Legge G. E.,, Hooven T. A.,, Klitz T. S.,, Mansfield J. S.,, Tjan B. S.(2002). Mr. Chips 2002: New insights from an ideal-observer model of reading. Vision Research, 42, 2219–2234. [DOI] [PubMed] [Google Scholar]
- Lingnau A.,, Albrecht T.,, Schwarzbach J.,, Vorberg D.(2014). Visual search without central vision—No single pseudofovea location is best. Journal of Eye Movement Research, 7(2), 1–14. [Google Scholar]
- McIlreavy L.,, Fiser J.,, Bex P. J.(2012). Impact of simulated central scotomas on visual search in natural scenes. Optometry and Vision Science, 89, 1385–1394, doi:http://dx.doi.org/10.1097/OPX.0b013e318267a914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek R. M.(2003). Competition between saccade goals in the superior colliculus produces saccade curvature. Journal of Neurophysiology, 89, 2577–2590, doi:http://dx.doi.org/10.1152/jn.00657.2002. [DOI] [PubMed] [Google Scholar]
- McPeek R. M.,, Keller E. L.(2001). Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vision Research, 41, 785–800, doi:http://dx.doi.org/10.1016/S0042-6989(00)00287-X. [DOI] [PubMed] [Google Scholar]
- Munoz D. P.,, Everling S.(2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience, 5, 218–228. [DOI] [PubMed] [Google Scholar]
- Najemnik J.,, Geisler W. S.(2005). Optimal eye movement strategies in visual search. Nature, 434, 387–391, doi:http://dx.doi.org/10.1038/nature03390. [DOI] [PubMed] [Google Scholar]
- Najemnik J.,, Geisler W. S.(2009). Simple summation rule for optimal fixation selection in visual search. Vision Research, 49, 1286–1294. [DOI] [PubMed] [Google Scholar]
- Port N. L.(2003). Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. Journal of Neurophysiology, 90, 1887–1903, doi:1http://dx.doi.org/0.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Renninger L. W.,, Coughlan J.,, Verghese P.,, Malik J.(2005). An information maximization model of eye movements. Advances in Neural Information Processing Systems, 17, 1121–1128. [PubMed] [Google Scholar]
- Renninger L. W.,, Dang L.,, Verghese P.,, Fletcher D. C.(2008). Effect of central scotoma on eye movement behavior. Journal of Vision, 8(6): 641, http://www.journalofvision.org/content/8/6/641, doi:10.1167/8.6.641. [Abstract] [Google Scholar]
- Renninger L. W.,, Verghese P.,, Coughlan J.(2007). Where to look next? Eye movements reduce local uncertainty. Journal of Vision, 7(3): 6,, 1–17, http://www.journalofvision.org/content/7/3/6, doi:10.1167/7.3.6. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Schutz A. C.,, Trommershauser J.,, Gegenfurtner K. R.(2012). Dynamic integration of information about salience and value for saccadic eye movements. Proceedings of the National Academy of Sciences, USA, 109, 7547–7552, doi:http://dx.doi.org/10.1073/pnas.1115638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A. C.,, Van Gisbergen J.,, Cools A. R.(1987). A parametric analysis of human saccades in different experimental paradigms. Vision Research, 27, 1745–1762, doi:http://dx.doi.org/10.1016/0042-6989(87)90104-0. [DOI] [PubMed] [Google Scholar]
- Snodgrass J. G.,, Vanderwart M.(1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6, 174–215. [DOI] [PubMed] [Google Scholar]
- Song J.-H.,, Nakayama K.(2008). Target selection in visual search as revealed by movement trajectories. Vision Research, 48, 853–861, doi:http://dx.doi.org/10.1016/j.visres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Spivey M. J.,, Grosjean M.,, Knoblich G.(2005). Continuous attraction toward phonological competitors. Proceedings of the National Academy of Sciences, USA, 102, 10393–10398, doi:http://dx.doi.org/10.1073/pnas.0503903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stigchel S.,, Bethlehem R. A. I.,, Klein B. P.,, Berendschot T. T. J. M.,, Nijboer T. C. W.,, Dumoulin S. O.(2013). Macular degeneration affects eye movement behavior during visual search. Frontiers in Psychology, 4, 579, doi:http://dx.doi.org/10.3389/fpsyg.2013.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoest W.,, Donk M.,, Van der Stigchel S.(2012). Stimulus-salience and the time-course of saccade trajectory deviations. Journal of Vision, 12(8): 16,, 1–13, http://www.journalofvision.org/content/12/8/16, doi:10.1167/12.8.16. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Verghese P.(2012). Active search for multiple targets is inefficient. Vision Research, 74, 61–71, doi:http://dx.doi.org/10.1016/j.visres.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Verghese P.,, Ghahghaei S.(2013). Immediate feedback improves saccade efficiency. Journal of Vision, 13(9): 302,.http://www.journalofvision.org/content/13/9/302, doi:10.1167/13.9.302. [Abstract] [Google Scholar]
- Walsh D. V.,, Liu L.(2014). Adaptation to a simulated central scotoma during visual search training. Vision Research, 96, 85–86. [DOI] [PubMed] [Google Scholar]
- Whittaker S. G.,, Cummings R. W.,, Swieson L. R.(1991). Saccade control without a fovea. Vision Research, 31, 2209–2218, doi:http://dx.doi.org/10.1016/0042-6989(91)90173-3. [DOI] [PubMed] [Google Scholar]
- Zhang S.,, Eckstein M. P.(2010). Evolution and optimality of similar neural mechanisms for perception and action during search. PLoS Computational Biology, 6(9), e1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.