Highlights
-
•
The patient was a 59-year-old man presenting with a swollen right submandibular gland. Laboratory tests revealed IgG4 levels of 176 mg/dl (reference range: 4.8–105).
-
•
Histological examination of the submandibular gland showed an adenoid cystic carcinoma with lymphocytic infiltration containing many IgG4-positive plasma cells in the tumor stroma.
Abbreviations: ACC, adenoid cystic carcinoma; IgG4-RD, IgG4-related disease
Keywords: Adenoid cystic carcinoma, IgG4-related disease, Plasma cells
Abstract
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is an inflammatory condition associated with elevated serum IgG4 levels and tissue infiltration by IgG4-expressing plasma cells. We present a case of adenoid cystic carcinoma (ACC) of the submandibular gland with possible involvement of IgG4-RD.
Presentation of case
The patient was a 59-year-old man presenting with a swollen right submandibular gland. Laboratory tests revealed IgG4 levels of 176 mg/dl (reference range: 4.8–105). An initial open biopsy for histological diagnosis showed chronic sialadenitis. The region was monitored on an outpatient basis, and finally the right submandibular was totally resected because malignant tumor could not be excluded. Histological examination of the submandibular gland showed an ACC with lymphocytic infiltration containing many IgG4-positive plasma cells in the tumor stroma.
Discussion
We have described a case that indicated a possible involvement of ACC with IgG4-RD. This allows us to speculate that longstanding IgG4-RD may progress to malignancy or infiltration of IgG4-positive plasma cells through the signals of tumor stimuli. Further investigations are required to determine the potential pathogenic mechanism underlying this unique tumor.
Conclusion
This case underscores that caution is needed in the diagnosis of masses with high serum IgG4 levels, as the differential diagnosis includes malignancy.
1. Introduction
Immunoglobulin G4-related diseases (IgG4-RDs) are a recently recognized group of diseases characterized by elevated serum IgG4 levels and prominent lymphoplasmacytic infiltration of IgG4-positive cells into multiple organs [1]. These conditions were first described in relation to type 1 autoimmune pancreatitis (AIP) [2]. As more patients with IgG4-related AlP were studied, it was recognized that these patients frequently (49–80% of cases) have other extrapancreatic sites involved with this process [3]. It is now accepted that this disease process typically involves multiple organs at varying times with or without pancreatic involvement [1,3,4].
Adenoid cystic carcinoma (ACC) is a distinctive malignant neoplasm that involves primarily the major and minor salivary glands, and that accounts for approximately 10% of all malignant tumors of the salivary glands [5]. In the present study, we report a case with possible involvement of ACC in association with IgG4-RD.
2. Case presentation
A 59-year-old man without a history of malignancy was referred to our institution because of a mass with associated pressure pain in his right submandibular region, which he had first noticed several months earlier. A physical examination demonstrated that the mass was elastic, hard and movable and was not attached to the overlying skin. No lingual nerve paralysis was observed. The serum amylase level was elevated to 247 IU/l (reference range: 38–125), the salivary amylase to 186 IU/l, and the pancreatic amylase to 61 IU/l (reference range: 16–49). The IgG4 levels were 176 mg/dl (reference range: 48–105). Although the white blood cells, eosinophil levels, and C-reactive protein levels were within the reference ranges, and his serum was negative for antinuclear antibody, anti-SSA and anti-SSB. A computed topographic (CT) scan (Fig. 1A–D) demonstrated a heterogeneous contrasting mass in the right submandibular gland that was 30 × 25 mm in size (Fig. 1B and D, arrowheads). Magnetic resonance imaging (MRI) scanning showed low signal intensity on the T1-weighted image (Fig. 2A and D), and high signal intensity on the short inversion time inversion-recovery (STIR) image (Fig. 2B and E) and fat-suppressed T1-weighted image (Fig. 2C and F). The clinical diagnosis was submandibular sialadenitis. Fine-needle aspiration cytology of the submandibular legion revealed inflammatory cells. To determine whether the disease diagnosis corresponded with sialadenitis, the patient underwent an initial open biopsy of the submandibular gland. Histological examination of the submandibular legion showed chronic sialadenitis. A diagnosis of AIP was denied by gastroenterological inspection. The region was followed-up in our outpatient department, but the diagnosis was not further clarified by diagnostic imaging. The right submandibular gland was totally resected at 12 months after the first medical exam day because a malignant submandibular gland tumor could not be excluded. Histological examination of the submandibular lesion showed ACC. The patient was treated with postoperative radiotherapy (62 Gy). The postoperative serum IgG4 level was 99.1 mg/dl at 3 months after surgery. During the follow-up over 12 months, the patient was healthy with no evidence of recurrence.
Fig. 1.
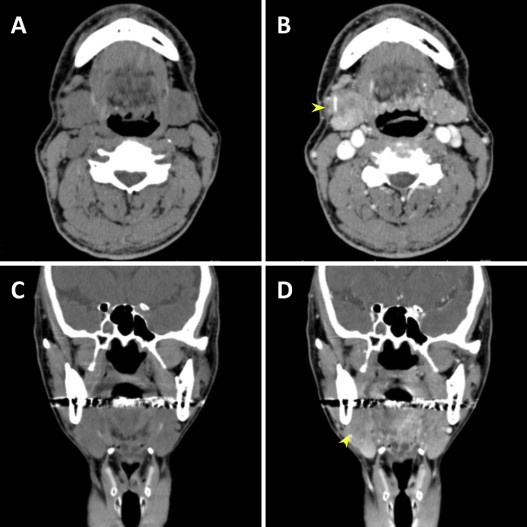
CT findings. (A and C) Plain CT image. (B and D) Contrast-enhanced CT image shows heterogeneous contrasting mass in the right submandibular gland (arrowheads).
Fig. 2.
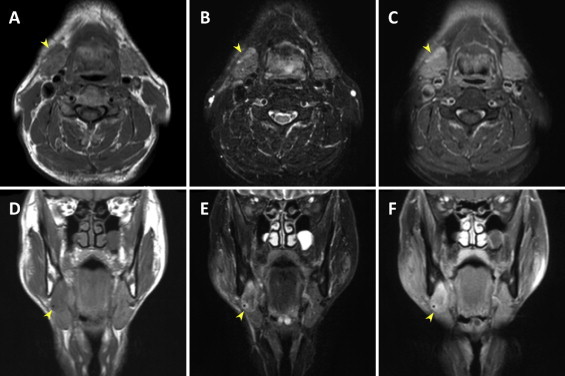
MRI findings. (A and D) T1-weighted image. (B and E) Short inversion time inversion-recovery (STIR) image. (C and F) Fat-suppressed T1-weighted image. Arrowheads, right submandibular gland lesion.
Macroscopically, the resected specimen consisted of a right submandibular gland measuring 46 × 33 × 25 mm with a highly developed vasculature on the surface (Fig. 3A). The histological sections of the submandibular gland (Fig. 3B and D) showed an infiltrative tumor composed of cribriform structures surrounded by dense fibrosis and abundant inflammatory cells. The plasma cells in the tumor stroma and the non-neoplastic area adjacent to the tumor were strongly immunoreactive for IgG4 (Fig. 3C and E) and IgG4 (Fig. 3F). The IgG4-positive plasma cells accounted for more than 40% of IgG-positive plasma cells.
Fig. 3.
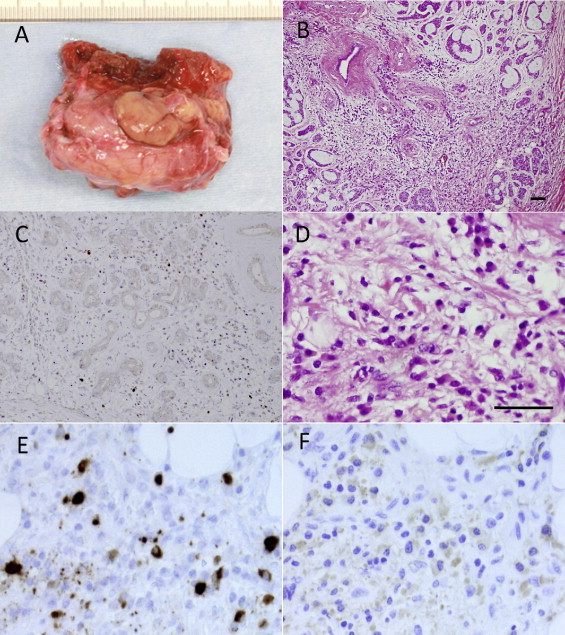
Macroscopic and histological findings of the resected tumor in the right submandibular gland. (A) Resected submandibular gland. (B) HE staining of the tumor, which was composed of islands of basaloid cells surrounding variably sized cyst-like spaces forming cribriform structures. The inflammatory infiltrate and stromal fibroblastic tissue were observed around the tumor (B and D). Numerous plasma cells with IgG4 (C and E) and IgG4 (F) were present in the tumor stromal tissue (C) and the inflammatory infiltrate adjacent to the tumor (E and F). Bar 25 μm.
3. Discussion
IgG4-RD is a recently recognized multi-organ system condition with pathological features that are largely consistent across a wide range of organ systems. It shows hyper-IgG4-γ-globulinemia and IgG4-positive plasma cell expansion in affected organs. In 2001, Hamano et al. [2] showed that the IgG4 serum level in AIP patients was increased, and this increase was ameliorated with corticosteroid therapy. Hamano et al. [6] later found that some AIP patients had retroperitoneal fibrosis with abundant IgG4-positive plasma cells. A subsequent case report [7] demonstrated retroperitoneal fibrosis, mediastinal fibrosis, and increased IgG4 levels without AIP, highlighting the spectrum of IgG4-RDs. Similar cases involving different sites have been reported, including inflammatory abdominal aortic aneurysms, inflammatory pseudotumors of the lung and breast, chronic sclerosing sialadenitis, chronic sclerosing dacryoadenitis, and cholangitis [8].
ACCs of the head and neck are relatively rare tumors, accounting for only about 10% of all salivary gland neoplasms [5]. ACCs are found both in the major salivary glands, including the submandibular glands, and in the minor salivary glands. These tumors are characterized by slow, local growth and rarely spread to the local lymph nodes. Pathologic features with perineural invasion have been correlated with prognosis [9–11]. In our case, perineural invasion was detected histologically in the resected samples, and postoperative radiation was therefore performed. Patients with ACC of the major salivary glands treated with surgery and radiation have excellent overall control rates: the 5 and 10 year local control rates are 94% and 73% [9,11,12]. Accordingly, ACC requires long-term follow-up to track the local and distant recurrence after initial diagnosis.
By immunohistochemistry, IgG4-positive plasma cells were significantly increased in the tumor stromal tissue (Fig. 3C) and the inflammatory infiltrate adjacent to the tumor (Fig. 3E). Kojima et al. [13] found that IgG4 plasma cell counts of more than 20/HPF were highly specific for a diagnosis of AIP. In the present study, >40/HPF of IgG4-positive plasma cells were found and accounted for more than 40% of IgG-positive cells. The serum levels of IgG4 of the patient were increased, but the patient showed no evidence of IgG4 systemic disease. At the present time, it is unclear whether this case of ACC developed in the submandibular gland with IgG4-related chronic sclerosing inflammation, or whether the prominent IgG4-positive plasma cells and stromal fibrosis represented inflammatory and immune reactions caused by the carcinoma cells.
Zen and Nakanuma [14] recently reported 7 (6%) malignancies in a series of 114 patients with systemic IgG4 fibrosclerosing disease; 4 developed before and 3 developed after the patient’s diagnosis of IgG4-RD. Several cases of pancreatic adenocarcinoma have arisen in association with IgG4-related fibrosclerosing pancreatitis [3], and other authors have described a pulmonary adenocarcinoma, a salivary duct carcinoma arising in IgG4 fibrosclerosing disease of the parotid gland [15,16]. Tian et al. suggested that the association of elevated IgG4-positive plasma cells with increased fibrosis in the sclerosing variant of mucoepidermoid carcinoma suggests a role of IgG4-positive plasma cells in fibrogenesis and may be a new concept related to sclerosis in cancer [17]. Interestingly, it has been reported that IgG4-positive plasma cells are a variable component of the intratumoral and peritumoral inflammatory response in various types of cancer [18]. The histological sections of 5 cases of ACC treated in our department showed peritumoral inflammation of the submandibular gland or the sublingual gland and were characterized by diffuse lymphoplasmacytic infiltration, IgG4-positive plasma cells and fibrosis, but no IgG4-positive plasma cells were observed (data not shown). Thus, the increased IgG4 in this case of ACC may represent a secondary response to a primary immune-mediated process caused by an unknown tumor-associated stimulus. Some cytokines, such as tissue interleukin (IL)-10 and transforming growth factor-β, are suggested to be involved in the process inducing increased IgG-4 positive cells and fibrosis [19]. It is possible that increased IL-10 and TGF-β in the tumor microenvironment might be involved in the pathogenesis of ACC. However, the biological significance of infiltrating IgG4-positive plasma cells and elevated serum IgG4 levels in patients with ACC remains to be elucidated.
4. Conclusion
Here, we have described a case that indicated a possible involvement of ACC with IgG4-RD. This allows us to speculate that longstanding IgG4-RD may progress to malignancy or infiltration of IgG4-positive plasma cells through the signals of tumor stimuli. Further investigations are required to determine the potential pathogenic mechanism underlying this unique tumor. This case underscores that caution is needed in the diagnosis of masses with high serum IgG4 levels, as the differential diagnosis includes malignancy.
Conflicts of interest
None.
Funding
None.
Consent
Informed consent was taken.
Ethical approval
Informed consent was taken.
Author contributions
Tsuyoshi Shimo contributed to data and writing. Mayumi Yao, Yuichiro Takebe, Yuko Ono, Kyoichi Obata, Naito Kurio, Soichiro Ibaragi, Norie Yoshioka, Koji Kishimoto, Yoshinobu Yanagi, Hitoshi Nagatsuka, and Akira Sasaki contributed to data collections.
References
- 1.Masaki Y., Dong L., Kurose N., Kitagawa K., Morikawa Y., Yamamoto M. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann. Rheum. Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 2.Hamano H., Kawa S., Horiuchi A., Unno H., Furuya N., Akamatsu T. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 3.Cheuk W., Chan J.K. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv. Anat. Pathol. 2010;17:303–332. doi: 10.1097/PAP.0b013e3181ee63ce. [DOI] [PubMed] [Google Scholar]
- 4.Kasashima S., Zen Y., Kawashima A., Konishi K., Sasaki H., Endo M. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am. J. Surg. Pathol. 2008;32:197–204. doi: 10.1097/PAS.0b013e3181342f0d. [DOI] [PubMed] [Google Scholar]
- 5.Bradley P.J. Adenoid cystic carcinoma of the head and neck: a review. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:127–132. doi: 10.1097/00020840-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Hamano H., Kawa S., Ochi Y., Unno H., Shiba N., Wajiki M. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 7.Zen Y., Sawazaki A., Miyayama S., Notsumata K., Tanaka N., Nakanuma Y. A case of retroperitoneal and mediastinal fibrosis exhibiting elevated levels of IgG4 in the absence of sclerosing pancreatitis (autoimmune pancreatitis) Hum. Pathol. 2006;37:239–243. doi: 10.1016/j.humpath.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Neild G.H., Rodriguez-Justo M., Wall C., Connolly J.O. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006;4:23. doi: 10.1186/1741-7015-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garden A.S., Weber R.S., Morrison W.H., Ang K.K., Peters L.J. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1995;32:619–626. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 10.Mendenhall W.M., Morris C.G., Amdur R.J., Werning J.W., Hinerman R.W., Villaret D.B. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–162. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 11.Kokemueller H., Eckardt A., Brachvogel P., Hausamen J.E. Adenoid cystic carcinoma of the head and neck–a 20 years experience. Int. J. Oral Maxillofac. Surg. 2004;33:25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 12.Gurney T.A., Eisele D.W., Weinberg V., Shin E., Lee N. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. Laryngoscope. 2005;115:1278–1282. doi: 10.1097/01.MLG.0000165381.64157.AD. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M., Sipos B., Klapper W., Frahm O., Knuth H.C., Yanagisawa A. Autoimmune pancreatitis: frequency, IgG4 expression, and clonality of T and B cells. Am. J. Surg. Pathol. 2007;31:521–528. doi: 10.1097/01.pas.0000213390.55536.47. [DOI] [PubMed] [Google Scholar]
- 14.Zen Y., Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am. J. Surg. Pathol. 2010;34:1812–1819. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 15.Geyer J.T., Deshpande V. IgG4-associated sialadenitis. Curr. Opin. Rheumatol. 2011;23:95–101. doi: 10.1097/BOR.0b013e3283413011. [DOI] [PubMed] [Google Scholar]
- 16.Gill J., Angelo N., Yeong M.L., McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Hum. Pathol. 2009;40:881–886. doi: 10.1016/j.humpath.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Tian W., Yakirevich E., Matoso A., Gnepp D.R. IgG4(+) plasma cells in sclerosing variant of mucoepidermoid carcinoma. Am. J. Surg. Pathol. 2012;36:973–979. doi: 10.1097/PAS.0b013e318258f018. [DOI] [PubMed] [Google Scholar]
- 18.Strehl J.D., Hartmann A., Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J. Clin. Pathol. 2011;64:237–243. doi: 10.1136/jcp.2010.085613. [DOI] [PubMed] [Google Scholar]
- 19.Jeannin P., Lecoanet S., Delneste Y., Gauchat J.F., Bonnefoy J.Y. IgE versus IgG4 production can be differentially regulated by IL-10. J. Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]


