Highlights
-
•
This case presentation, emphasizes the typical manifestations of hepatic but associated asymptomatic pericardial HCs.
-
•
Hepatic hydatid cyst presenting as a painless abdominal lump preceded painful presentation for a few months.
-
•
Asymptomatic pericardial hydatid cyst could have presented with pericardial tamponade due to rupture, if ignored further.
-
•
The origin of the pericardial hydatid cyst could have been due to trans diaphragmatic passage of scolices from the hepatic location, as the usual pulmonary involvement was absent.
Keywords: Hydatid cyst of liver, Hydatid cyst of pericardium
Abstract
Hydatid disease in human beings, as in all intermediate hosts, manifest as hydatid cyst (HC). It is an important cyclozoonotic disease, endemic in various sheep and cattle raising areas of the world, including India. The tapeworm commonly involved is Echinococcus granulosus. HC can occur almost anywhere in the body, most common organs being liver and lungs, and are usually solitary. In 25% of cases combination of liver HC with HC in other extra pulmonary locations are found. Cardiac HCs comprise of 0.5–2% of all HC cases. Within the heart, HCs are usually situated in the left or right ventricle and rarely found in the peri-cardium. Pericardial HC does not produce symptoms and is often painless and silent, until the cysts grow to a large size over the years, when the usual complications develop, such as cyst rupture, cardiac compression, atrial fibrillation, and even sudden death. We describe the case of a 39 year old house wife, of rural origin, with proximity to livestock, who had an asymptomatic pericardial HC along with a symptomatic hepatic HC. She clinically presented with an abdominal lump for one year with recent onset of abdominal pain for 1 month, when radiological imaging confirmed the diagnosis of an unruptured hepatic HC and a pericardial HC. The patient recovered after pericardiectomy along with excision of the HC over the left ventricle and enucleation of hepatic HC, by thoracoabdominal approach. She is doing well after 5 years of followup without recurrence.
1. Case report
A 39 year old lady, from a remote rural location of Gangetic Delta of South West Bengal, presented to us with gradually increasing swelling of the upper abdomen noted for 1 year and chronic abdominal pain for 1 month. A large, mildly tender and non pulsatile lump was present in the left upper quadrant of abdomen, moving with respiration without an upper palpable margin. She was hemodynamically stable without any respiratory distress. There was pallor, but no icterus, dependent edema, jugular venous engorgement and ascitis. Both heart and breath sounds were normal. Chest X-ray (Fig. 1A) showed an increased cardiothoracic ratio with a bulge along the left heart border and an area of increased opacity in left lower zone. Ultrasonograpy (USG) of abdomen revealed a cystic lesion in the left lobe of liver and also suggested a cyst in the pericardium. Echocardiography confirmed the presence of a localized cyst in the pericardial cavity over the left ventricle without any pressure effect and communication to the ventricular chambers and effusion. The wall of the cardiac chambers were free of any space occupying lesion. Computerized tomogram (CT) of the abdomen and thorax showed a 14 cm by 8 cm oval cyst in left lobe of liver (Fig. 1C) and an elliptical cystic lesion of 12 cm by 5 cm in the pericardial cavity overlying the left ventricle (LV) (Fig. 1B and D). Serology was positive for hydatid disease. There was eosinophilia, but other routine investigations were normal. Preoperative diagnosis was hydatid cyst (HC) of liver and pericardium. Oral albendazole tablets 400 mg twice daily was started during the preoperative period.
Fig. 1.
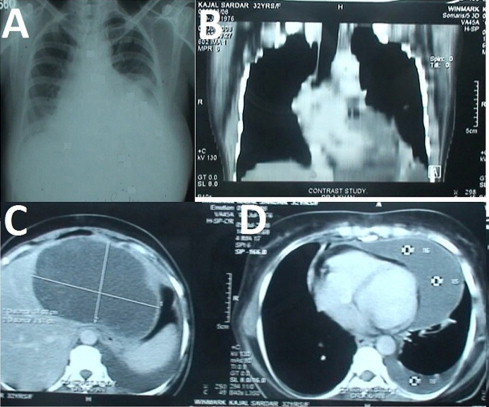
(A) Chest X-ray showing and increased cardiothoracic ratio with a bulging along the left border of heart. (B) Shows longitudinal section of thoracic CT showing the pericardial cyst over the left heart border. (C) Shows the cyst in the left lobe of liver. (D) Shows the transverse section of the CT showing the elliptical pericardial hydatid cyst over the left ventricle.
2. Operation
Exploration was done by a left thoracoabdominal approach under general anesthesia. There was no pulmonary adhesion. The pericardial hydatid cyst (PHC) was situated mainly over the left ventricle and partly over the right. The surrounding area was isolated with large swabs soaked with 5% povidone iodine solution which was also injected into the cyst. The parietal pericardium was thick and after incising the pericardium (Fig. 2A) approximately 500 cc of fluid was drained. The pericardium along with the contents of HC, were dissected off the ventricular wall (Fig. 2B) and excised, leaving behind a clear myocardial surface (Fig. 2C and D). The hepatic hydatid cyst (HHC) was enucleated with usual precautions and the cavity was packed with omentum (Fig. 2C). Wound closure was done in the usual fashion and recovery was uneventful. Histopathology confirmed the diagnosis of HC. The preoperative schedule of oral albendazole tablets 400 mg twice daily was continued for 6 months. There is no recurrence during 5 years of followup.
Fig. 2.
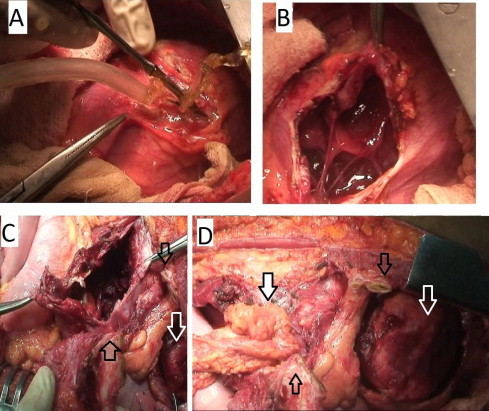
(A) Pericardial cyst being incised after injection of diluted 5% povidone iodine into the pericardial cystic cavity. Clear fluid under pressure being sucked out. (B) The thickened pericardium is being incised to expose the heart. The bands between the pericardium and the epicardium are shown, which are remnants of the smaller secondary hydatid cysts, and are being dissected and excised along with fibrous pericardium to expose the clean epicardial surface (shown by transparent white arrow in the figure C and D in the right hand corners). (C) Shows the liver cyst after clearance. (D) Shows omentum tuked into the liver cyst as shown by the black bordered white arrow. The divided costal arch (transparent black arrows in both C and D) with the attached diaphragm separating the liver and the heart shown, before thoracoabdominal wound closure.
3. Discussion
Cystic echinococcosis (hydatidosis) is a cyclozoonotic infection of cosmopolitan distribution, caused commonly by the larval stages of tapeworm Echinococcus granulosus. This is endemic in the Indian subcontinent and is prevalent where livestock is raised in association with dogs. HCs are most common in the liver (65%) as it is the site of first filtration of the hexacanth Echinococcus embryos [1,2] after intestinal absorption. Hematogenous spread of the embryos which escape hepatic capillary filtration, or protoscolices from mature HHC which may leak into systemic circulation, can pass through the right heart to form HCs in the lungs. Passage of the embryos after absorption from intestines through the lymphatic route and thoracic duct [1], and even inhalation of the eggs of contaminated air in susceptible individuals [3], has also been proposed to form isolated pulmonary HCs. Pulmonary HCs are found in 25% of cases [1,2] and an important source of cardiac HCs. Involvement of the diaphragm by HC from the adjacent upper lobes of liver and transdiaphragmatic migration to thoracic cavity occurs in 0.6–16% of cases of HHC [2] and infect thoracic organs. Incidence of cardiac HC is rare (0.5–2%) even in countries where hydatid disease is endemic [1,2]. Origin of the cardiac HC is hematogenous. Embryos may enter the coronary arteries and grow to form intramyocardial cysts [1,2,4]. The commonly affected cardiac chambers are the left ventricle (50–60%) followed by right ventricle (15%), while PHCs comprise 10–15% of these cardiac HCs [1,2]. The intramyocardial HCs produce early symptoms as they grow and their life span is shortened due to kinetic pressure exerted by the myocardium [4]. The same kinetic pressure by myocardial contractility favors their passage, usually towards the epicardial surface [4], and less frequently towards the subendocardial region with risk of intracavitory rupture. Ultimatley, a few of these subepicardial HCs perforate the epicardium and grow it into a large asymptomatic PHC, in the pericardial space, by stretching the fibrous pericardium [1,2,4]. Partial rupture of PHC can produce constrictive pericarditis; whereas, complete rupture can lead to tamponade [1,2,4]. Unruptured PHCs at a later stage, can produce pressure effect over the heart, like right ventricular outflow tract or great vessel obstruction [1,4], coronary artery compression with consequent ischemia [1,2]. Contents of these cysts is clear during the early stages [4] and may become cheesy due to degenerative changes later. In our case the PHC was localized over the left ventricular aspect and contained approximately 500 cc of clear fluid without any signs and symptoms of pressure effect produced during its long existence, probably counterbalanced by high left ventricular pressure. The PHC could be excised in beating heart in our case due to its favourable anterolateral location. Concomitant myocardial HC or subendocardial HC would require removal under cardiopulmonary bypass [1,4].
PHCs like cardiac HCs are usually found in association with pulmonary HCs because of hematogenous or contiguous spread from pulmonary HC [2]. Conversely, cardiac HC may also be the primary source, with metastatic spread to pulmonary and other remote organs, by hematogenous route [5]. The combination of a mature, large PHC and a HHC, as in our case, can not be explained by the known mechanisms of hematogenous or lymphatic spread from a HHC, without sparing the lungs. Whether the combination is just incidental due to separate primary infestation, by infective larvae [5] at different times, is open to conjecture. One plausible explanation is, contiguous spread of infective protoscolices from a primary HHC through trans diaphragmatic migration, due to small leakage [2] in the HHC wall, into the adjacent pericardial cavity to form a PHC.
In our case, abdominal pain, which was the presenting symptom, was produced by stretching of the hepatic capsule, by the expanding HHC in the left lobe of liver and was of recent origin. Pericardial effusion, usually from a leaking PHC, can also be caused by transdiaphragmatic rupture of a HHC [2], and produce abdominal pain due to hepatic congestion [1]. But it was not so in our case as both the cysts were intact. The abdominal lump was present for about a year, but was ignored by the patient till appearance of pain. Such indifference to asymptomatic abdominal lumps is common in the rural areas of Indian subcontinent and other developing countries. An increased cardiothoracic ratio in chest X-ray led to final diagnosis of combined HHC and PHC, by echocardiography and computerized tomogram.
4. Conclusion
This case presentation, emphasizes the manifestations of a symptomatic hepatic hydatid cyst along with an associated asymptomatic pericardial hydatid cyst, diagnosed by radiological imaging. This is of importance to both physicians and surgeons because appropriate management can be formulated for a successful clinical outcome.
Conflict of interest
There is no conflict of interest.
Funding
The authors declare they have no funding support.
Ethical approval
Our research is approved by the Ethics Committee of Medical College Hospitals, Kolkata, West Bengal, India.
Authors’ contribution
Each of the authors for preparing this case report, Dr. Kallol Dasbaksi (Corresponding author), Dr. Suranjan Haldar, Dr. Kaushik Mukherjee and Dr. Plaban Mukherjee have contributed to the study concept or design, data collection, data analysis or interpretation, for writing the paper.
Consent
Patient’s consent has been obtained to publish this report for academic interest.
Guarantor
Dr. Kallol Dasbaksi.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Yaliniz H., Tokcan A., Salih O.K., Ulus T. Surgical treatment of cardiac hydatid disease: a report of 7 cases. Texas Heart Inst. J. 2006;33(3):333–339. [PMC free article] [PubMed] [Google Scholar]
- 2.Polat P., Kantarci M., Alper F., Suma S., Koruyucu M.B., Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–494. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 3.Golzari S.E., Sokouti Pericyst M. The outermost layer of hydatid cyst. World J. Gastroenterol. 2014;20(February (5)):1377–1378. doi: 10.3748/wjg.v20.i5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Gomez F., Duran H., Tamames S., Perrote J.L., Blanes A. Cardiac echinococcosis: clinical picture and complications. Br. Heart J. 1973;35(12):1326–1331. doi: 10.1136/hrt.35.12.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmane J.V. Multiple pulmonary hydatid cysts. Postgrad. Med. J. 1967;43:774–778. doi: 10.1136/pgmj.43.506.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


