Abstract
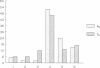
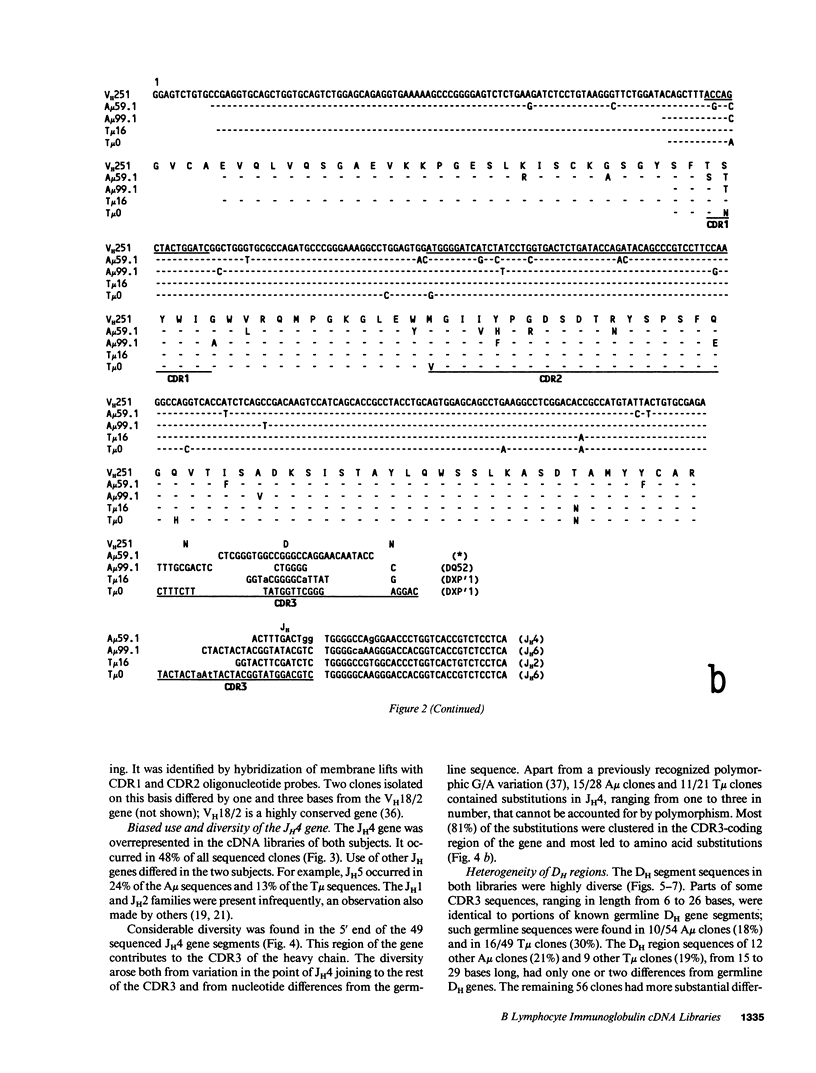
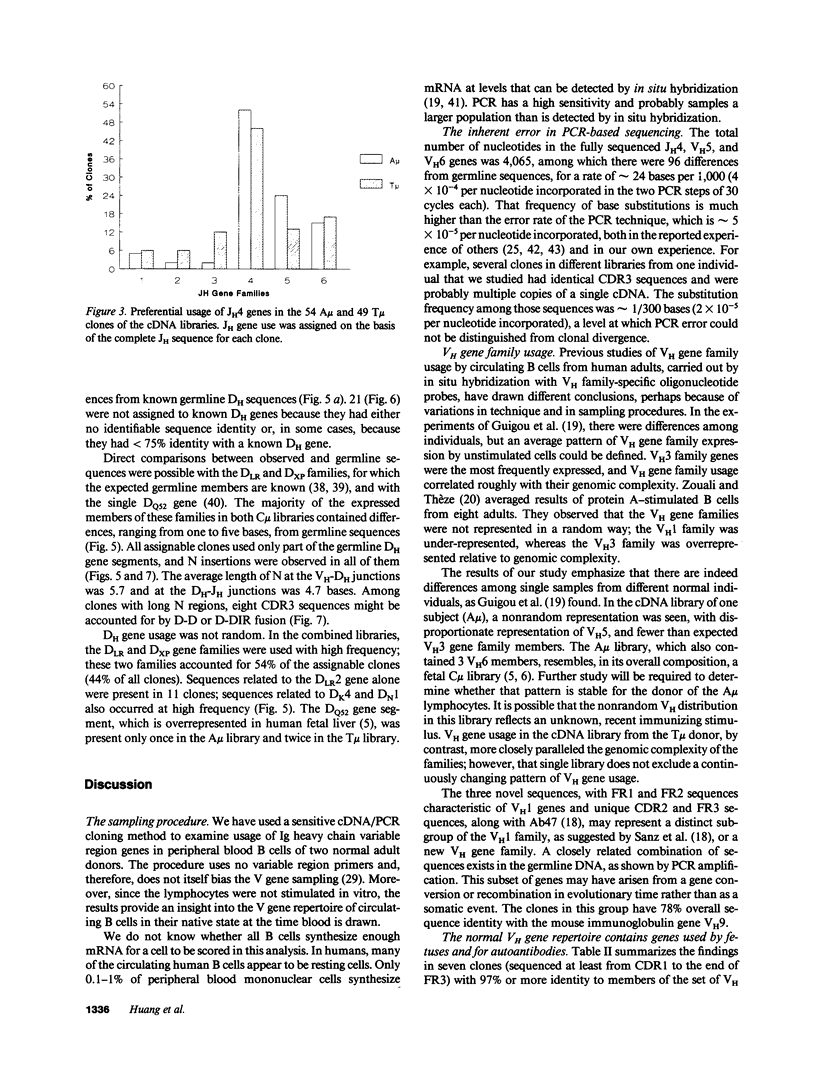
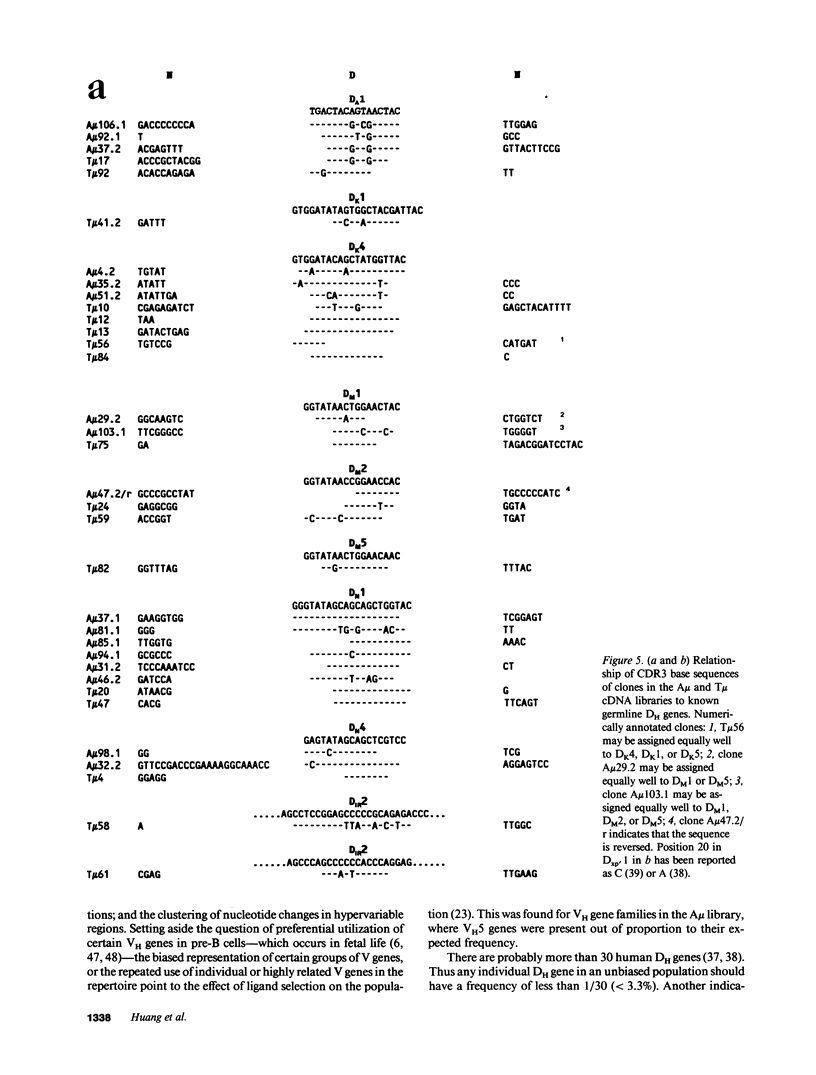
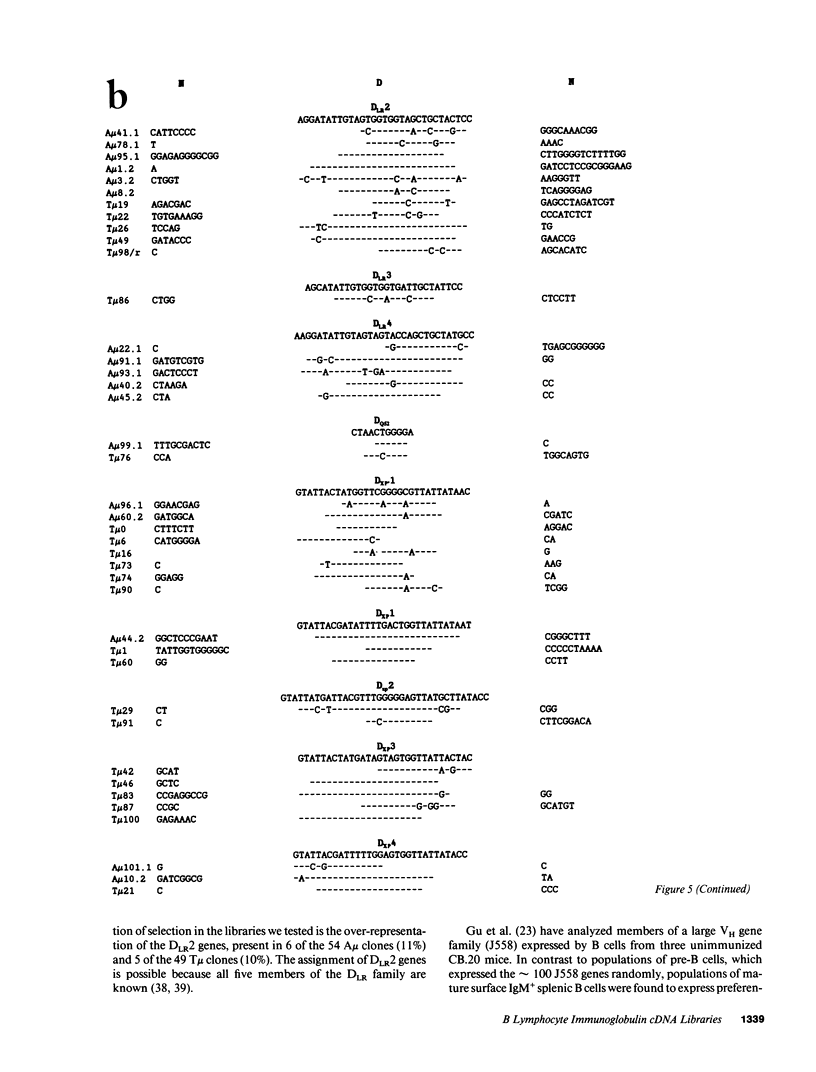
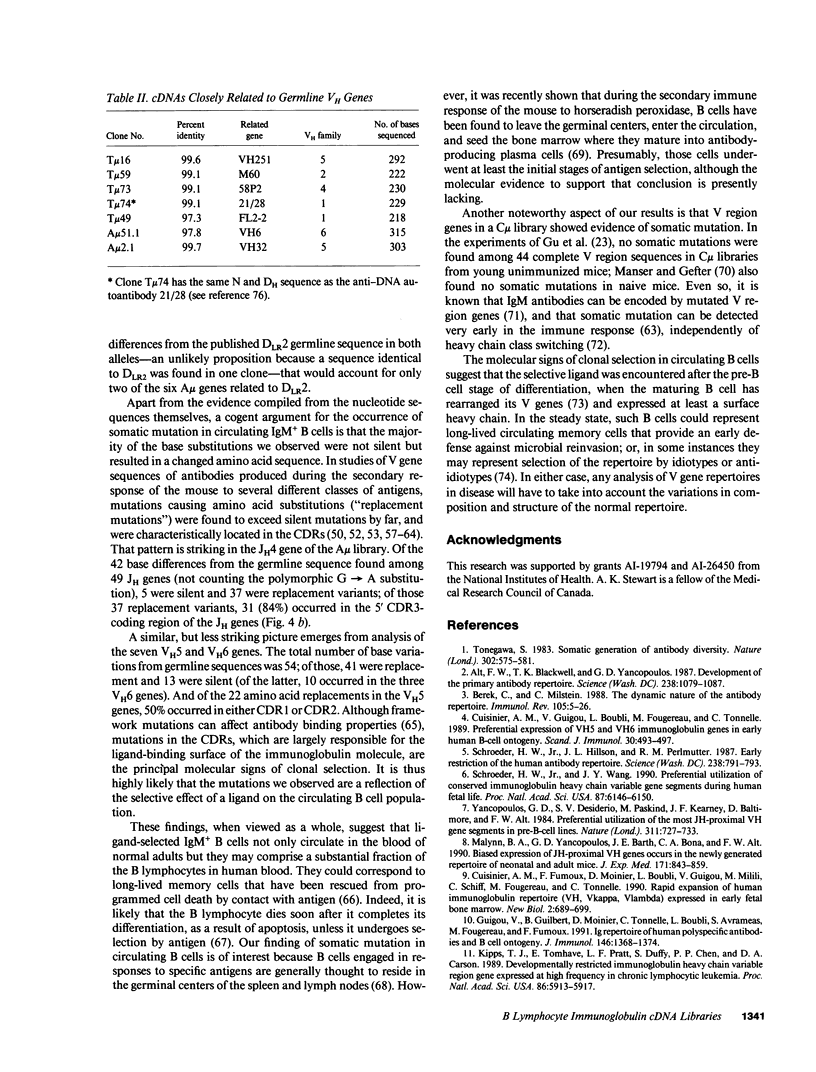
cDNA libraries for IgM heavy chain variable regions were prepared from unmanipulated peripheral blood lymphocytes of two healthy people. Partial sequencing of 103 clones revealed VH gene family use and complete CDR3 and JH sequences. The libraries differed in the two subjects. In one person's cDNA the VH5 family was overexpressed and the VH3 family underexpressed relative to genomic complexity. In the second person's cDNA, VH3 was most frequently expressed. In both libraries, JH4 was most frequent. VH segments of several clones were closely related to those in fetal repertoires. However, there was also evidence of mutation in many cDNAs. Three clones differed from the single nonpolymorphic VH6 germline gene by 7-13 bases. Clones with several differences from VH5 germline gene VH251 were identified. CDR3 segments were highly diverse. JH portions of several CDR3's differed from germline JH sequences. 44% of the clones had DH genes related to the DLR and DXP families, most with differences from germline sequences. In 11 DLR2-related sequences, several base substitutions could not be accounted for by polymorphism. Thus, circulating IgM-producing B cell populations include selected clones, some of which are encoded by variable region gene segments that have mutated from the germline form.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Atkinson P. M., Lampman G. W., Furie B. C., Naparstek Y., Schwartz R. S., Stollar B. D., Furie B. Homology of the NH2-terminal amino acid sequences of the heavy and light chains of human monoclonal lupus autoantibodies containing the dominant 16/6 idiotype. J Clin Invest. 1985 Apr;75(4):1138–1143. doi: 10.1172/JCI111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs L. A., Sanz I. E., Teale J. M. Comparison of D, JH, and junctional diversity in the fetal, adult, and aged B cell repertoires. J Immunol. 1991 Mar 15;146(6):1996–2004. [PubMed] [Google Scholar]
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988 Oct;105:5–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaison G., Kuntz J. L., Pasquali J. L. Molecular analysis of V kappa III variable regions of polyclonal rheumatoid factors during rheumatoid arthritis. Eur J Immunol. 1991 May;21(5):1221–1227. doi: 10.1002/eji.1830210519. [DOI] [PubMed] [Google Scholar]
- Buluwela L., Albertson D. G., Sherrington P., Rabbitts P. H., Spurr N., Rabbitts T. H. The use of chromosomal translocations to study human immunoglobulin gene organization: mapping DH segments within 35 kb of the C mu gene and identification of a new DH locus. EMBO J. 1988 Jul;7(7):2003–2010. doi: 10.1002/j.1460-2075.1988.tb03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Chien N. C., Pollock R. R., Desaymard C., Scharff M. D. Point mutations cause the somatic diversification of IgM and IgG2a antiphosphorylcholine antibodies. J Exp Med. 1988 Mar 1;167(3):954–973. doi: 10.1084/jem.167.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin J. L., Berry J., Flaherty D., Dunnick W. Somatic evolution of diversity among anti-phosphocholine antibodies induced with Proteus morganii. J Immunol. 1987 May 1;138(9):3060–3068. [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Rudikoff S. Evidence for gene conversion among immunoglobulin heavy chain variable region genes. J Exp Med. 1984 Mar 1;159(3):773–782. doi: 10.1084/jem.159.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Staudt L. M., Kavaler J., Schwartz D., Gerhard W. U., Weigert M. G. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. J Immunol. 1990 Apr 1;144(7):2795–2801. [PubMed] [Google Scholar]
- Crain M. J., Sanders S. K., Butler J. L., Cooper M. D. Epstein-Barr virus preferentially induces proliferation of primed B cells. J Immunol. 1989 Sep 1;143(5):1543–1548. [PubMed] [Google Scholar]
- Cuisinier A. M., Fumoux F., Moinier D., Boubli L., Guigou V., Milili M., Schiff C., Fougereau M., Tonnelle C. Rapid expansion of human immunoglobulin repertoire (VH, V kappa, V lambda) expressed in early fetal bone marrow. New Biol. 1990 Aug;2(8):689–699. [PubMed] [Google Scholar]
- Cuisinier A. M., Guigou V., Boubli L., Fougereau M., Tonnelle C. Preferential expression of VH5 and VH6 immunoglobulin genes in early human B-cell ontogeny. Scand J Immunol. 1989 Oct;30(4):493–497. doi: 10.1111/j.1365-3083.1989.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986 Oct;5(10):2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker D. J., Boyle N. E., Koziol J. A., Klinman N. R. The expression of the Ig H chain repertoire in developing bone marrow B lineage cells. J Immunol. 1991 Jan 1;146(1):350–361. [PubMed] [Google Scholar]
- Dersimonian H., Long A., Rubinstein D., Stollar B. D., Schwartz R. S. VH genes of human autoantibodies. Int Rev Immunol. 1990;5(3-4):253–264. doi: 10.3109/08830189009056733. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Dilosa R. M., Maeda K., Masuda A., Szakal A. K., Tew J. G. Germinal center B cells and antibody production in the bone marrow. J Immunol. 1991 Jun 15;146(12):4071–4077. [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991 Aug;1(1):17–24. doi: 10.1101/gr.1.1.17. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Burlen O., Coutinho A. Selection of antibody repertoires by anti-idiotypes can occur at multiple steps of B cell differentiation. J Immunol. 1988 Jun 15;140(12):4097–4102. [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guigou V., Cuisinier A. M., Tonnelle C., Moinier D., Fougereau M., Fumoux F. Human immunoglobulin VH and VK repertoire revealed by in situ hybridization. Mol Immunol. 1990 Sep;27(9):935–940. doi: 10.1016/0161-5890(90)90161-r. [DOI] [PubMed] [Google Scholar]
- Guigou V., Guilbert B., Moinier D., Tonnelle C., Boubli L., Avrameas S., Fougereau M., Fumoux F. Ig repertoire of human polyspecific antibodies and B cell ontogeny. J Immunol. 1991 Feb 15;146(4):1368–1374. [PubMed] [Google Scholar]
- Guillaume T., Rubinstein D. B., Young F., Tucker L., Logtenberg T., Schwartz R. S., Barrett K. J. Individual VH genes detected with oligonucleotide probes from the complementarity-determining regions. J Immunol. 1990 Sep 15;145(6):1934–1945. [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Stollar B. D. Construction of representative immunoglobulin variable region cDNA libraries from human peripheral blood lymphocytes without in vitro stimulation. J Immunol Methods. 1991 Aug 9;141(2):227–236. doi: 10.1016/0022-1759(91)90149-a. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988 Dec 20;7(13):4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991 May 1;173(5):1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. Comparison of the fetal and adult functional B cell repertoires by analysis of VH gene family expression. J Exp Med. 1988 Aug 1;168(2):589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A., Nickerson K. G., Liao J., Grossbard L., Osserman E. F., Glickman E., Chess L., Robbins J. B., Schneerson R., Yang Y. H. A human monoclonal macroglobulin with specificity for alpha(2----8)-linked poly-N-acetyl neuraminic acid, the capsular polysaccharide of group B meningococci and Escherichia coli K1, which crossreacts with polynucleotides and with denatured DNA. J Exp Med. 1986 Aug 1;164(2):642–654. doi: 10.1084/jem.164.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Tefferi A., Meisenholder G., Banks P. M., Carson D. A. CD5-positive B-cell malignancies frequently express cross-reactive idiotypes associated with IgM autoantibodies. Am J Pathol. 1990 Apr;136(4):809–816. [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Pratt L. F., Duffy S., Chen P. P., Carson D. A. Developmentally restricted immunoglobulin heavy chain variable region gene expressed at high frequency in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5913–5917. doi: 10.1073/pnas.86.15.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8206–8210. doi: 10.1073/pnas.85.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. S., Malipiero U. V., Lebecque S. G., Gearhart P. J. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J Exp Med. 1989 Jun 1;169(6):2007–2019. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. L., Keohavong P., Dias C., Thilly W. G. Optimization of the polymerase chain reaction with regard to fidelity: modified T7, Taq, and vent DNA polymerases. PCR Methods Appl. 1991 Aug;1(1):63–69. doi: 10.1101/gr.1.1.63. [DOI] [PubMed] [Google Scholar]
- Logtenberg T. Properties of polyreactive natural antibodies to self and foreign antigens. J Clin Immunol. 1990 May;10(3):137–140. doi: 10.1007/BF00917912. [DOI] [PubMed] [Google Scholar]
- Logtenberg T., Young F. M., Van Es J. H., Gmelig-Meyling F. H., Alt F. W. Autoantibodies encoded by the most Jh-proximal human immunoglobulin heavy chain variable region gene. J Exp Med. 1989 Oct 1;170(4):1347–1355. doi: 10.1084/jem.170.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A. A., Mueller J., Andre-Schwartz J., Barrett K. J., Schwartz R., Wolfe H. High-specificity in-situ hybridization. Methods and application. Diagn Mol Pathol. 1992 Mar;1(1):45–57. doi: 10.1097/00019606-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Gefter M. L. The molecular evolution of the immune response: idiotope-specific suppression indicates that B cells express germ-line-encoded V genes prior to antigenic stimulation. Eur J Immunol. 1986 Nov;16(11):1439–1444. doi: 10.1002/eji.1830161120. [DOI] [PubMed] [Google Scholar]
- Manser T., Huang S. Y., Gefter M. L. Influence of clonal selection on the expression of immunoglobulin variable region genes. Science. 1984 Dec 14;226(4680):1283–1288. doi: 10.1126/science.6334361. [DOI] [PubMed] [Google Scholar]
- Panka D. J., Mudgett-Hunter M., Parks D. R., Peterson L. L., Herzenberg L. A., Haber E., Margolies M. N. Variable region framework differences result in decreased or increased affinity of variant anti-digoxin antibodies. Proc Natl Acad Sci U S A. 1988 May;85(9):3080–3084. doi: 10.1073/pnas.85.9.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Andris J., Capra J. D. Heavy chain variable region gene utilization in human antibodies. Int Rev Immunol. 1990;5(3-4):231–238. doi: 10.3109/08830189009056731. [DOI] [PubMed] [Google Scholar]
- Pascual V., Capra J. D. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv Immunol. 1991;49:1–74. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Roes J., Hüppi K., Rajewsky K., Sablitzky F. V gene rearrangement is required to fully activate the hypermutation mechanism in B cells. J Immunol. 1989 Feb 1;142(3):1022–1026. [PubMed] [Google Scholar]
- Sablitzky F., Wildner G., Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 1985 Feb;4(2):345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I., Casali P., Thomas J. W., Notkins A. L., Capra J. D. Nucleotide sequences of eight human natural autoantibody VH regions reveals apparent restricted use of VH families. J Immunol. 1989 Jun 1;142(11):4054–4061. [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990 Aug 23;346(6286):749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte M. E., Ebeling S. B., Akkermans K. E., Gmelig-Meyling F. H., Logtenberg T. Antibody specificity and immunoglobulin VH gene utilization of human monoclonal CD5+ B cell lines. Eur J Immunol. 1991 May;21(5):1115–1121. doi: 10.1002/eji.1830210505. [DOI] [PubMed] [Google Scholar]
- Shan H., Shlomchik M., Weigert M. Heavy-chain class switch does not terminate somatic mutation. J Exp Med. 1990 Aug 1;172(2):531–536. doi: 10.1084/jem.172.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuldiner A. R., Nirula A., Roth J. Hybrid DNA artifact from PCR of closely related target sequences. Nucleic Acids Res. 1989 Jun 12;17(11):4409–4409. doi: 10.1093/nar/17.11.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Teale J. M. B cell immune repertoire diversifies in a predictable temporal order in vitro. J Immunol. 1985 Aug;135(2):954–958. [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Trepicchio W., Jr, Barrett K. J. Eleven MRL-lpr/lpr anti-DNA autoantibodies are encoded by genes from four VH gene families: a potentially biased usage of VH genes. J Immunol. 1987 Apr 1;138(7):2323–2331. [PubMed] [Google Scholar]
- Walter M. A., Surti U., Hofker M. H., Cox D. W. The physical organization of the human immunoglobulin heavy chain gene complex. EMBO J. 1990 Oct;9(10):3303–3313. doi: 10.1002/j.1460-2075.1990.tb07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U., Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J Exp Med. 1990 Dec 1;172(6):1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Young F., Tucker L., Rubinstein D., Guillaume T., André-Schwartz J., Barrett K. J., Schwartz R. S., Logtenberg T. Molecular analysis of a germ line-encoded idiotypic marker of pathogenic human lupus autoantibodies. J Immunol. 1990 Oct 15;145(8):2545–2553. [PubMed] [Google Scholar]
- Zouali M., Theze J. Probing VH gene-family utilization in human peripheral B cells by in situ hybridization. J Immunol. 1991 Apr 15;146(8):2855–2864. [PubMed] [Google Scholar]