Abstract
Aim:
The objective of this study was to evaluate prognostic impact of clinical factors on outcome of renal function in septic and non-septic acute kidney injury (AKI) patients.
Methods:
The prospective, observational, clinical study was performed at Nephrology Clinic and Clinic for Infectious Diseases, University Clinical Centre Sarajevo. One hundred patients with diagnosis of AKI were enrolled in the study, and divided into two groups: septic and non-septic AKI patients. Clinical parameters included causes and type of AKI, pre-existing comorbidities and different treatment modalities. Patients were followed up until discharge or death. Renal function outcome was defined by creatinine clearance values at discharge.
Results:
Septic AKI patients had significantly longer hospital stay (p=0.03), significantly worse renal function outcome (p<0.001), and higher burden of comorbidities (70.6% vs. 60.6%), compared to non-septic patients. Septic AKI patients were almost three times less likely to receive renal replacement therapy (8.8% vs. 24.4%) and they had significant delay in initiation of dialysis (p=0.03). By multivariate analysis, sepsis (95% CI 0.128-0.967, p=0.043) and hypertension (95% CI 0.114-0.788, p=0.015) were independent predictors of adverse renal function outcome in AKI patients. Postrenal type of AKI was independent predictor of renal function recovery in non-septic AKI patients (95% CI 1.174-92.264, p=0.035), while Failure, as third class of AKI, was independent predictor of non-recovered renal function only in septic AKI patients (95% CI 0.026 to 0.868, p=0.034).
Conclusion:
Septic AKI patients are clinically distinct compared to non-septic AKI patients with different prognostic factors and poorer renal function outcome.
Keywords: acute kidney injury, septic etiology, prognostic factors, renal function outcome
1. INTRODUCTION
Acute kidney injury (AKI) is a syndrome with various etiologies, followed by numerous comorbidities, which makes predicting its outcome very complicated. AKI often occurs in the older population of patients with pre-existing chronic kidney disease (CKD), and it is associated with increased risk for dialysis (1).
The causes of AKI are multi-factorial, but sepsis has consistently been the most common contributing factor, accounting for approximately 50% of all cases, especially in the intensive care units (ICU) (2). Recent evidence suggests that septic AKI could be characterized by a distinct pathophysiology (3). Regrettably, not many clinical studies have investigated clinical characteristics, profile and renal function outcome of septic in comparison to non-septic AKI patients (4, 5). In addition, other authors have focused only on the clinical outcome of dialysis dependent septic AKI patients in the ICU, and they defined renal function recovery as independence from dialysis at discharge (6). However, a significant proportion of AKI patients is not in the ICU, nor dialyzed, and may require alternate definitions for assessing renal recovery (7). This encourages us to define renal function outcome according to the values of creatinine clearance at discharge, and to focus on clinical differences between septic and non-septic AKI patients and their prognostic significance for renal function outcome.
The objective of this study was to investigate clinical characteristics of septic compared to non-septic AKI patients, and to assess predictive value of clinical prognostic factors on renal function outcome of septic and non-septic AKI patients.
2. MATERIALS AND METHODS
A prospective, single-center, observational study was conducted from January, 2008 to December, 2012 at Nephrology Clinic and Clinic for Infectious Diseases, University Clinical Centre Sarajevo. The Human Research Ethics Committee approved the study prior to the commencement. One hundred patients included in this study were adults (age ≥18 years) with hospital stay ≥24 hours, and diagnosis of AKI. Exclusion criteria were prior kidney transplant, end-stage kidney disease, and initiation of renal replacement therapy (RRT) before hospital admission.
Demographic informations included age, gender and dates of admission. Informations on kidney function included serum urea, serum creatinine and creatinine clearance. Clinical data encompassed origin of AKI (septic and non-septic), type of AKI (prerenal, intrinsic and postrenal), comorbid conditions and different treatment modalities as well as renal function outcome and in-hospital mortality. Causative factors of non-septic AKI were also identified.
AKI was defined according to the consensus definition of the RIFLE criteria with three stages (Risk, Injury, Failure) of progressively higher creatinine (8). When pre-admission serum creatinine was unavailable, it was estimated by the Modification of Diet in Renal Disease equation, as recommended by the Acute Dialysis Quality Initiative Working Group (9).
Septic origin of AKI was diagnosed in those patients who had recognized source of infection, regardless of whether the blood culture was positive or not, and if they had verified increased serum creatinine. Criteria for the diagnosis of sepsis and septic shock were defined according to consensus American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (10).
Patients were followed-up until hospital discharge or death. Outcome of renal function was defined according to the values of creatinine clearance as recovered (creatinine clearance >60 mL/min) and non-recovered (creatinine clearance <60 mL/min) with impaired renal function. Creatinine clearance was determined from measurement of creatinine in a 24-hour urine specimen and serum specimen.
Statistical analysis
Descriptive statistics, Student’s t-test (for variables with normal distribution) and Mann Whitney U test (for variables without normal distribution) were used to compare mean values between groups. A multivariate logistic regression was performed to evaluate the impact of the variables on renal function outcome. P values <0.05 were considered significant.
3. RESULTS
A total of 100 patients with diagnosis of AKI were included in the study. Considering etiology, patients were divided into two groups: patients with AKI of non-septic etiology (66 patients) and patients with AKI of septic etiology (34 patients). Characteristics of septic and non-septic AKI patients are summarized in Table 1. There was no evidence of statistically significant difference in mean values of age and gender between these two groups of patients, while hospital stay was significantly longer in septic AKI patients (p=0.03). In the group of non-septic AKI patients, prerenal type was recognized in 30.3% of patients, while intrinsic and postrenal type were detected in 51.5% and 18.2% of non-septic AKI patients, respectively. Pre-existing chronic kidney disease had 10.6% of non-septic AKI patients and 2.3 greater proportion of septic AKI patients (23.5%). Comorbid conditions were present in 70.6% and 60.6% of septic and non-septic patients, respectively. In the group of septic AKI patients, only 8.8% patients underwent RRT, while 24.4% of non-septic AKI patients were treated with RRT. The mean hospital stay prior to the RRT commencement was significantly longer in septic AKI patients when compared to non-septic AKI patients (10.6 days vs. 2 days, p=0.03). Average values of creatinine clearance at discharge were significantly lower in the group of AKI patients with septic etiology (46.49±3.8 mL/min) than in the group of AKI patients with non-septic etiology (74.52±3.82 mL/min, p<0.001). Septic AKI patients also had significantly greater proportion of hospital mortality in comparison to non-septic AKI patients (14.7% vs. 3.03%, p=0.04).
Table 1.
Characteristics of acute kidney injury patients according to etiology. Data are presented as median and interquartile range or as means and standard deviation. AKI–acute kidney injury; n–number; ns–non significant; RIFLE–Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease; CKD–chronic kidney disease; RRT–renal replacement therapy
Figure 1 gives an overview of the most common causes of non-septic AKI: lower obstruction of the urinary tract (18.18%), Hantavirus hemorrhagic fever with renal syndrome (HFRS) (13.64%), hypovolemia due to acute enterocolitis (13.64%) and nephrotoxic drug-related acute tubular necrosis (ATN) (10.61%).
Figure 1.
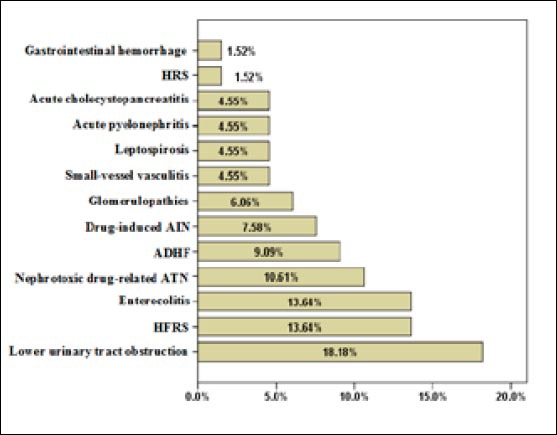
Etiology of non-septic acute kidney injury. HRS–Hepatorenal syndrome; AIN–Acute interstitial nephritis; ADHF–Acute decompensated heart failure; ATN–Acute tubular necrosis; HFRS–Hemorrhagic fever with renal syndrome
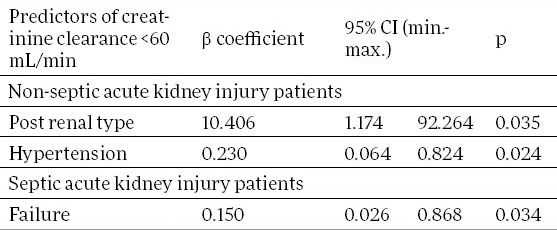
We monitored factors statistically significant in predicting outcome of renal function in all patients with AKI, and in the groups of septic and non-septic AKI patients, separately. Table 2 shows the results of multivariate logistic regression analysis. Factors statistically significant in predicting non-recovering of renal function in all AKI patients were sepsis (95% confidence interval 0.128 to 0.967, p=0.043) and hypertension (95% confidence interval 0.114 to 0.788, p=0.015). Failure was an independent predictor of non-recovered renal function in the group of septic AKI patients (95% confidence interval 0.026 to 0.868, p=0.034; Table 3). In the group of non-septic AKI patients, only hypertension was independent predictor of renal function non-recovery (95% confidence interval 0.064 to 0.824, p=0.024; Table 3). However, in this, non-septic AKI group of patients, postrenal type ARI was independently associated with the renal function recovery. Patients with postrenal type of AKI had 10 times more probability to achieve renal function recovery (95% confidence interval 1.174 to 92.264, p=0.035; Table 3).
Table 2.
Significant independent predictors of non-recovered renal function in acute kidney injury patients. CI–confidence interval
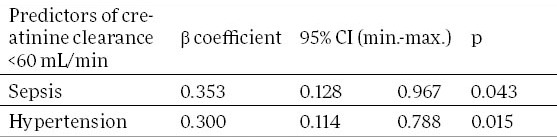
Table 3.
Significant independent predictors of renal function outcome in non-septic and septic acute kidney injury patients. CI–confidence interval
4. DISCUSSION
Sepsis is a well-known risk factor for the development of AKI, but our understanding of the pathophysiology of septic AKI is limited. Recent study implies that septic AKI may represent unique form of AKI: hyperemic AKI (3). In present study, sepsis has proven to be etiological factor of AKI development in 34% patients. Bagshaw and Daher with their associates (4, 11) estimated that 41.5% and 47.5% of patients had sepsis as a contributing factor to AKI, respectively. Accompanied with heart failure, ATN and surgical procedures, sepsis was one of the four most common causes of AKI development in the BEST Kidney (12) and PICARD study (13). Our study revealed that the most common causes of non-septic AKI were lower urinary tract obstruction (18.2%), hypovolemia due to enterocolitis (13.64%), HFRS (13.64%), nephrotoxic drug-related ATN (10.61%) and decompensated heart failure (9.09%). In comparison to the BEST Kidney (12) and PICARD studies (13), present study has shown a higher prevalence of HFRS, which can be explained by endemic occurrence of HFRS in the Balkans with epidemic outbreaks and sporadic cases that have been recorded yearly since the disease was first recognized (14). The prevalence of nephrotoxic drug-related ATN in our study was lower than in BEST kidney study and PICARD study (12, 13), but almost the same as in the study of Daher with coworkers (11).
Both conceptually and diagnostically, the various causes of AKI are divided broadly into three anatomic categories: prerenal, intrinsic, and postrenal. Since AKI of septic origin is more likely intrinsic type, we analyzed prevalence of prerenal, intrinsic and postrenal type of AKI only in the group of non-septic AKI patients. Prerenal type of AKI was present in 30.3% of non-septic AKI patients in our study. Similarly, prerenal azotemia was singled out as a separate etiological factor of AKI development in the study of other authors (15) who found prerenal type in 38.9% of AKI cases.
AKI, as an isolated phenomenon, is not common. More than 80% of patients with severe AKI of critical illness have associated respiratory and circulatory failure (11). We analyzed presence of comorbidities in both followed groups of patients. In accordance with previous reports (11, 16), our results have shown that septic AKI patients have a higher prevalence of comorbid illnesses compared to non-septic patients. The main comorbid conditions in non-septic AKI patients were hypertension (31.8%) and diabetes mellitus (25.8%). In septic AKI group of patients, almost every second patient had hypertension (44.1%), and more than a third of patients had diabetes mellitus (38.2%). Other authors have also confirmed that hypertension and diabetes mellitus were among the five most common comorbid conditions in AKI (11).
Recent data have suggested that rates of RRT utilization in AKI are increasing (17). However, only 19% of all AKI patients included in our study were treated with RRT, which is similar with prevalence (12%) found in recent survey conducted in 10 Italian ICU (18). Present study detected that need for dialysis was greater in patients with non-septic AKI, compared to septic AKI patients, but without statistical significance (24.2% vs. 8.8%), which is consistent with previous reports (19). However, other authors (4) found no difference in proportion of RRT in septic and non-septic groups of patients. Our patients with septic AKI had 5.3 times longer hospital stay before initiation of RRT compared with non-septic AKI patients (10.6 vs. 2 days, p=0.03). This significant delay in initiation of RRT was also registered in the study of Bagshaw and associates (4).
Our findings also suggest that septic AKI patients have significantly worse outcome of renal function (measured by creatinine clearance values at the discharge) and that they have significantly longer hospital stay in comparison to non-septic AKI patients, which is consistent with data of other authors (18). By multivariate logistic regression, sepsis was significant predictor of non-recovering of kidney function in AKI patients. This negative prognostic impact of sepsis can be explained by the pathophysiological effect of sepsis on the kidney with significant damage at the level of the tubules (3). Using multivariate analysis, Failure (as third, the most difficult class of AKI), was independently associated with non-recovering of kidney function only in the group of septic AKI patients in present study. Although our results confirmed that septic AKI patients had significantly greater proportion of mortality when compared with non-septic AKI patients (14.7% vs. 3.03%, p=0.04), septic etiology was not proved to be predictor of mortality. Maybe this can be explained with rather low overall mortality rate in our study (7%). Unlike our results, sepsis has shown to be strong predictor of death in other studies (4, 5).
In accordance with recent findings (19), we found that hypertension was independent predictor of adverse renal function outcome in all AKI patients and in the group of non-septic AKI patients separately. This is not unexpected since systemic hypertension is potent contributor to the development of arteriosclerosis, tubulointerstitial fibrosis and glomerulosclerosis, all of which may hasten the decline in kidney function. Opposite to hypertension, postrenal type of AKI proved to be an independent predictor of renal function recovery in non-septic AKI group. A possible reason for this finding could be the fact that the pathophysiology of lower urinary tract symptoms is the result of bladder outlet obstruction, mainly associated with benign prostatic hyperplasia with no great kidney affection.
5. CONCLUSION
Septic AKI patients are clinically distinct compared to non-septic AKI patients with poorer renal function outcome. Significant prognostic factor of adverse renal function outcome in septic AKI is class Failure, while significant predictor of poor renal function outcome in non-septic AKI is hypertension. These findings highlight the importance of identification of clinical risk factors separately in septic and non-septic AKI patients, since those patients seem to have not only pathophysiological, but also clinical differences. Close monitoring of AKI patients due to sepsis should be recommended in order to improve renal function outcome of these high-risk AKI patients.
Footnotes
CONFLICT OF INTEREST: NON DECLARED.
REFERENCES
- 1.Wang IK, Wang ST, Lin CL, Chen TC, Chang HY, Kuo HL, et al. Early prognostic factors in patients with acute renal failure requiring dialysis. Ren Fail. 2006;28:43–49. doi: 10.1080/08860220500461245. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z, Mu DW, Guo L, Li XM, Lun LD. Etiological factors, prognostic assessment, and outcomes of patients with acute kidney injury and multiple organ dysfunction syndrome. Genet Mol Res. 2014;13:8378–8384. doi: 10.4238/2014.October.20.13. [DOI] [PubMed] [Google Scholar]
- 3.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36:198–203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z, Ye H, Shen X, Chao H, Wu X, Yang J. Continuous venovenous hemofiltration versus extended daily hemofiltration in patients with septic acute kidney injury: a retrospective cohort study. Crit Care. 2014;18:R70. doi: 10.1186/cc13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macedo E, Bouchard J, Mehta R. Renal recovery following acute kidney injury. Curr Opin Crit Care. 2010;14:660–665. doi: 10.1097/MCC.0b013e328317ee6e. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup: Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Uchino S, Cruz D, Bellomo R, Morimatsu H, Morgera S, et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24:2739–2744. doi: 10.1093/ndt/gfp159. [DOI] [PubMed] [Google Scholar]
- 10.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 11.Daher EF, Marques CN, Lima RS, Silva GB, Júnior, Barbosa AS, Barbosa ES, et al. Acute kidney injury in an infectious disease intensive care unit-an assessment of prognostic factors. Swiss Med Wkly. 2008;138:128–133. doi: 10.4414/smw.2008.12062. [DOI] [PubMed] [Google Scholar]
- 12.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 14.Avšič Županc T, Korva M, Markotić A. HFRS and hantaviruses in the Balkans/South-East Europe. Virus Res. 2014;187:27–33. doi: 10.1016/j.virusres.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Cruz DN, Bolgan I, Parezella MA, Bonello M, de Cal M, Corradi V, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 16.Parmar A, Langenberg C, Li W, May CN, Bellomo R, Bagshaw SM. Epidemiology of septic acute kidney injury. Current Drug Targets. 2009;10:1169–1178. doi: 10.2174/138945009789753183. [DOI] [PubMed] [Google Scholar]
- 17.Shneider AG, Bagshaw SM. Effects of renal replacement therapy on renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:35–41. doi: 10.1159/000363671. [DOI] [PubMed] [Google Scholar]
- 18.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiol. 2011;77:1072–1083. [PubMed] [Google Scholar]
- 19.Harel Z, Bell CM, Dixon SN, McArthur E, James MT, Garg AX, et al. Predictors of progression to chronic dialysis in survivors of severe acute injury: a competing risk study. BMC Nephrol. 2014;15:114. doi: 10.1186/1471-2369-15-114. [DOI] [PMC free article] [PubMed] [Google Scholar]


