Abstract
Aim:
The aim of prenatal diagnostics is to provide information of the genetic abnormalities of the fetus early enough for the termination of pregnancy to be possible. Chromosomal abnormalities can be detected in an unborn child through the use of cytogenetic, molecular- cytogenetic and molecular methods. In between them, central spot is still occupied by cytogenetic methods. In cases where use of such methods is not informative enough, one or more molecular cytogenetic methods can be used for further clarification. Combined use of the mentioned methods improves the quality of the final findings in the diagnostics of chromosomal abnormalities, with classical cytogenetic methods still occupying the central spot.
Material and methods:
Conducted research represent retrospective-prospective study of a four year period, from 2008 through 2011. In the period stated, 1319 karyotyping from amniotic fluid were conducted, along with 146 FISH analysis.
Results:
Karyotyping had detected 20 numerical and 18 structural aberrations in that period. Most common observed numerical aberration were Down syndrome (75%), Klinefelter syndrome (10%), Edwards syndrome, double Y syndrome and triploidy (5% each). Within observed structural aberrations more common were balanced chromosomal aberrations then non balanced ones. Most common balanced structural aberrations were as follows: reciprocal translocations (60%), Robertson translocations (13.3%), chromosomal inversions, duplications and balanced de novo chromosomal rearrangements (6.6% each).
Conclusion:
With non- balanced aberrations observed in the samples of amniotic fluid, non- balanced translocations, deletions and derived chromosomes were equally represented. Number of detected aneuploidies with FISH, prior to obtaining results with karyotyping, were 6.
Keywords: amniotic fluid, G bending, karyotype, FISH
1. INTRODUCTION
One of the greatest advances in medical genetics was the development of prenatal diagnostic methods that use different invasive and non-invasive techniques. The aim of prenatal diagnosis is to prevent the birth of children with genetic diseases, i.e. main objective is the birth of a healthy and normal offspring. This type of diagnostics is mainly focused on the identification of chromosomopathies applying cytogenetic, molecular-cytogenetic and molecular methods. Chromosomopathies represent a deviation from the normal number or structure of chromosomes. The frequency of chromosomal aberrations in the live-born population is approximately 0.40% (1:250)–0.65% (1:154) (1). Aneuploidies, especially those involving chromosomes 13, 18, 21, X and Y, represent more than 80% of chromosomal abnormalities detected in prenatal diagnostics (2). Abortions that occur up to 2nd week of gestation have a rate of 78% for chromosomal aberrations. In abortions that occur up to the 20th week of gestation, the rate of chromosomal aberrations decreases to 62%. The rate of chromosomal aberrations decrease further to 6% for abortion after the 20th week of gestation, and in live born this rate is 0.5%. Cytogenetic methods take central place in prenatal diagnosis. The number and structure of chromosomes are examined by classic cytogenetics method, karyotype analysis/karyotyping. The most widespread method is chromosome banding by GTG-banding method or G-bands, where the chromosome slides are treated with the enzyme trypsin and dyed with nonflourescent dye–Giemsa. Typical cytogenetic analysis is based on a resolution of 300-550 bands, and prometaphase analysis of chromosomes is based on resolution of 850 or more bands (1, 2). Digitized or photographic presentation of karyotype, i.e. all analyzed chromosomes from a single cell paired and arranged in groups according to ISCNI standards, is called cariogram. The minimum number of bands in the metaphase chromosome, obtained by GTG-banding method, is 300-400. Using high-resolution technique, in metaphase chromosome can be obtained from 500 to 550 bands. These methods are often not sufficiently informative, and for a complete and accurate analysis of chromosomes must be applied one or more techniques of molecular cytogenetics. Fluorescent in situ hybridization combines cytogenetic and molecular techniques for the determination of chromosomes and their aberrations. The basic principle is the detection of target DNA molecules labeled with a fluorescent probe. While the methods of classical cytogenetics need cultivation of cells, FISH can be applied to non-cultivated cells, i.e. the interphase nucleus. Non-cultivated cells with interphase nuclei (e.g. amniocytes) are used for the rapid detection (24 to 48 hours) of most common aneuploidies in humans, such as trisomies of chromosome 13, 18, 21 as well as the X and Y chromosomes. Using probes for listed aneuploidies, there can not be simultaneously detect other possible numerical and structural changes in chromosomes, and classical cytogenetics methods are needed for a complete analysis of chromosomes.
2. MATERIALS AND METHODS
Subjects
During the four-year period from May 13, 2008 to December 31, 2011 in cytogenetic laboratory, Organizational Unit Clinical Pathology and Cytology, Clinical Center University of Sarajevo, had been received and processed 1323 samples of amniotic fluid. There were 15 samples from twin pregnancies, and the total number of amniotic fluid received samples for the same period was 1 338 samples. It was indicated a karyotyping analysis for all amniotic fluid samples. During the period 2008-2011, the number of failed cytogenetic analysis of amniotic fluid samples, due to a failed cultivation, was 19 (1.5%). Since the karyotype wasn’t done for 19 of 1338 received amniotic fluid samples, the number of karyotyping of amniotic fluid in the period 2008 to 2011 was 1319. For most of 19 samples with failed cultivation (12 samples), due to the impossibility of karyotype determination, there were performed molecular-cytogenetic analysis after their cultivation. At six samples of amniotic fluid, sampling and karyotype analysis were repeated (irregularly sampled, contamination, suspicion of mosaicism or in vitro changes in the process of cultivation, unrepresentative sample). In 146 of 1338 samples of the amniotic fluid, there were indicated, except karyotype analysis, FISH molecular-cytogenetic analysis, or for some samples during analysis of the karyotype there were need for additional FISH analysis.
Methods
All amniotic fluid samples were processed and analyzed according to a standardized protocol for processing these samples. Cultivation of amniotic fluid sample for chromosomal analysis was performed by the method of long-term cell cultures, where “flask” technique of cultivation was used. For cultivating of samples, commercial medium for amniotic fluid cultivation was used (Amniomed and Amniomax, Euroclone and GIBCO–Invitrogen manufacture). Karyotype analysis from the amniotic fluid were performed by G-bending technique–routine technique in classical cytogenetics. Karyotype analysis was performed using a microscope and karyotyping software system Cytovision. In each karyotype analysis there were analyzed 20 cells per sample of amniotic fluid, where the number of chromosomes, the sex and chromosome structure were determined. One of the analyzed metaphases was digitally processed, to obtain patient’s cariogram which was issued in printed form with karyotype finding. All analyzed metaphases from one patient were stored in the karyotyping software system. Slides for karyotyping analysis were also stored in laboratory archive (3, 4). In 146 samples of amniotic fluid except classical cytogenetic analysis, there were performed molecular-cytogenetic analysis of fluorescent in situ hybridization (FISH). Samples of amniotic fluid analyzed by this method were processed and analyzed according to standardized protocols for FISH method. For FISH analysis, there were used AneuVysion (Vysis) commercial DNA test kits for enumeration of chromosomes 13, 18, 21, X and Y. AneuVysion kit consists of two sets of probes. One set consists of locus-specific probes for chromosomes 13 and 21, a second set consists of centromeric probes for chromosomes 18, X and Y. FISH analysis of amniotic fluid samples was performed on fluorescent microscope with its Cytovision software. Analysis was performed according to standard protocols for FISH analysis. For each analyzed sample, there were counted at least 30 interphase nuclei, where the signals for the target chromosome (13, 18, 21, X and Y) were analyzed. One of the analyzed nuclei was digitally processed and issued in printed form with FISH analysis finding. Patient’s analyzed interphase nuclei were stored in the software system, similar to the procedure for karyotyping from the amniotic fluid. Slides for FISH analysis goes into the laboratory records too (4).
3. RESULTS
There were found 20 karyotypes with numerical aberrations in the 1319 analyzed amniotic fluid samples. The largest number of identified numerical aberrations were autosomal chromosome aneuploidies. It was found 15 trisomies of chromosome 21 (Down syndrome) and one trisomy of chromosome 18 (Edwards syndrome). Monosomies of autosomal chromosomes were not identified in the prenatal diagnostics in samples of amniotic fluid. There were found the three samples with aneuploidy of sex chromosomes, trisomies of sex chromosomes (two Klinefelter’s syndromes, and one XYY syndrome). Monosomies of sex chromosomes weren’t established. Polyploidies were rare, and only one sample with triploidy was found (Table 1). The most frequent numerical chromosome aberration was Down syndrome 75%, then Edvardsov syndrome 5%, Klinefelter’s syndrome 10%, syndrome of double Y chromosome 5%, and triploidy 5% (Figure 1). In the 1319 amniotic fluid analyzed samples, there were about 18 karyotypes with structural chromosomal aberrations. The largest number of structural aberrations were balanced chromosomal changes. There were found 15 balanced chromosomal changes, while there have been three unbalanced chromosomal changes. In the case of balanced changes, there were also found identical balanced changes in chromosomes from peripheral blood in the child’s parents, except in one de novo balanced change where the child’s parents had a normal karyotype.
Table 1.
Distribution of numerical chromosomal abnormalities in the analyzed karyotypes from the amniotic fluid
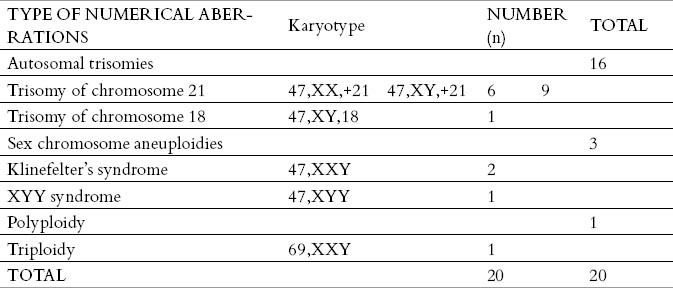
Figure 1.
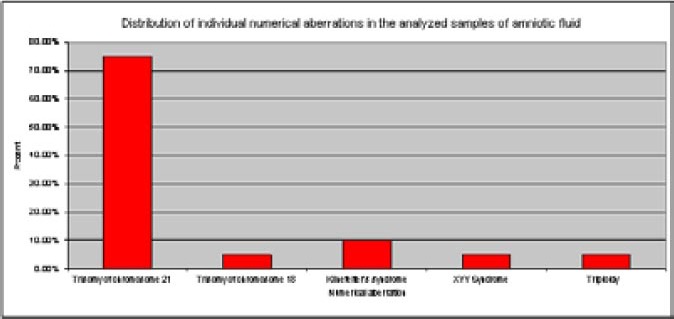
Distribution of individual numerical chromosomal aberrations in the analyzed samples of amniotic fluid
During the study period, there were determined 20 patients with pericentric inversion of chromosome 9 with karyotype 46, XX, inv (9) (p12q13) and 46, XY, inv (9) (p12q13) in 1319 samples of amniotic fluid, which were considered as population variant of the normal karyotype, and this type of karyotype was counted in the results of normal karyotypes. The majority of 15 karyotypes with established balanced chromosomal changes were reciprocal translocations, including Robertsonian translocation – 11 samples, two samples had inversions of chromosomes, one had duplication, and one sample had complex de novo unbalanced karyotype. From three determined unbalanced karyotype, one sample was based on an unbalanced translocation, one sample was with derived chromosome, and one was with a chromosomal deletion (Table 2). In the group of balanced structural aberrations, reciprocal translocations were the most common and represented 60%, while the Robertsonian translocations occurred rarely with an incidence of 13.3%. Inversions of chromosomes 9 and 12 are represented with 6.6% each, and the inversions of other chromosomes were not determined. In one case, there was determined complex de novo balanced karyotype that included two chromosomal changes, reciprocal translocation and inversion of chromosomes, 6.6%. In the group of prenatally detected unbalanced chromosome rearrangements, there was found equal proportion of chromosomal deletions and derived chromosomes with 33.33% each (Figure 2). The proportion of balanced changes in the total amniotic fluid sample was 1.13%, and the proportion of unbalanced changes was 0.21%, i.e. the overall incidence of structural chromosome aberration in the analyzed samples of amniotic fluid was 1.34%. Calculating both numerical and structural chromosomal aberrations identified in the analyzed karyotypes from the amniotic fluid, the number of aberrant (abnormal karyotypes) in the analyzed samples of amniotic fluid was 2.9%.
Table 2.
Distribution of structural chromosomal aberrations in the analyzed karyotypes from the amniotic fluid
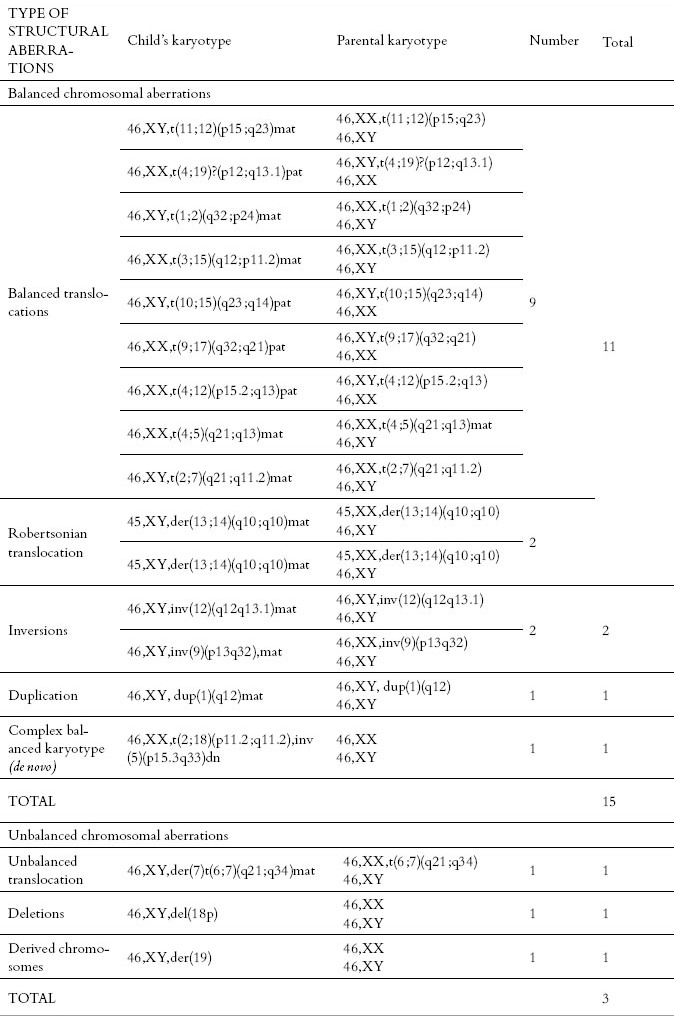
Figure 2.
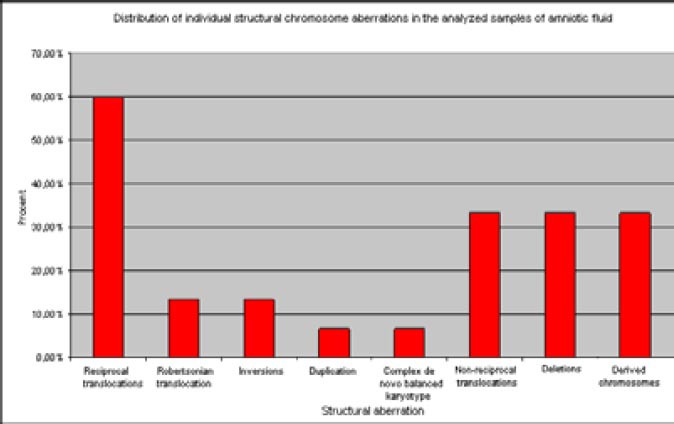
Distribution of individual structural chromosomal aberrations in the analyzed samples of amniotic fluid
There were determined 20 numerical chromosomal aberrations (1.51%), 15 balanced (1.13%) and 3 unbalanced structural chromosomal aberrations (0.22%). The number of normal karyotypes in the analyzed samples of amniotic fluid was 1 281 samples, 97.1% (Figure 3). Besides the classical cytogenetic analysis of amniotic fluid samples, there was performed molecular-cytogenetic analysis of Fluorescent in situ hybridization (FISH). The number of these analyzes for the same period was the 146. The number of successfully realized FISH analysis was 121, while the number of FISH analysis that could not be interpreted due to insufficient sample or unclear signals was 20 samples. The number of aneuploidies detected by FISH method of all indicated 146 FISH analysis, and before karyotype results were available, was 5 (Table 3).
Figure 3.

Share of aberrant karyotypes in the analyzed samples of amniotic fluid
Table 3.
Presentation of number of the performed FISH analysis on amniotic fluid samples, and the number of aneuploidies detected by this method before obtaining cariogram results.

4. DISCUSSION
In the four-year period (2008-2011) of using the prenatal diagnosis method, there was found 1.5% of pathological karyotypes with numerical aberrations, which coincides with the results of other authors who describe the frequency of numerical aberrations detection in different genetic centers from 1 to 7% (5, 6, 7, 8, 9). In the sample of prenatal pathological karyotypes with numerical aberrations in this research, most frequently occur autosomal chromosome aberrations 80%, followed by sex chromosome aneuploidies 15% and polyploidies 5%. The most frequent individually present numerical aberration is Down syndrome 75%, then Edwards syndrome 5%, Klinefelter’s syndrome 10%, syndrome of double chromosome Y 5%, and triploidy 5%. The research results are comparable with the results of other authors since most studies show that Down syndrome is the most common and clinically most significant aneuploidy in prenatal cytogenetic analysis (about 40-70%), followed by Edwards syndrome (15-20%) and Patau syndrome (2-10%), the other abnormalities occur less frequently (10, 11, 6, 7, 12, 13). In the group of prenatally detected structural rearrangements, there were detected 18 structural aberrations, i.e. 1.34%. Inversions of chromosome 9 are not included because they are considered as variants of normal karyotype, and the percentage of normal karyotypes is 98.7%. The proportion of balanced changes in the total amniotic fluid sample is 1.13%, and the proportion of unbalanced changes is 0.21%. In the published results of other authors, unbalanced structural chromosome abnormalities has 0.06% (1:1 640) live newborns, and balanced rearrangements has 0.52% (1:190) live births (14, 15). Taking into account both numerical and structural chromosomal aberrations, proportion of aberrant karyotypes in the analyzed amniotic fluid samples in this study is 2.9%, which is consistent with results from other studies that describe the frequency of detection of abnormal karyotypes with 1-7% (5, 6, 7, 8. 9). In prenatal detected aberrant karyotypes, there were 20 (52.7%) numerical aberrations, and 18 (47.3%) structural aberrations. In the group of structural aberrations more frequent were balanced aberration 15 (83%), while there were 3 (17%) unbalanced. Besides classical cytogenetic analysis, it was performed molecular cytogenetic analysis of Fluorescent in situ hybridization. The advantage of FISH analysis is that, far before the final findings of the karyotype, it can determine the presence of common aneuploidies. It should be noted that even if the FISH analysis finding indicates the presence of aneuploidy, any further decisions of the course of pregnancy should not be taken without final confirmation of the findings by karyotyping method. The lack FISH analysis is that the structural changes of chromosomes can not be detected, e.g. FISH analysis of the sample that was made before karyotyping showed normal results of FISH analysis for deletion of p arm of chromosome 18 in one of the samples of amniotic fluid, because for this the type of analysis we used centromeric probe for chromosome 18. Benefits of molecular-cytogenetic analysis of Fluorescent in situ hybridization in the detection of most frequent aneuploidies in prenatal diagnosis are efficiency, quick results, reliability. The lack of this type of analysis is the strict specificity of applied analysis to just a certain type of aberrations. Molecular cytogenetic methods are a good supplement to conventional cytogenetic methods and combined use of these methods significantly contributes to improvement of cytogenetic laboratories working quality in prenatal diagnosis of chromosomopathies. Combined use of these methods can significantly reduce the number of children born with one of chromosomopathies, but golden standard of cytogenetic analysis in prenatal diagnosis of chromosomopathies still remains classical cytogenetic methods.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Gilbert-Barnes E, Debitch-Spicer D. Embryo & Fetal patology. Cembrige University Press; 2006. [Google Scholar]
- 2.Gilbert-Barnes E. Poter's Patology of the fetus, infant and child. Elsevier; 2007. [Google Scholar]
- 3.Barić I, Stavljenić-Rukavina A. Racionalna dijagnostika nasljednih i prirođenih bolesti. Zagreb: Medicinska naklada; 2005. [Google Scholar]
- 4.Wagner RD. Diagnostic Cytogenetic. Berlin Heilderberg New York: Springer-Verlag; 1999. [Google Scholar]
- 5.Methew T, Navsaria D, Verma RS. Prenatal cytogenetic diagnosis of 1400 consecutive amniocentesis. Gynecol Obstet Invest. 1992;34:122–123. doi: 10.1159/000292741. [DOI] [PubMed] [Google Scholar]
- 6.Park SY, Kim JW, Kim YM, Kim JM, Lee MH, Lee BY, et al. Frequencies of Fetal Chromosomal Abnormalities at Prenatal Diagnosis: 10 years experiences in a single institution. J Korean Med Sci. 2001;16(3):290–293. doi: 10.3346/jkms.2001.16.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Methew T, Navsaria D, Verma RS. Prenatal cytogenetic diagnosis of 1400 consecutive amniocentesis. Gynecol Obstet Invest. 1992;34:122–123. doi: 10.1159/000292741. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Zhang XH, Liang MY, Ren MH. Prenatal cytogenetic diagnosis study of 2782 cases of high-risk pregnant women. Chin Med J. 2010;123(4):423–430. [PubMed] [Google Scholar]
- 9.Sener KT, Durak B, Tanir HM, Tepeli E, Kaya M, Artan S. Amnioocentesis Results in 7 Years Period in Our Clinic. Perinatal Journal. 2006;14(4):170–175. [Google Scholar]
- 10.Park IY, Kwon JY, Kim YH, Kim M, Shin JC. Maternal age-specific rates of fetal chromosomal abnormalities at 16-20 weeks’ gestation in Korean pregnant women >or=35 years of age. Fetal Diagn Ther. 2010;27(4):214–221. doi: 10.1159/000309136. [DOI] [PubMed] [Google Scholar]
- 11.Karaoguz MY, Bal F, Yakut T, Ercelen NO, Ergun MA, Gokcen AB, et al. Cytogenetic results of amniocentesis materials: incidence of abnormal karyotypes in the Turkish collaborative study. Genet Couns. 2006;17(2):219–230. [PubMed] [Google Scholar]
- 12.Seeds JW. Diagnostic mid trimester amniocentesis: how safe? Am J Obstet Gynecol. 2004;191:608–616. doi: 10.1016/j.ajog.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein E, Lenchner E, Donnenfeld A, Barnhard Y, Seubert D, Divon MY. Advanced maternal age as a sole indication for genetic amniocentesis;risk-benefit analysis based on a large database reflecting the current common practice. J Perinat Med. 2009;37(2):99–102. doi: 10.1515/JPM.2009.032. [DOI] [PubMed] [Google Scholar]
- 14.Gardner RJ, Sutherland GR. Chromosome Abnormalities and Genetic Counseling. 3rd ed. New York: Oxford University Press; 2004. [Google Scholar]
- 15.Beana N, De Vigan C, Cariati E, Clementi M, Stoll C, Caballin MR, et al. Prenatal Detectin of Rare Chromozomal Autosomal Abnormalities in Europe. Am J Med Genet. 2003;118A:319–327. doi: 10.1002/ajmg.a.10104. [DOI] [PubMed] [Google Scholar]


