Abstract
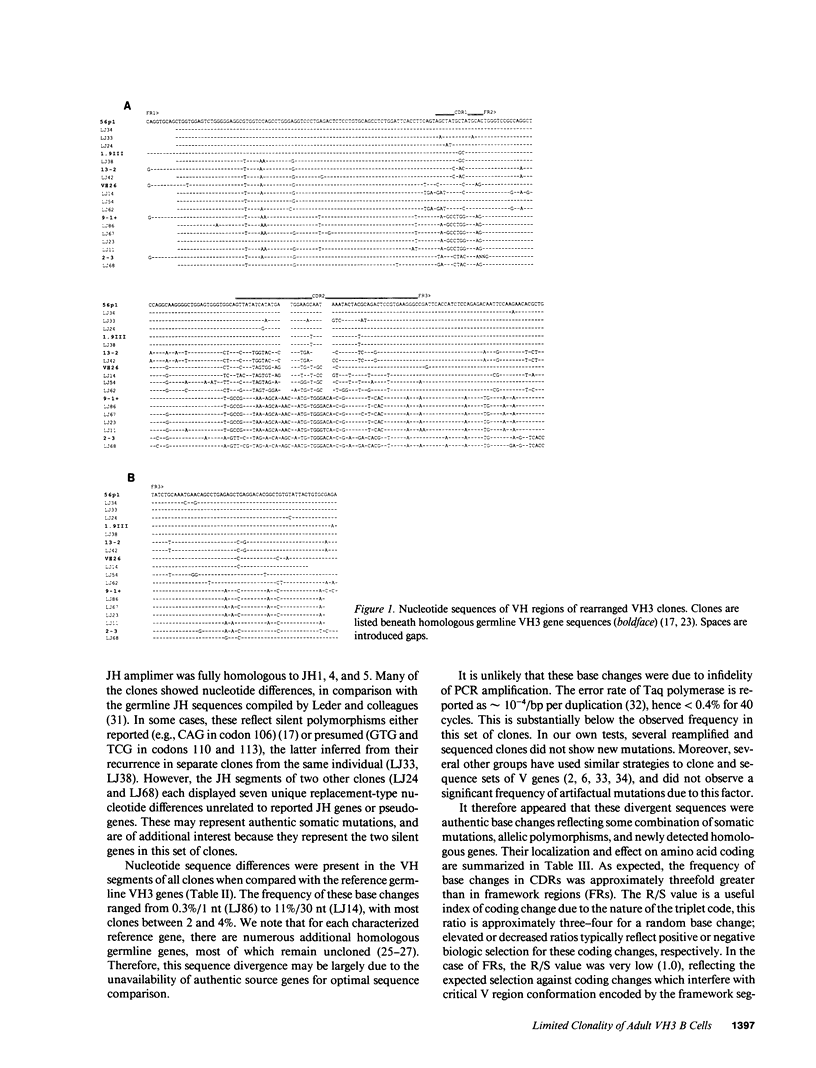
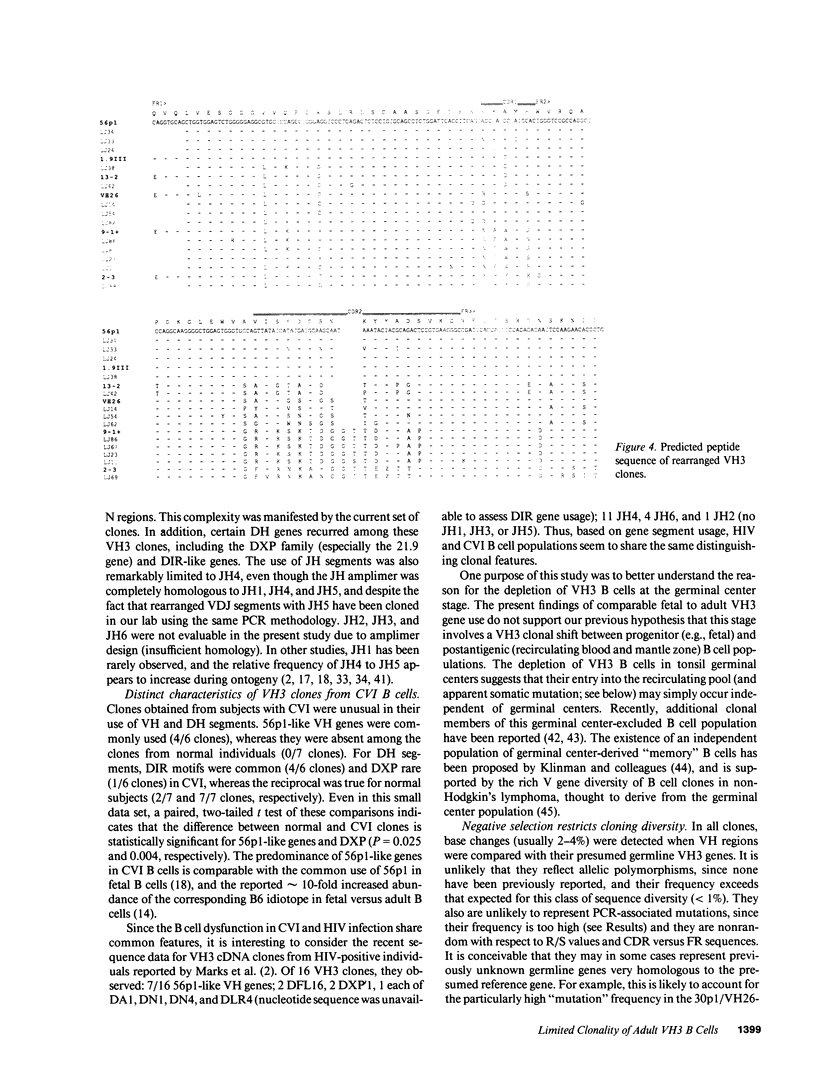
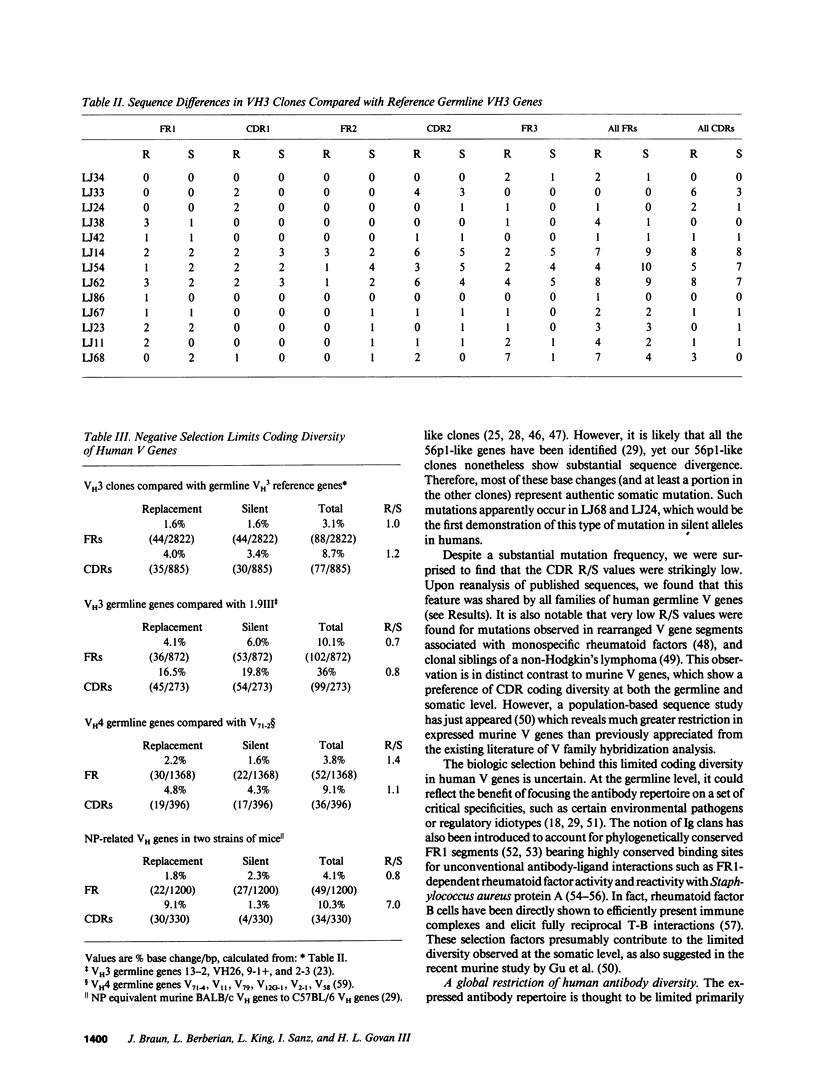
The large VH3 family of human immunoglobulin genes is commonly used throughout B cell ontogeny. However, B cells of the fetus and certain autoantibody-producing clones are restricted to a recurrent subset of VH3 genes, and VH3 B cells are deficient in certain immunodeficiency diseases. In this study, we have sequenced a set of rearranged VH3 genes generated by genomic polymerase chain reaction (PCR) from normal adults and those with common variable immunodeficiency (CVI). In both groups, all cones were readily identifiable with the fetal VH3 subset, and were further distinguished by limited DH motifs and exclusive use of JH4. In CVI, the residual population of VH3 B cells were notable for predominant use of 56p1-like VH genes. All clones displayed sequence divergence (including somatic mutation) with evidence of strong selection against complementarity-determining region (CDR) coding change. A survey of other V gene families indicates that human V gene diversity may be restricted in general by germline mechanisms. These findings suggest that the expressed antibody repertoire in the human adult may be much smaller than anticipated, and selected by processes in part distinct from the paradigm of maximal antigen-binding diversity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Shackelford P. G., Quinn A., Carroll W. L. Restricted Ig H chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991 Sep 1;147(5):1667–1674. [PubMed] [Google Scholar]
- Axelrod O., Silverman G. J., Dev V., Kyle R., Carson D. A., Kipps T. J. Idiotypic cross-reactivity of immunoglobulins expressed in Waldenström's macroglobulinemia, chronic lymphocytic leukemia, and mantle zone lymphocytes of secondary B-cell follicles. Blood. 1991 Apr 1;77(7):1484–1490. [PubMed] [Google Scholar]
- Bangs L. A., Sanz I. E., Teale J. M. Comparison of D, JH, and junctional diversity in the fetal, adult, and aged B cell repertoires. J Immunol. 1991 Mar 15;146(6):1996–2004. [PubMed] [Google Scholar]
- Berberian L., Valles-Ayoub Y., Sun N., Martinez-Maza O., Braun J. A VH clonal deficit in human immunodeficiency virus-positive individuals reflects a B-cell maturational arrest. Blood. 1991 Jul 1;78(1):175–179. [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Galbraith L., Valles-Ayoub Y., Saxon A. Human immunodeficiency resulting from a maturational arrest of germinal center B cells. Immunol Lett. 1991 Mar;27(3):205–208. doi: 10.1016/0165-2478(91)90152-z. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Kipps T. J., Radoux V., Liu F. T., Carson D. A. Isolation and characterization of human VkIII germ-line genes. Implications for the molecular basis of human VkIII light chain diversity. J Immunol. 1987 Sep 1;139(5):1727–1733. [PubMed] [Google Scholar]
- Chen P. P. Structural analyses of human developmentally regulated Vh3 genes. Scand J Immunol. 1990 Mar;31(3):257–267. doi: 10.1111/j.1365-3083.1990.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Berry J., Flaherty D., Dunnick W. Somatic evolution of diversity among anti-phosphocholine antibodies induced with Proteus morganii. J Immunol. 1987 May 1;138(9):3060–3068. [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Galili N., Trela M., Levy R., Sklar J. Single cell origin of bigenotypic and biphenotypic B cell proliferations in human follicular lymphomas. J Exp Med. 1988 Feb 1;167(2):582–597. doi: 10.1084/jem.167.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Givol D. Conservation and divergence of immunoglobulin VH pseudogenes. EMBO J. 1983;2(10):1795–1800. doi: 10.1002/j.1460-2075.1983.tb01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. J., Mageed R. A., Silverman G. J., Chen P. P., Kozin F., Erger R. A., Jefferis R., Carson D. A. The incidence of a new human cross-reactive idiotype linked to subgroup VHIII heavy chains. Mol Immunol. 1990 Jan;27(1):87–94. doi: 10.1016/0161-5890(90)90063-6. [DOI] [PubMed] [Google Scholar]
- Cuisinier A. M., Guigou V., Boubli L., Fougereau M., Tonnelle C. Preferential expression of VH5 and VH6 immunoglobulin genes in early human B-cell ontogeny. Scand J Immunol. 1989 Oct;30(4):493–497. doi: 10.1111/j.1365-3083.1989.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Deane M., Norton J. D. Immunoglobulin heavy chain variable region family usage is independent of tumor cell phenotype in human B lineage leukemias. Eur J Immunol. 1990 Oct;20(10):2209–2217. doi: 10.1002/eji.1830201009. [DOI] [PubMed] [Google Scholar]
- Deenen G. J., Van Balen I., Opstelten D. In rat B lymphocyte genesis sixty percent is lost from the bone marrow at the transition of nondividing pre-B cell to sIgM+ B lymphocyte, the stage of Ig light chain gene expression. Eur J Immunol. 1990 Mar;20(3):557–564. doi: 10.1002/eji.1830200315. [DOI] [PubMed] [Google Scholar]
- Fish S., Zenowich E., Fleming M., Manser T. Molecular analysis of original antigenic sin. I. Clonal selection, somatic mutation, and isotype switching during a memory B cell response. J Exp Med. 1989 Oct 1;170(4):1191–1209. doi: 10.1084/jem.170.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. F., Cho E. A., Goldman J., Carmack C. E., Besa E. C., Hardy R. R., Silberstein L. E. The role of clonal selection in the pathogenesis of an autoreactive human B cell lymphoma. J Exp Med. 1991 Sep 1;174(3):525–537. doi: 10.1084/jem.174.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Tarlinton D., Müller W., Rajewsky K., Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991 Jun 1;173(6):1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigou V., Cuisinier A. M., Tonnelle C., Moinier D., Fougereau M., Fumoux F. Human immunoglobulin VH and VK repertoire revealed by in situ hybridization. Mol Immunol. 1990 Sep;27(9):935–940. doi: 10.1016/0161-5890(90)90161-r. [DOI] [PubMed] [Google Scholar]
- Hayzer D. J. Immunoglobulin lambda light chain evolution: Igl and Igl-like sequences form three major groups. Immunogenetics. 1990;32(3):157–174. doi: 10.1007/BF02114969. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986 Dec;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Duffy S. F. Relationship of the CD5 B cell to human tonsillar lymphocytes that express autoantibody-associated cross-reactive idiotypes. J Clin Invest. 1991 Jun;87(6):2087–2096. doi: 10.1172/JCI115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Levy N. S., Malipiero U. V., Lebecque S. G., Gearhart P. J. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J Exp Med. 1989 Jun 1;169(6):2007–2019. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S., Avnur Z., Levy R. Mutational hot spots in Ig V region genes of human follicular lymphomas. J Exp Med. 1988 Aug 1;168(2):475–489. doi: 10.1084/jem.168.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ L., Decker D. J., Klinman N. R. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989 Dec 22;59(6):1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- Liu M. F., Robbins D. L., Crowley J. J., Sinha S., Kozin F., Kipps T. J., Carson D. A., Chen P. P. Characterization of four homologous L chain variable region genes that are related to 6B6.6 idiotype positive human rheumatoid factor L chains. J Immunol. 1989 Jan 15;142(2):688–694. [PubMed] [Google Scholar]
- Logtenberg T., Schutte M. E., Inghirami G., Berman J. E., Gmelig-Meyling F. H., Insel R. A., Knowles D. M., Alt F. W. Immunoglobulin VH gene expression in human B cell lines and tumors: biased VH gene expression in chronic lymphocytic leukemia. Int Immunol. 1989;1(4):362–366. doi: 10.1093/intimm/1.4.362. [DOI] [PubMed] [Google Scholar]
- Loh D. Y., Bothwell A. L., White-Scharf M. E., Imanishi-Kari T., Baltimore D. Molecular basis of a mouse strain-specific anti-hapten response. Cell. 1983 May;33(1):85–93. doi: 10.1016/0092-8674(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Lydyard P. M., Quartey-Papafio R. P., Bröker B. M., MacKenzie L., Hay F. C., Youinou P. Y., Jefferis R., Mageed R. A. The antibody repertoire of early human B cells. III. Expression of cross-reactive idiotopes characteristic of certain rheumatoid factors and identifying V kappa III, VHI, and VHIII gene family products. Scand J Immunol. 1990 Dec;32(6):709–716. doi: 10.1111/j.1365-3083.1990.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Tristem M., Karpas A., Winter G. Oligonucleotide primers for polymerase chain reaction amplification of human immunoglobulin variable genes and design of family-specific oligonucleotide probes. Eur J Immunol. 1991 Apr;21(4):985–991. doi: 10.1002/eji.1830210419. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Shin E. K., Hirabayashi Y., Nagaoka H., Yoshida M. C., Zong S. Q., Honjo T. Organization of variable region segments of the human immunoglobulin heavy chain: duplication of the D5 cluster within the locus and interchromosomal translocation of variable region segments. EMBO J. 1990 Aug;9(8):2501–2506. doi: 10.1002/j.1460-2075.1990.tb07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olee T., Yang P. M., Siminovitch K. A., Olsen N. J., Hillson J., Wu J., Kozin F., Carson D. A., Chen P. P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Invest. 1991 Jul;88(1):193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Capra J. D. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv Immunol. 1991;49:1–74. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- Pascual V., Randen I., Thompson K., Sioud M., Forre O., Natvig J., Capra J. D. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germ line genes. J Clin Invest. 1990 Oct;86(4):1320–1328. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Victor K., Lelsz D., Spellerberg M. B., Hamblin T. J., Thompson K. M., Randen I., Natvig J., Capra J. D., Stevenson F. K. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991 Jun 15;146(12):4385–4391. [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Roosnek E., Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991 Feb 1;173(2):487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989 Apr 15;142(8):2778–2783. [PubMed] [Google Scholar]
- Sasso E. H., Van Dijk K. W., Milner E. C. Prevalence and polymorphism of human VH3 genes. J Immunol. 1990 Oct 15;145(8):2751–2757. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Structure and evolution of mammalian VH families. Int Immunol. 1990;2(1):41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri F., Mageed R. A., Maziak B. R., Jefferis R. Expression of VHIII-associated cross-reactive idiotype on human B lymphocytes. Association with staphylococcal protein A binding and Staphylococcus aureus Cowan I stimulation. J Immunol. 1991 Feb 1;146(3):936–940. [PubMed] [Google Scholar]
- Tutter A., Riblet R. Conservation of an immunoglobulin variable-region gene family indicates a specific, noncoding function. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7460–7464. doi: 10.1073/pnas.86.19.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Ayoub Y., Govan H. L., 3rd, Braun J. Evolving abundance and clonal pattern of human germinal center B cells during childhood. Blood. 1990 Jul 1;76(1):17–23. [PubMed] [Google Scholar]
- Walter M. A., Surti U., Hofker M. H., Cox D. W. The physical organization of the human immunoglobulin heavy chain gene complex. EMBO J. 1990 Oct;9(10):3303–3313. doi: 10.1002/j.1460-2075.1990.tb07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization in colonies derived from B and pre-B cells detected by the RNA colony blot assay. EMBO J. 1986 Dec 20;5(13):3475–3481. doi: 10.1002/j.1460-2075.1986.tb04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Wasserman R., Reichard B. A., Shane S., Caton A. J., Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J Exp Med. 1991 Feb 1;173(2):395–407. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Malynn B. A., Alt F. W. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med. 1988 Jul 1;168(1):417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F., Tucker L., Rubinstein D., Guillaume T., André-Schwartz J., Barrett K. J., Schwartz R. S., Logtenberg T. Molecular analysis of a germ line-encoded idiotypic marker of pathogenic human lupus autoantibodies. J Immunol. 1990 Oct 15;145(8):2545–2553. [PubMed] [Google Scholar]
- Zouali M., Theze J. Probing VH gene-family utilization in human peripheral B cells by in situ hybridization. J Immunol. 1991 Apr 15;146(8):2855–2864. [PubMed] [Google Scholar]
- van Es J. H., Gmelig Meyling F. H., van de Akker W. R., Aanstoot H., Derksen R. H., Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991 Feb 1;173(2):461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


