Abstract
Playing a musical instrument is an intense, multisensory, and motor experience that usually commences at an early age and requires the acquisition and maintenance of a range of sensory and motor skills over the course of a musician’s lifetime. Thus, musicians offer an excellent human model for studying behavioral-cognitive as well as brain effects of acquiring, practicing, and maintaining these specialized skills. Research has shown that repeatedly practicing the association of motor actions with specific sound and visual patterns (musical notation), while receiving continuous multisensory feedback will strengthen connections between auditory and motor regions (e.g., arcuate fasciculus) as well as multimodal integration regions. Plasticity in this network may explain some of the sensorimotor and cognitive enhancements that have been associated with music training. Furthermore, the plasticity of this system as a result of long term and intense interventions suggest the potential for music making activities (e.g., forms of singing) as an intervention for neurological and developmental disorders to learn and relearn associations between auditory and motor functions such as vocal motor functions.
Keywords: brain plasticity, diffusion tensor imaging, morphometry, motor, auditory, Melodic Intonation Therapy, Auditory–Motor Mapping Training (AMMT)
1 INTRODUCTION
Musicians with extensive music training and playing experience provide an excellent model for studying plasticity of the human brain. The demands placed on the nervous system by music making are unique and provide a uniquely rich multisensory and motor experience to the player. As confirmed by neuroimaging studies, playing music depends on a strong coupling of perception and action mediated by sensory, motor, and multimodal integration regions distributed throughout the brain (e.g., Schlaug et al., 2010a; Zatorre et al., 2007). A violinist, for example, must execute a myriad of complex skills which includes translating visual analysis of musical notation into motor movements, coordinating multisensory information with bimanual motor activity, developing fine-motor skills mostly of their nondominant hand coupled with metric precision, and monitoring auditory feedback to fine-tune a performance in progress.
This chapter summarizes research on the effects of musical training on brain or-ganization. Musical training usually commences at an early age, and requires the acquisition and maintenance of a range of skills over the course of a musician’s lifetime. In the past, much research has focused on how musical training shapes the healthy brain, more recent studies provide evidence that music making activities induces brain plasticity to help overcome neurological impairments. Both neurode-velopmental disorders (e.g., stuttering, speech-motor acquired brain injuries; e.g., stroke patients with motor and communication deficits, patients with Parkinson’s disease) and neurodevelopmental disorders (e.g., stuttering, speech difficulties in individuals with autism) and acquired brain injuries (e.g., stroke patients with motor and communication deficits, patients with Parkinson’s disease) are examples of such impairments.
2 BEHAVIORAL STUDIES: THE EFFECTS OF MUSICAL TRAINING ON COGNITIVE PERFORMANCE
Over the past 20 years, a large plethora of research has referenced the beneficial effects of musical training on cognitive development in children. Cross-sectional studies have shown that musically trained children are better than musically untrained children on a range of auditory and motor abilities, such as pitch and rhythmic discrimination (Forgeard et al., 2008), melodic contour perception (Morrongiello and Roes, 1990), and finger sequencing (Forgeard et al., 2008).
Many studies have examined whether or not musical training leads to enhance-ment of other cognitive skills. For example, similarities between music and language suggest that musical training may lead to enhanced language abilities. Studies with children showed a positive association between pitch perception and reading abilities (Anvari et al., 2002), and years of musical training predicted increased verbal recall (Jakobson et al., 2003) and reading skills (Butzlaff, 2000). Additionally, musically trained children showed superior auditory, finger tapping, and vocabulary skills when compared to their musically untrained counterparts (Schlaug et al., 2005), who were matched on age, handedness, and socioeconomic status. Improvements in mathematical and spatial skills have also been implicated, although their relationship with musical training remains unclear (e.g., Forgeard et al., 2008; Hetland, 2000; Vaughn, 2000). Recently, Kraus et al. (2014) showed that having a group of children engage in a music enrichment program for 2 years improved their neurophysiological processing of speech sounds which was not seen in a wait-list control group or after only 1 year of music classes.
It is not unexpected that musical training induces domain-specific adaptations in terms of improved sensorimotor and auditory abilities. However, what remains to be determined is whether or not training in the musical domain might enhance function in an untrained domain. In one study, for example, the level of engagement in musical practice during childhood predicted academic performance at university level (Schellenberg, 2006). These differences in performance persisted even when variables such as socioeconomic status and parent education were controlled. One potential mechanism for this association is the effects of musical practice on general executive function (Schellenberg and Peretz, 2008), although recent research has not provided support for this hypothesis (Schellenberg, 2011). Another hypothesis is that of cross-modal transfer of plasticity: long-term musical training leads to changes in polymodal integration regions (e.g., regions surrounding the intraparietal sulcus), which may alter task performance in other domains (Wan and Schlaug, 2010). Playing music, for example, leads to changes in the intraparietal sulcus, and this region is implicated in numerical representation and operations (Cohen Kadosh et al., 2007; Dehaene et al., 1998; Piazza et al., 2007; Pinel et al., 2004). Accordingly, adaptations in brain regions that are involved in musical tasks may have an effect on mathematical performance because of shared neural resources involved in the mental manipulation of symbolic representation. Further research examining the mechanisms underlying the associations between musical training and cognitive skills is clearly warranted.
Although cross-sectional studies provide information about the potential benefits of musical training on cognitive functions, longitudinal studies allow stronger inferences to be made within a group of individuals. The reason is that longitudinal studies minimize the possible influence of preexisting factors such as socioeconomic status, home support, and available resources, which be responsible for some of the differences between musicians and nonmusicians. Longitudinal studies have also provided evidence that musical training has positive implications for cognitive functioning. For example, children who received 1 year of instrumental musical training showed superior verbal memory skills compared to children who had discontinued training (Ho et al., 2003). Considering that this study was done in Hong Kong, one might speculate that superior verbal memory skills could be due to an enhancement in memory for the pitches of lexical tones. However, another study showed an increase in IQ comparing children who participated in a 36-week music program to children who received drama lessons (Schellenberg, 2004). Interestingly, children who practiced singing during the music program had greater increase in IQ compared to those who played the keyboard. In two other longitudinal studies, children who received music lessons were compared to children who received painting lessons. After 8 weeks of training, there were clear differences in electrophysiology between the two groups (reduction of late positive component to strong pitch incongruities in the music group), despite no differences in their ability to perform a language perception task (Moreno and Besson, 2006). In a subsequent study, children allocated to the music and painting groups were tested before and after 6 months of training (Moreno et al., 2009). For children who received music lessons, there were improvements in reading and language perception abilities, while no such improvement was observed in children who received painting lessons. These behavioral enhancements in the musically trained children were accompanied by changes in the amplitudes of specific event-related potential components associated with music and speech. A recent study also reported that a specialized weekly instrumental program in a socioeconomically disadvantaged school led to significantly improved learning and immediate recall for verbal information after 1 year of instruction, but no such benefits were observed in children who underwent a standard classroom music program and those who underwent juggling training for a year (Rickard et al., 2010). However, when a standard classroom music program in a non-disadvantaged school was compared with standard drama and art programs, there were no significant benefits of music instruction on cognitive abilities over other instructions (Rickard et al., 2011). The absence of cognitive effects in this latter study could be due to the class-based nature of the program, which made it less likely to adapt instruction for the wide range abilities in the students and be equally engaging for all. Furthermore, classroom-based studies are often difficult to conduct because it is challenging to find an appropriate “control” instruction program, to randomly allocate students into the experimental conditions, and to match students on preexisting abilities.
3 IMAGING STUDIES: THE EFFECTS OF MUSICAL TRAINING ON BRAIN ORGANIZATION
Musical training in childhood has profound effects on both the structural and functional organization of the brain. The first study that examined structural differences between musicians and nonmusicians reported larger anterior corpus callosum in musicians (Schlaug et al., 1995a), a finding that has since been replicated by different research groups using different methodological approaches (Hyde et al., 2009; Lee et al., 2003; Oztürk et al., 2002). Specifically, musicians who began training at an early age (≤7 years) had a significantly larger corpus callosum compared to musicians who commenced training later. When cortical motor regions were examined, a similar finding was observed. In particular, the depth of the central sulcus, often used as a marker of primary motor cortex size, was larger on both hemispheres, but more pronounced on the right hemisphere for musicians compared to nonmusicians, possibly due to years of manual motor practice emphasizing the nondominant hand, while the dominant hand undergoes some form of fine-motor training in every adult writing with the right hand and using the right hand for skilled sensorimotor tasks (Amunts et al., 1997; Schlaug, 2001). As was observed for the corpus callosum, there was a positive correlation between the size of the primary motor cortex and the onset of instrumental musical training (used as a surrogate for intensity and duration of training).
Structural brain differences have been reported in musicians who play different instruments (Bangert et al., 2006). For keyboard players, the omega sign of the precentral gyrus, which is associated with hand and finger movement representation, was found to be more prominent on the left hemisphere for keyboard players, but was more prominent on the right hemisphere for string players. This structural difference is likely to reflect an adaptation to the specific demands of different musical instruments. One brain region that differentiates musical experts from novices is the pla-num temporale, or secondary auditory cortex, which occupies the posterior plane of the superior temporal gyrus (Schlaug, 2001; Schlaug et al., 1995a,b; Zatorre et al., 1998). A pronounced leftward asymmetry of the planum temporale was linked to the ability to perceive absolute pitch. More recently, it was also demonstrated that in musicians with absolute pitch, the posterior superior temporal gyrus is connected to a region within the middle temporal gyrus which has been associated with categorical perception (Loui et al., 2010). Thus, the connections between the posterior superior temporal gyrus and the middle temporal gyrus may play a role in determining whether or not someone develops absolute pitch in addition to early exposure to music. Other areas showing structural differences between musicians and nonmusi-cians include the Heschl’s gyrus, or primary auditory cortex (Schneider et al., 2005a), Broca’s area, and the inferior frontal gyrus in general (Gaser and Schlaug, 2003a,b; Sluming et al., 2002), as well as the cerebellum (Hutchinson et al., 2003), and areas in the superior parietal lobule (Gaser and Schlaug, 2003a). These structural differences appear to be more pronounced in those musicians who began training early in life (Elbert et al., 1995; Schlaug et al., 1995b) and who practiced with greater intensity (Gaser and Schlaug, 2003b; Schneider et al., 2005b).
In addition to structural alterations, intensive musical training has also been associated with an expansion of the functional representation of finger or hand maps, as demonstrated in magnetoencephalography studies. For example, the somatosensory representations of the playing fingers of string players were found to be larger than those of nonmusicians (Pantev et al., 2001). This effect was more pronounced for the fifth digit, which was rarely used in the nonmusician group. Musicians who had begun training early in life (<13 years) demonstrated larger cortical representation of their left fifth digit compared to those who started to play their instruments later, who, in turn, had larger representations than nonmusicians. In addition to these enhanced somatosensory representations, musicians have larger representations for tones than do nonmusicians. In one study, musicians who had started playing at a young age demonstrated the largest cortical representations (Pantev et al., 1998), and this enlargement was evident for piano tones but not for pure tones. In contrast, a study by Schneider et al. (2002) reported increased representation for pure tones, up to twice as large in professional musicians compared to nonmusicians. In that study, amateur musicians showed an intermediate increase over nonmusicians, but only for tones less than 1000 Hz. In a longitudinal study, violin students showed a larger cortical response to violin sounds compared to other sounds after only 1 year of training, whereas this difference was not observed in musically untrained children (Fujioka et al., 2006).
A large body of research has used functional magnetic resonance imaging (fMRI) to compare musicians and nonmusicians. Differences in activity have been observed across many brain regions when individuals were asked to perform musical tasks involving discrimination (e.g., Foster and Zatorre, 2010; Koelsch et al., 2005), working memory (e.g., Gaab and Schlaug, 2003; Gaab et al., 2006), or production (Bangert et al., 2006; Kleber et al., 2010). Despite the heterogeneity of the tasks used, an area that was commonly activated in many of these studies was the posterior superior temporal gyrus, which is important for spectrotemporal processing as well as auditory–motor transformations (Warren et al., 2005). Indeed, a recent study identified the left superior temporal gyrus as the region that is linked with musical training, in terms of cumulative practice hours (Ellis et al., 2013).
A relatively new technique that can be used to study brain differences between musicians and nonmusicians is diffusion tensor imaging (DTI). This technique provides information about white matter microstructures (i.e., orientation and direction of axons and their degree of myelination) by measuring diffusion properties of water molecules. Some studies reported lower fractional anisotropy (FA, a measure of the directionality of water diffusion) in the internal capsule (Schmithorst and Wilke, 2002), corticospinal tract (Imfeld et al., 2009), and a portion of the arcuate fasciculus (Halwani et al., 2011) of musicians compared to nonmusicians. In contrast, higher FA in the internal capsules has also been observed. For example, Bengtsson et al. (2005) have reported that the number of practice hours during childhood is positively correlated with increased FA values, not only in the internal capsule but also in the corpus callosum and the superior longitudinal fasciculus.
Rüber et al. (2013) recently assessed diffusivity measures of different corticosp-inal motor tracts of 10 keyboard players, 10 string players, and 10 nonmusicians. When compared with nonmusicians, FA values of right-hemispheric motor tracts were significantly higher in both musician groups, whereas left-hemispheric motor tracts showed significantly higher FA values only in the keyboard players. Voxel-wise FA analysis found a group effect in white matter underlying the right motor cortex. Diffusivity measures of fibers originating in the primary motor cortex correlated with the maximal tapping rate of the contralateral index finger across all groups. It was argued that the observed between-group diffusivity differences might represent an adaptation to the specific motor demands of the respective musical instrument. The discrepancy in published studies between higher and lower FA values of known tracts in response to intense training may reflect the different mechanisms by which different brain regions and brain systems can remodel. Variations in FA across and within individuals over time can be influenced by factors such as fiber density, axon diameter, myelination, axon collateral sprouting, cell membrane density, and fiber coherence. Higher FA values has been thought to reflect more aligned fibers in a particular tract, while lower FA values does not only indicate less alignment of fibers, but could also mean more axonal sprouting and more branching of axons the closer the tract is to the cortical target region (see Wan et al., 2014). Future developments in DTI methodologies are likely to generate further interest in the music neuroscience community to utilize this technique (see also Fig. 1).
FIGURE 1.
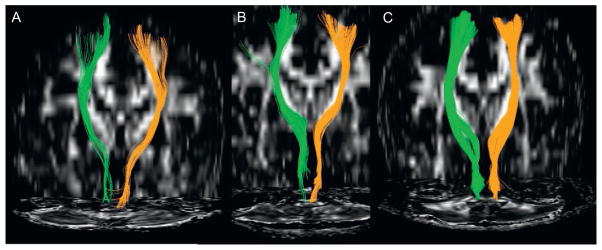
Corticospinal tracts of both hemispheres (green; dark gray in the print version = left) show a child nonmusician (A), an adult nonmusician (B), and an adult musician (C). A comparison of A and B shows the maturational changes that the corticospinal tract undergoes from childhood to adulthood. A comparison of A/B to C shows the additional adaptation of this important motor tract in an adult keyboard player whose requirements are to make fast, precise, and coordinated fine finger movements.
4 AUDITORY–MOTOR INTERACTIONS UNDERLIE MUSIC AND LANGUAGE LEARNING
Playing a musical instrument is a complex sensorimotor activity that simultaneously engages multiple brain regions. The interactions between auditory and motor brain regions are in particular important for both music learning and speech learning. Whether one is learning how a note is played or how a word is pronounced, both tasks involve the association of sounds with articulatory actions associated with auditory feedback. Several studies have shown that merely listening to a melody that one has learned to play on a keyboard (i.e., where a sound-motor map has been established) can activate a motor network, which includes the inferior frontal gyrus, in addition to auditory brain regions. However, listening to a melody that one has not learned to play (i.e., where a sound-motor map has not been established) does not activate the inferior frontal gyrus (e.g., Lahav et al., 2007; Meister et al., 2004) (see also Fig. 2). A more recent study showed that modulation of activity in premotor cortex is associated with increased performance when novices learned to play a melody on a keyboard (Chen et al., 2012). Presumably, the reduced activity in the dorsal auditory action stream is related to increase processing efficiency as individuals acquire auditory–motor associations.
FIGURE 2.
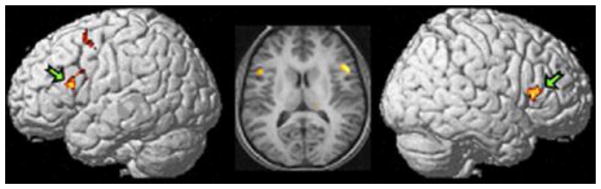
Mapping of sounds to finger actions. Activation spots show significant brain actions when subjects listened to short melodies that they had learned to play on a keyboard subtracted from a condition that had subjects listen to short melodies that were equally familiar, but were never mapped to keyboard actions. It was concluded that the posterior inferior frontal region (Broca’s region on the left and Broca’s homologue on the right) plays a critical role in the mapping of sounds to actions.
Figure is adapted from Lahav et al. (2007).
5 MUSIC-BASED TREATMENTS TO MODULATE BRAIN PLASTICITY: MELODIC INTONATION THERAPY AND AUDITORY–MOTOR MAPPING TRAINING
As described, intensive musical training can lead to modifications in brain structure and function. Recent research has demonstrated that training-induced plasticity is not restricted to the developing brain, but that intensive skill learning in adulthood can also lead to plastic changes. Even for older adults, skill learning appears to preserve gray and white matter structures during the normal ageing process when the brain generally undergoes substance loss (e.g., Boyke et al., 2008; Sluming et al., 2002).
The malleability of the human brain across the lifespan has important implications for the development of rehabilitation techniques, particularly for overcoming impairments associated with neurological disorders. Here, we describe the ongoing research in our laboratory that tests the therapeutic potential of music-based interventions in facilitating speech output in chronic stroke patients with aphasia and in completely nonverbal children with autism. Both disorders are characterized by marked impairments in speech production, and the utility of these interventions (Melodic Intonation Therapy (MIT) for stroke patients, and Auditory–Motor Mapping Training (AMMT) for children with autism) may lie in our understanding of how music and language are processed in the brain.
A large body of neuroimaging research has demonstrated that music and language share brain networks (e.g., Koelsch, 2005; Koelsch et al., 2002; Ozdemir et al., 2006; Patel et al., 1998; Schon et al., 2004) and that active and intensive training with music may assist language recovery and acquisition. In particular, fMRI studies have reported activation of Broca’s area (a classical language area in the brain including the posterior inferior frontal gyrus) during music perception tasks (e.g., Koelsch et al., 2002; Tillmann et al., 2003), active music tasks such as singing (e.g., Ozdemir et al., 2006), and imagining playing an instrument (e.g., Baumann et al., 2007; Meister et al., 2004). Moreover, a common network appears to support the sen-sorimotor components for both speaking and singing (e.g., Kleber et al., 2010; Ozdemir et al., 2006; Pulvermuller, 2005) (see also Fig. 3).
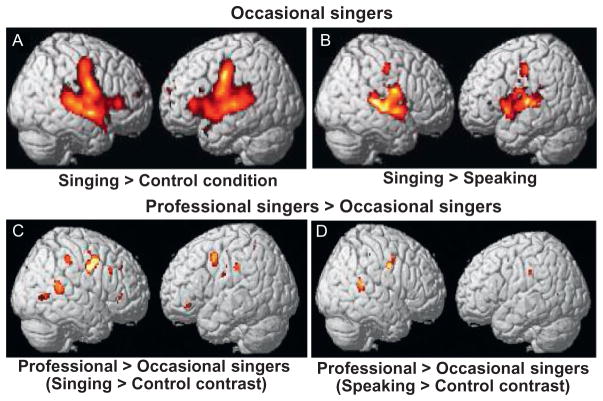
FIGURE 3.
Activation pattern of an overt singing and speaking task contrasting occasional singers with professional singers. Professional singers showed additional activations in temporal, parietal, sensorimotor, and inferior frontal regions on both sides of the brain (right more than left), which was not only seen in the highly controlled singing task but also transferred to the speaking control task (for details on the fMRI task and data analysis, see Ozdemir et al., 2006).
Understanding the extent to which the neural substrates of speaking and singing are distinct depends on an understanding of the lateralization of speech function in the brain. Specifically, speech can be decomposed according to time scale. For example, formant transitions, and consonant-vowel (CV) transitions, are regarded as the fast components of speech (tens of milliseconds), whereas processing syllables and the prosody are regarded as the slow components of speech (hundreds of milliseconds) (Abrams et al., 2008; Poeppel, 2003). Considering a delay of more than 25 ms for interhemispheric transfer in humans, this necessitates a localization of functions involving the resolution of very fine and rapid temporal changes in the signal to one hemisphere (Aboitiz et al., 1992; Ringo et al., 1994). Tasks that involve short temporal integration windows (tens of milliseconds) would preferentially recruit the left hemisphere (Poeppel, 2003), whereas tasks involving temporal integration windows on the order of hundreds of milliseconds may recruit homologous structures in the right hemisphere (Abrams et al., 2008; Poeppel, 2003). Consistent with this functional localization, neuroimaging studies have shown that tasks involving the rapid articulation of phonemes (such as CV transitions) and the modulation of prosody are correlated with fronto-temporal activation patterns that show a right more than left lateralization (Meyer et al., 2002).
5.1 MELODIC INTONATION THERAPY
The ability to sing in humans is evident from infancy and does not depend on formal vocal training, although it can be enhanced by training (Dalla Bella et al., 2007; Halwani et al., 2011; Kleber et al., 2010; Siupsinskiene and Lycke, 2011; Zarate and Zatorre, 2008). Given the behavioral similarities between singing and speaking, as well as the shared and distinct neural correlates of both, researchers have begun to examine whether forms of singing can be used to treat speech-motor impairments associated with acquired and congenital neurological disorders (Wan et al., 2010b).
The most obvious neurological condition that could benefit from a singing-type intervention is aphasia. Aphasia is a common and devastating complication of stroke or traumatic brain injury that results in the loss of ability to produce and/or comprehend language. It has been estimated that between 24% and 52% of acute stroke patients have some form of aphasia if tested within 7 days of their stroke; 12% of survivors still have significant aphasia at 6 months after stroke (Wade et al., 1986). The nature and severity of language dysfunction depends on the location and extent of the brain lesion. Accordingly, aphasia can be classified broadly into fluent or nonfluent. Fluent aphasia often results from a lesion involving the posterior superior temporal lobe known as Wernicke’s area. Patients who are fluent exhibit articulated speech with relatively normal utterance length. However, their speech may be completely meaningless to the listener with errors in syntax and grammar. These patients typically also have severe speech comprehension deficits. In contrast, nonfluent aphasia results most commonly from a lesion in the left frontal lobe, involving the left posterior inferior frontal region known as Broca’s area. Patients who are nonfluent tend to have relatively intact comprehension for conversational speech, but have marked impairments in articulation and speech production. It has been observed for more than 100 years that patients with severe nonfluent aphasia can often sing phrases that they cannot speak (Gerstman, 1964; Geschwind, 1971; Keith and Aronson, 1975). This clinical observation formed the basis for developing an intervention which has been referred to as MIT.
It is now understood that there can be two routes to recovery from aphasia. In patients with small lesions in the left hemisphere, there tend to be recruitment of both left-hemispheric, perilesional cortex, and only variable involvement of right-hemispheric homologous regions during the recovery process (Heiss and Thiel, 2006; Heiss et al., 1999; Hillis, 2007; Rosen et al., 2000). In contrast, for patients with large left-hemispheric lesions involving language-related regions of the fronto-temporal lobes, their only path to recovery may be through recruitment of homologous language and speech-motor regions in the right hemisphere (Rosen et al., 2000; Schlaug et al., 2008). For these patients, therapies that specifically stimulate the homologous right-hemispheric regions have the potential to facilitate the language recovery process beyond the limitations of natural recovery (Rosen et al., 2000; Schlaug et al., 2008, 2009). It has been argued that MIT, which emphasizes melody and contour, engages a sensorimotor network on the unaffected hemisphere (Albert et al., 1973b; Schlaug et al., 2010b; Sparks and Holland, 1976). The two unique components of MIT are the (1) intonation of words and simple phrases using a melodic contour that follows the prosody of speech and the (2) rhythmic tapping of the left-hand tapping which accompanies the production of each syllable and serves as a catalyst for fluency.
The intonation component of MIT was intended to engage the right hemisphere, which has a dominant role in processing spectral information (Albert et al., 1973a; Meyer et al., 2002; Schlaug et al., 2010b; Zatorre and Belin, 2001) and is more sen-sitive than the left hemisphere to the slow temporal features in acoustic signals (Abrams et al., 2008; Zatorre and Gandour, 2008). The fronto-temporal cortices of both hemispheres can be involved in both singing and speaking, although singing tends to show stronger right-hemisphere activations compared to speaking (Bohland and Guenther, 2006; Ozdemir et al., 2006). Thus, the slower rate of articulation as-sociated with intonation enhancing the prosodic and contour aspects of the stimulus may increase the involvement of the right hemisphere. The left-hand tapping component of MIT not only serves as a metronome but can also facilitate auditory–motor mapping (Lahav et al., 2007) and engages a sensorimotor network that controls both hand and articulatory movements (Meister et al., 2009).
To date, a few studies using MIT have produced positive outcomes in patients with nonfluent aphasia. These outcomes range from improvements on the Boston Diagnostic Aphasia Examination (Goodglass and Kaplan, 1983; see also Bonakdarpour et al., 2000), to improvements in articulation and phrase production (Wilson et al., 2006) after treatment. The effectiveness of this intervention is further demonstrated in a recent study that examined transfer of language skills to untrained contexts. Schlaug et al. (2008) compared the effects of MIT with a control intervention (speech repetition) on picture naming performance and measures of propositional speech. After 40 daily sessions, both therapy techniques resulted in significant improvement on all outcome measures, but the extent of this improvement was far greater for the patient who underwent MIT compared to the one who underwent the control therapy.
The therapeutic effect of MIT is evident in several neuroimaging studies showing reorganization of brain functions. Not only did MIT result in increased activation in a right-hemisphere network involving the premotor, inferior frontal, and temporal lobes (Schlaug et al., 2008), but also the white matter structure that connects these regions, the arcuate fasciculus, underwent noticeable microstructural remodeling (Schlaug et al., 2009). This remodeling is most prominent in the white matter underlying the posterior inferior frontal gyrus, which further highlights the potential role of the Broca homologue in the right hemisphere for the relearning of mapping sounds to actions and the selection of motor plans through reciprocal connections with premo-tor and motor areas (Schlaug et al., 2009; Zheng et al., 2011).
5.2 AUDITORY–MOTOR MAPPING TRAINING
AMMT is an intonation-based speech therapy that has been developed in our laboratory specifically for nonverbal children with Autism Spectrum Disorder (ASD). ASD is a developmental condition that affects 1 in 110 children, and one of the core diagnostic features relates to impairments in language and communication. In fact, up to 25% of the individuals with ASD lack the ability to communicate with others using speech sounds, and many of them have limited vocabulary in any modality including sign language (Koegel, 2000; Turner et al., 2006). Although the ability to communicate verbally is considered to be a positive prognostic indicator for children with ASD (Luyster et al., 2007), there are extremely few techniques that can reliably produce improvements in speech output in nonverbal children with ASD.
AMMT is a therapy technique that aims to facilitate speech output and vocal pro-duction in nonverbal children with ASD (Wan et al., 2010a). Briefly, AMMT in-volves two main components: (1) intonation of words/phases and (2) motor activities. Intonation (or singing) is known to engage a bilateral network between frontal and temporal regions, which overlaps with components of the putative mirror neuron system (Meister et al., 2003, 2004; Ozdemir et al., 2006). It has been argued that a dysfunctional mirror neuron system underlies some of the language deficits in autism (Iacoboni and Dapretto, 2006). The presumed mirror neuron system consists of, among others, the posterior inferior frontal regions, which also play a critical role in auditory–motor mapping. Our preliminary imaging findings suggest that the arcuate fasciculus may show a reversed pattern of asymmetry in completely nonverbal children with ASD compared to typically developing children (Wan et al., 2012). Motor activity (through bimanual tapping tuned drums) not only captures the child’s interest but also engages or primes the sensorimotor network that controls orofacial and articulatory movements in speech (e.g., Bangert et al., 2006; Dambeck et al., 2006; Meister et al., 2003, 2006a,b). The sound produced by the tuned drums may also facilitate the auditory–motor mapping that is critical for meaningful vocal communication.
A recent proof-of-concept study showed that AMMT had a significant therapeutic effect on the speech output of six completely nonverbal children (Wan et al., 2011). In that study, each child was enrolled into an intensive 40-session program over an 8-week period. Using a single-subject multiple-baseline design, the speech (CV) production of each child before treatment was compared to that observed during treatment and also to the immediate posttreatment assessment. Follow-up assessments enabled us to establish that the effects were lasting beyond the cessation of the daily AMMT treatments. After therapy, all children showed significant improvements in their ability to articulate words and phrases, and this ability even generalized to items that were not practiced during therapy sessions. Most importantly, these skills were maintained during the 8-week follow-up assessment. A larger-scale clinical trial is currently underway to examine whether AMMT produces superior results compared to non-intonation speech therapy.
6 CONCLUDING REMARKS
Emerging research over the last 20 years has shown that long-term music training and the associated sensorimotor skill learning can be a strong stimulus for neuroplastic changes. These changes can occur in both the developing and the adult brain, and affect both white and gray matter, as well as cortical and subcortical structures. Active musical activities lead to a strong coupling of perception and action mediated by sensory, motor, and multimodal brain regions and affect important sound relay stations in the brainstem and thalamus. Active musical activities make rehabilitation and restorative neurotherapies more enjoyable and can remediate impaired neural processes or neural connections by engaging and linking brain regions with each other.
Although music-based interventions have intuitive appeal, it is critical that devel-opments are grounded on a neurobiological understanding of how particular brain systems can be engaged by music listening and music making activities and what music offers beyond the traditional approaches. The efficacy of these experimental interventions should be assessed quantitatively and objectively, as one would require from any other experimental intervention. A strong neuroscientific basis, combined with compelling data from randomized clinical trials, are important steps in establishing effective music therapies that will enhance brain recovery processes and ameliorate the effects of neurological disorders.
Acknowledgments
G. S. gratefully acknowledges support from NIH (1RO1 DC008796, 3R01DC008796-02S1, R01 DC009823, P50-HD-73912), the family of Rosalyn and Richard Slifka, and the family of Tom and Suzanne McManmon.
Some parts of this review article contain an updated version of a previous review, which appeared in 2012 in Psychology of Music (Wan and Schlaug, Brain Plasticity Induced by Musical Training, 2012, pp. 565–582).
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J Neurosci. 2008;28(15):3958–3965. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973a;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973b;29(2):130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jancke L, Steinmetz H, Schleicher A, Dabringhaus A, et al. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5(3):206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anvari SH, Trainor LJ, Woodside J, Levy BA. Relations among musical skills, phonological processing, and early reading ability in preschool children. J Exp Child Psychol. 2002;83(2):111–130. doi: 10.1016/s0022-0965(02)00124-8. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, et al. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage. 2006;30 (3):917–926. doi: 10.1016/j.neuroimage.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Baumann S, Koeneke S, Schmidt CF, Meyer M, Lutz K, Jäncke LA. A network for audio-motor coordination in skilled pianists and non-musicians. Brain Res. 2007;1161:65–78. doi: 10.1016/j.brainres.2007.05.045. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neu-rosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32 (2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Eftekharzadeh A, Ashayeri H. Preliminary report on the effects of melodic intonation therapy in the rehabilitation of Persian aphasic patients. Iran J Basic Med Sci. 2000;25:156–160. [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzlaff R. Can music be used to teach reading? J Aesthet Educ. 2000;34:167–178. [Google Scholar]
- Chen JL, Rae C, Watkins KE. Learning to play a melody: an fMRI study examining the formation of auditory-motor associations. Neuroimage. 2012;59 (2):1200–1208. doi: 10.1016/j.neuroimage.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Kaas A, Henik A, Goebel R. Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron. 2007;53 (2):307–314. doi: 10.1016/j.neuron.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Dalla Bella S, Giguere JF, Peretz I. Singing proficiency in the general population. J Acoust Soc Am. 2007;121(2):1182–1189. doi: 10.1121/1.2427111. [DOI] [PubMed] [Google Scholar]
- Dambeck N, Sparing R, Meister IG, Wienemann M, Weidemann J, Topper R, et al. Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Res. 2006;1072(1):194–199. doi: 10.1016/j.brainres.2005.05.075. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. Abstract representations of numbers in the animal and human brain. Trends Neurosci. 1998;21(8):355–361. doi: 10.1016/s0166-2236(98)01263-6. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270 (5234):305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Norton A, Overy K, Winner E, Alsop D, Schlaug G. Differentiating maturational and training influences on fMRI activation during music processing. Neuroimage. 2013;75:97–107. doi: 10.1016/j.neuroimage.2012.01.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgeard M, Winner E, Norton A, Schlaug G. Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One. 2008;3 (10):e3566. doi: 10.1371/journal.pone.0003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NE, Zatorre RJ. A role for the intraparietal sulcus in transforming musical pitch information. Cereb Cortex. 2010;20 (6):1350–1359. doi: 10.1093/cercor/bhp199. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Ross B, Kakigi R, Pantev C, Trainor LJ. One year of musical training affects development of auditory cortical-evoked fields in young children. Brain. 2006;129 (Pt 10):2593–2608. doi: 10.1093/brain/awl247. [DOI] [PubMed] [Google Scholar]
- Gaab N, Schlaug G. Musicians differ from nonmusicians in brain activation despite performance matching. Ann N Y Acad Sci. 2003;999:385–388. doi: 10.1196/annals.1284.048. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gaser C, Schlaug G. Improvement-related functional plasticity following pitch memory training. Neuroimage. 2006;31 (1):255–263. doi: 10.1016/j.neuroimage.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003a;23(27):9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Gray matter differences between musicians and nonmusicians. Ann N Y Acad Sci. 2003b;999:514–517. doi: 10.1196/annals.1284.062. [DOI] [PubMed] [Google Scholar]
- Gerstman HL. A case of aphasia. J Speech Hear Disord. 1964;29:89–91. doi: 10.1044/jshd.2901.89. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Current concepts: aphasia. N Engl J Med. 1971;284(12):654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. 2. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Halwani GF, Loui P, Ruber T, Schlaug G. Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front Psy-chol. 2011;2:156. doi: 10.3389/fpsyg.2011.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98(1):118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45(4):430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hetland L. Learning to make music enhances spatial reasoning. J Aesthet Educ. 2000;34(3–4):179–238. [Google Scholar]
- Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69 (2):200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- Ho YC, Cheung MC, Chan AS. Music training improves verbal but not visual memory: cross-sectional and longitudinal explorations in children. Neuropsychology. 2003;17:439–450. doi: 10.1037/0894-4105.17.3.439. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LH, Gaab N, Schlaug G. Cerebellar volume of musicians. Cereb Cortex. 2003;13 (9):943–949. doi: 10.1093/cercor/13.9.943. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, et al. Musical training shapes structural brain development. J Neurosci. 2009;29(10):3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46 (3):600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Jakobson LS, Cuddy LL, Kilgour AR. Time tagging: a key to musicians’ superior memory. Music Percept. 2003;20:307–313. [Google Scholar]
- Keith RL, Aronson AE. Singing as therapy for apraxia of speech and aphasia: report of a case. Brain Lang. 1975;2(4):483–488. doi: 10.1016/s0093-934x(75)80085-x. [DOI] [PubMed] [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M. The brain of opera singers: experience-dependent changes in functional activation. Cereb Cortex. 2010;20 (5):1144–1152. doi: 10.1093/cercor/bhp177. [DOI] [PubMed] [Google Scholar]
- Koegel LK. Interventions to facilitate communication in autism. J Autism Dev Disord. 2000;30(5):383–391. doi: 10.1023/a:1005539220932. [DOI] [PubMed] [Google Scholar]
- Koelsch S. Neural substrates of processing syntax and semantics in music. Curr Opin Neurobiol. 2005;15(2):207–212. doi: 10.1016/j.conb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Gunter TC, von Cramon DY, Zysset S, Lohmann G, Friederici AD. Bach speaks: a cortical “language-network” serves the processing of music. Neuroimage. 2002;17 (2):956–966. [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Schulze K, Alsop D, Schlaug G. Adults and children processing music: an fMR1 study. Neuroimage. 2005;25 (4):1068–1076. doi: 10.1016/j.neuroimage.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kraus N, Slater J, Thompson E, Hornickel J, Strait DL, Nicol T, White-Schwoch T. Music enrichment programs improve the neural encoding of speech in at-risk children. J Neurosci. 2014;34:11913–11918. doi: 10.1523/JNEUROSCI.1881-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav A, Saltzman E, Schlaug G. Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J Neurosci. 2007;27(2):308–314. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Chen Y, Schlaug G. Corpus callosum: musician and gender effects. Neuroreport. 2003;14:205–209. doi: 10.1097/00001756-200302100-00009. [DOI] [PubMed] [Google Scholar]
- Loui P, Li HC, Hohmann A, Schlaug G. Enhanced cortical connectivity in absolute pitch musicians: a model for local hyperconnectivity. J Cogn Neurosci. 2010;23:1015–1026. doi: 10.1162/jocn.2010.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Qiu S, Lopez K, Lord C. Predicting outcomes of children referred for autism using the MacArthur-Bates communicative development inventory. J Speech Lang Hear Res. 2007;50(3):667–681. doi: 10.1044/1092-4388(2007/047). [DOI] [PubMed] [Google Scholar]
- Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, Topper R. Motor cortex hand area and speech: implications for the development of language. Neuropsychologia. 2003;41 (4):401–406. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- Meister IG, Krings T, Foltys H, Boroojerdi B, Müller M, Töpper R, et al. Playing piano in the mind—an fMRI study on music imagery and performance in pianists. Brain Res Cogn Brain Res. 2004;19(3):219–228. doi: 10.1016/j.cogbrainres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Meister IG, Sparing R, Foltys H, Gebert D, Huber W, Topper R, et al. Functional connectivity between cortical hand motor and language areas during recovery from aphasia. J Neurol Sci. 2006a;247(2):165–168. doi: 10.1016/j.jns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Meister IG, Wienemann M, Buelte D, Grunewald C, Sparing R, Dambeck N, et al. Hemiextinction induced by transcranial magnetic stimulation over the right temporo-parietal junction. Neuroscience. 2006b;142 (1):119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Meister IG, Buelte D, Staedtgen M, Boroojerdi B, Sparing R. The dorsal premo-tor cortex orchestrates concurrent speech and fingertapping movements. Eur J Neurosci. 2009;29:2074–2082. doi: 10.1111/j.1460-9568.2009.06729.x. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp. 2002;17(2):73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Besson M. Musical training and language-related brain electrical activity in children. Psychophysiology. 2006;43 (3):287–291. doi: 10.1111/j.1469-8986.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Moreno S, Marques C, Santos A, Santos M, Castro SL, Besson M. Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb Cortex. 2009;19 (3):712–723. doi: 10.1093/cercor/bhn120. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, Roes CL. Developmental-changes in childrens perception of musical sequences—effects of musical training. Dev Psychol. 1990;26(5):814–820. [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33 (2):628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Oztürk AH, Tascioglu B, Aktekin M, Kurtoglu Z, Erden I. Morphometric comparison of the human corpus callosum in professional musicians and non-musicians by using in vivo magnetic resonance imaging. J Neuroradiol. 2002;29:29–34. [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392 (6678):811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- Pantev C, Engelien A, Candia V, Elbert T. Representational cortex in musicians. Plastic alterations in response to musical practice. Ann N Y Acad Sci. 2001;930:300–314. [PubMed] [Google Scholar]
- Patel AD, Gibson E, Ratner J, Besson M, Holcomb PJ. Processing syntactic relations in language and music: an event-related potential study. J Cogn Neurosci. 1998;10(6):717–733. doi: 10.1162/089892998563121. [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53 (2):293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41 (6):983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time”. Speech Comm. 2003;41(1):245–255. [Google Scholar]
- Pulvermuller F. Brain mechanisms linking language and action. Nat Rev Neurosci. 2005;6(7):576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Rickard N, Vasquez J, Murphy F, Gill A, Toukhsati S. Benefits of a classroom based instrumental music program on verbal memory of primary school children: a longitudinal study. Aust J Music Educ. 2010;2010(1):36–47. [Google Scholar]
- Rickard N, Bambrick C, Gill A. Absence of widespread psychosocial and cognitive effects of school-based music instruction in 10–13 year old students. Int J Music Educ. 2011:1–20. [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55 (12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rüber T, Lindenberg R, Schlaug G. Differential adaptation of descending motor pathways in musicians. Cereb Cortex. 2013 doi: 10.1093/cercor/bht331. http://dx.doi.org/10.1093/cercor/bht331 Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Schellenberg EG. Music lessons enhance IQ. Psychol Sci. 2004;15:511–514. doi: 10.1111/j.0956-7976.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Schellenberg EG. Long-term positive associations between music lessons and IQ. J Educ Psychol. 2006;98(2):457–468. [Google Scholar]
- Schellenberg EG. Examining the association between music lessons and intelligence. Br J Psychol. 2011;102(3):283–302. doi: 10.1111/j.2044-8295.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- Schellenberg EG, Peretz I. Music, language and cognition: unresolved issues. Trends Cogn Sci. 2008;12(2):45–46. doi: 10.1016/j.tics.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians: a model for functional and structural plasticity. Ann N Y Acad Sci. 2001;930:281–299. [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H. In vivo evidence of structural brain asymmetry in musicians. Science. 1995a;267 (5198):699–701. doi: 10.1126/science.7839149. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang YX, Staiger JF, Steinmetz H. Increased corpus-callosum size in musicians. Neuropsychologia. 1995b;33 (8):1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, Winner E. Effects of music training on brain and cognitive development. Ann N Y Acad Sci. 2005;1060:219–230. doi: 10.1196/annals.1360.015. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. From singing to speaking: why patients with Broca’s aphasia can sing and how that may lead to recovery of expressive language functions. Music Percept. 2008;25:315–323. doi: 10.1525/MP.2008.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white matter tracts of chronic aphasic patients undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Altenmüller E, Thaut M. Music listening and music making in the treatment of neurological disorders and impairments. Music Percept. 2010a;27(249–250) [Google Scholar]
- Schlaug G, Norton A, Marchina S, Zipse L, Wan CY. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol. 2010b;5(5):657–665. doi: 10.2217/fnl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321(1–2):57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci. 2002;5(7):688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- Schneider P, Sluming V, Roberts N, Bleeck S, Rupp A. Structural, functional, and perceptual differences in Heschl’s gyrus and musical instrument preference. Ann N Y Acad Sci. 2005a;1060:387–394. doi: 10.1196/annals.1360.033. Neurosciences and Music II: From Perception to Performance. [DOI] [PubMed] [Google Scholar]
- Schneider P, Sluming V, Roberts N, Scherg M, Goebel R, Specht HJ, et al. Structural and functional asymmetry of lateral Heschl’s gyrus reflects pitch perception preference. Nat Neurosci. 2005b;8(9):1241–1247. doi: 10.1038/nn1530. [DOI] [PubMed] [Google Scholar]
- Schon D, Magne C, Besson M. The music of speech: music training facilitates pitch processing in both music and language. Psychophysiology. 2004;41 (3):341–349. doi: 10.1111/1469-8986.00172.x. [DOI] [PubMed] [Google Scholar]
- Siupsinskiene N, Lycke H. Effects of vocal training on singing and speaking voice characteristics in vocally healthy adults and children based on choral and nonchoral data. J Voice. 2011;25:e177–e189. doi: 10.1016/j.jvoice.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N. Voxel-based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage. 2002;17 (3):1613–1622. doi: 10.1006/nimg.2002.1288. [DOI] [PubMed] [Google Scholar]
- Sparks RW, Holland AL. Method: melodic intonation therapy for aphasia. J Speech Hear Disord. 1976;41(3):287–297. doi: 10.1044/jshd.4103.287. [DOI] [PubMed] [Google Scholar]
- Tillmann B, Janata P, Bharucha JJ. Activation of the inferior frontal cortex in musical priming. Cogn Brain Res. 2003;16(2):145–161. doi: 10.1016/s0926-6410(02)00245-8. [DOI] [PubMed] [Google Scholar]
- Turner LM, Stone WL, Pozdol SL, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10 (3):243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Vaughn K. Music and mathematics: modest support for the oft-claimed relationship. J Aesthet Educ. 2000;34(3–4):149–166. [Google Scholar]
- Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49 (1):11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16 (5):566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Demaine K, Zipse L, Norton A, Schlaug G. From music making to speaking: engaging the mirror neuron system in autism. Brain Res Bull. 2010a;82(3–4):161–168. doi: 10.1016/j.brainresbull.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Rueber T, Hohmann A, Schlaug G. The therapeutic effects of singing in neurological disorders. Music Percept. 2010b;27(4):287–295. doi: 10.1525/mp.2010.27.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Bazen L, Baars R, Libenson A, Zipse L, Zuk J, et al. Auditory-motor mapping training as an intervention to facilitate speech output in non-verbal children with autism: a proof of concept study. PLoS One. 2011;6 (9):e25505. doi: 10.1371/journal.pone.0025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Marchina S, Norton A, Schlaug G. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Ann N Y Acad Sci. 2012;1252:332–337. doi: 10.1111/j.1749-6632.2012.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang. 2014;136:1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Wise RJ, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28(12):636–643. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Parsons K, Reutens DC. Preserved singing in aphasia: a case study of the efficacy of the melodic intonation therapy. Music Percept. 2006;24:23–36. [Google Scholar]
- Zarate JM, Zatorre RJ. Experience-dependent neural substrates involved in vocal pitch regulation during singing. Neuroimage. 2008;40 (4):1871–1887. doi: 10.1016/j.neuroimage.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11 (10):946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Gandour JT. Neural specializations for speech and pitch: moving beyond the dichotomies. Philos Trans R Soc Lond B Biol Sci. 2008;363(1493):1087–1104. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R, Perry DW, Beckett CA, Westbury CF, Evans AC. Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci USA. 1998;95(6):3172–3177. doi: 10.1073/pnas.95.6.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nat Rev Neurosci. 2007;8(7):547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wan CY, Marchina S, Norton A, Schlaug G. Intensive therapy induces white matter changes in stroke patients with aphasia. Paper presented at the 17th Annual Meeting of the Organization for Human Brain Mapping.2011. [Google Scholar]