Abstract
Aim
The aim of the present study was to investigate the relative importance of a wide array of patient demographic, procedural, anatomic and perioperative variables as potential risk factors for early saphenous vein graft (SVG) thrombosis after coronary artery bypass graft (CABG) surgery.
Methods
The patency of 611 SVGs in 291 patients operated on at four different hospitals enrolled in the Reduction in Graft Occlusion Rates (RIGOR) study was assessed six months after CABG surgery by multidetector computed tomography coronary angiography or clinically-indicated coronary angiography. The odds of graft occlusion versus patency were analyzed using multilevel multivariate logistic regression with clustering on patient.
Results
SVG failure within six months of CABG surgery was predominantly an all-or-none phenomenon with 126 (20.1%) SVGs totally occluded, 485 (77.3%) widely patent and only 16 (2.5%) containing high-grade stenoses. Target vessel diameter ≤1.5 mm (adjusted OR 2.37, P=0.003) and fetnale gender (adjusted OR 2.46, P=0.01) were strongly associated with early SVG occlusion. In a subgroup analysis of 354 SVGs in which intraoperative graft blood flow was measured, lower mean flow was also significantly associated with SVG occlusion when analyzed as a continuous variable (adjusted OR 0.984, P=0.006) though not when analyzed dichotomously, <40 mL/min versus ≥40 mL/min (adjusted OR 1.86, P=0.08).
Conclusion
Small target vessel diameter, female gender and low mean graft blood flow are significant risk factors for SVG thrombosis within six months of CABG surgery in patients on postoperative aspirin therapy. This information may be useful in guiding revascularization strategies in selected patients.
Keywords: Saphenous vein, Thrombosis, Coronary artery bypass, Angiography
Autologous saphenous vein grafts (SVGs) continue to be widely used conduits for coronary artery bypass graft (CABG) surgery. Despite advances in surgical technique and perioperative management, SVGs suffer significantly higher failure rates than arterial grafts. In two recent large multicenter trials, SVG occlusion rates of 25–45% were observed during the first 12–18 months after surgery.1, 2 This has clinical implications as patients with SVG failure within two years of surgery suffer greater rates of death, myocardial infarction and need for repeat revascularization than patients with patent SVGs.3, 4
The proximate cause of SVG failure is a function of graft age. Early SVG failure (<6 months) is predominantly due to occlusive thrombosis, whereas late SVG failure (>1 year) is primarily the result of accelerated atherosclerosis.5 SVG thrombosis is a multifactorial process resulting from factors inherent to the graft, such as endothelial injury/dysfunction and altered blood flow, as well as systemic factors, such as inflammation and hypercoagulation. Aspirin therapy has been shown to reduce SVG occlusion in the first year after surgery by nearly half and is now recommended for all patients undergoing CABG surgery.6
Prior studies have identified a number of anatomic variables that affect SVG patency. These include the location, diameter and quality of the native target vessel as well as the method of harvest, diameter, number of distal anastomosis, mean blood flow and time since implantation of the SVG.7–16 Several of these risk factors were identified in studies conducted without prospective angiography and/or prior to adoption of routine postoperative aspirin therapy. Given advancements in operative technique and perioperative management, along with demographic shifts in the types of patients referred for CABG surgery, the relative importance of many of these risk factors is unclear in a contemporary patient population. The goal of the present study was to comprehensively investigate the relative associations of patient demographic, procedural, anatomic and perioperative variables to early SVG thrombosis in patients receiving early postoperative aspirin therapy.
Materials and methods
Patient population
The Reduction in Graft Occlusion Rates (RIGOR) study is a multicenter observational study of 368 subjects undergoing first-time CABG surgery between 2003 and 2006 designed to investigate the association between anti-platelet factor 4/heparin antibody induction and other thrombotic risk factors and early SVG occlusion. A detailed description of the study design, patient characteristics and principal findings has been previously published.17 Any patient ≥18 years of age undergoing first-time CABG surgery with implantation of at least one SVG was eligible for enrollment. Patients with an anticipated requirement for postoperative oral anticoagulation or antiplatelet therapy other than aspirin were excluded, although those placed on these agents for unforeseen postoperative conditions (e.g., atrial fibrillation) continued in the study. All patients were administered aspirin (300–325 mg) within 24 hours of surgery. At hospital discharge, patients were given a supply of 325 mg enteric-coated aspirin and instructed to take one tablet daily for six months unless directed otherwise by their physician. Pill counts were performed at each postoperative encounter. Demographic, historical, procedural, clinical and laboratory data were recorded for all patients. At the time of surgery, the method of harvest, quality, diameter, type and number of distal anastomosis were recorded for each implanted SVG. Target vessel diameter was measured and the presence of atherosclerosis and/or calcification at the site of distal anastomosis was recorded. Measurement of SVG blood flow was performed at the discretion of the surgeon.
Assessment of SVG patency
SVG patency was assessed six months after CABG surgery by multidetector computed tomography Coronary angiography (CTA) using 16–64 row detector scanners (Aquilion; Toshiba Medical Systems Corporation, Otawara, Japan) as previously described.17 Patients with renal insufficiency that precluded CTA were offered the option of magnetic resonance coronary angiography. Data from clinically-driven invasive coronary angiograms could be used for the primary endpoint analysis if performed within six weeks of the anticipated six-month follow up visit or if it was the only assessment of SVG patency prior to an adverse clinical endpoint. SVG patency was ultimately determined in 297 (81%) of the 368 RIGOR study enrollees. The imaging modalities employed to assess SVG patency in these enrollees and reasons for not being able to assess SVG patency in the remaining 71 enrollees have been previously reported.17 Angiographic images underwent 2- and 3-dimensional reconstruction using a Vitrea workstation (Vital Images, Minnetonka, MN, USA) and were analyzed by two reviewers blinded to outcome. Each segment of “Y-grafts” and “skip grafts” were considered as separate SVGs according to the Society of Thoracic Surgeons (STS) criteria. SVGs were scored as patent (containing stenoses of 0–75%), significantly diseased (containing stenoses of 76–99%) or occluded (containing a 100% stenosis). There was 98% concordance in assessment of SVG patency between reviewers. In cases of discordance, a third reviewer adjudicated all SVGs in that patient. Any SVG treated with percutaneous coronary revascularization prior to scheduled CTA was scored according to the maximal extent of stenosis observed within that vein graft during the six-month follow-up period.
Statistical analysis
Data from 291 patients who survived the index hospitalization and underwent SVG patency assessment by CTA or invasive coronary angiography were included in the present analysis. Because so few SVGs were significantly diseased (containing stenoses of 76–99%), univariate analyses were performed for the outcome of odds of SVG occlusion (100% stenosis) versus patency (≤75% stenosis) using those variables deemed biologically plausible or supported by the literature.
Categorical covariates were tested for collinearity using χ2 or Fisher’s exact tests, while continuous variables were similarly tested using Pearson’s correlation coefficients. Association between categorical and continuous variables was tested using logistic regression. For pairs of collinear variables that non-significant when tested together in the model, that variable with the greatest explanatory power was retained to balance parsimony and model fit. To test the possibility that SVG outcome changed over the course of the study, we constructed a variable for study period dichotomized by median date of surgery. By individually entering interaction terms with time period into the model, the potential for a change in effect of these variables across the two time periods was tested. The possibility of a difference in effect of the variables between men and women was similarly investigated. Those interaction terms that were statistically significant were retained in the final model.
To account for hierarchical clustering of predictors, multilevel effect models with random intercepts were constructed. A four-level model was initially explored, analyzing SVG outcome with random intercepts for patient, surgeon, hospital and multi-segmented SVGs (i.e., skip and Y-grafts) considered as single grafts. Because random intercepts on surgeon, hospital and multi-segmented SVGs were not significant, the analysis thereafter included clustering on patient alone. Logistic regression was employed using variables that were biologically plausible, previously identified as affecting the outcome or had a P value <0.2 in univariate analysis. The Akaike Information Criterion (AIC) was used for model comparison. All analyses were performed using Statat/MP 10.0 (College Station, TX, USA).
Results
Patient characteristics
Data on 627 SVGs, implanted in 291 patients by 17 different surgeons at 4 different clinical sites were used for the present analyses. The baseline and operative characteristics of these subjects (Table I) were comparable to patients undergoing isolaled CABG surgery in the United States as reported in the STS National Database.
Table I.
Baseline characteristics.
| Variable | RIGOR study (N.=291) |
2007 STS database (N.=154,188) |
|---|---|---|
| Age (median, IQR) | 63.0 (56.0–70.5) | 65.0 (57.0–73.0) |
| Male (%) | 78.7 | 73.0 |
| Caucasian race (%) | 86.9 | 85.1 |
| Body mass index (%) | ||
| <18.5 kg/m2 | 1.0 | 0.8 |
| >30.0 kg/m2 | 39.9 | 40.5 |
| Current smoker (%) | 24.0 | 24.7 |
| Former smoker (%) | 69.1 | NA |
| Diabetes mellitus (%) | 35.4 | 39.0 |
| Dyslipidemia (%) | 84.5 | 80.6 |
| Hypertension (%) | 85.2 | 82.7 |
| Congestive heart failure (%) | 12.4 | 13.2 |
| Peripheral arterial disease (%) | 11.7 | 15.1 |
| Cerebrovascular disease (%) | 8.6 | 13.8 |
| Myocardial infarction within 7 days (%) | 21.7 | 24.5 |
| Previous percutaneous coronary intervention (%) | 20.6 | 23.5 |
| Preoperative creatinine (median mg/dL, IQR) | 1.0(0.8–1.1) | 1.0(0.9–1.2) |
| Extent of coronary artery disease (%) | ||
| 3 vessel disease (>50% stenoses) | 83.2 | 76.0 |
| Left main (>50% stenosis) | 39.2 | 30.6 |
| Surgery status (%) | ||
| Elective | 38.2 | 46.4 |
| Urgent | 60.8 | 48.7 |
| Emergent | 1.0 | 4.6 |
| First-time CABG (%) | 100.0 | 96.1 |
| Isolated CABG (%) | 91.7 | 100.0 |
| Cardiopulomary bypass (%) | 97.6 | 79.6 |
| Patients with internal mammary artery graft (%) | 96.9 | 93.8 |
| Number of total grafts per patient (%) | ||
| 1 | 1.0 | 4.6 |
| 2 | 18.9 | 15.7 |
| 3 | 45.0 | 36.1 |
| 4 | 25.1 | 30.2 |
| ≥5 | 10.0 | 13.2 |
| Discharge medications (%) | ||
| Aspirin | 100 | 93.8 |
| Other antiplatelet agent | 10.3 | 28.8 |
| Oral anticoagulation | 10.0 | 9.1 |
| ACE inhibitor | 50.9 | 42.8 |
| Beta-blocker | 98.6 | 90.1 |
| Lipid lowering agent | 96.2 | 88.3 |
IQR: interquanile range; CABG: coronary artery bypass graft surgery; ACE: angiorensin converting enzyme.
SVG patency
SVG patency was assessed a median of 189 days after surgery (25th and 75th percentiles 182 and 202 days, respectively) in 276 patients (601 SVGs) by CTA (Figure 1) and in 15 patients (26 SVGs) by invasive coronary angiography. SVG failure in the first 6 months after CABG surgery was essentially an all-or-Done phenomenon (Figure 2A) with 485 (77.3%) SVGs patent and 126 (20.1%) totally occluded. Over 80% of SVGs classified as patent contained no or only minor luminal irregularities (<30% stenoses). Only 16 (2.5%) SVGs contained high-grade (76–99%) stenoses that would be expected to impede blood flow, consistent with the concept that thrombosis, not atherosclerosis or neointimal hyperplasia, is the predominant cause of early SVG failure. In 210 patients with implantation of ≥2 SVGs, the proportion of occluded SVGs within individual patients was uniformly distributed (Figure 1B), suggesting that graft and/or target vessel-specific factors appear to be important determinants of graft patency.
Figure 1.
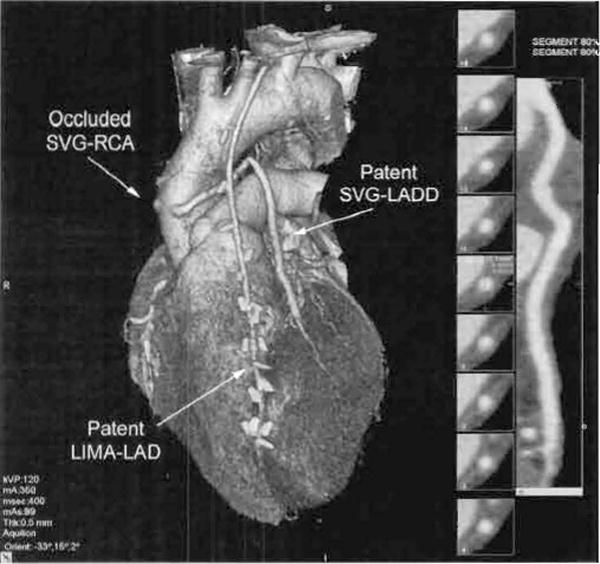
Representative CTA (0.5 mm, 64 detector rmv) reconstruction demonstrating the appearance of a patent SVG to the left anterior descending diagonal coronary artery (SVG-LADD), a patent left internal mammary graft to the left anterior descending coronary artery (LIMA-LAD) and an occluded SVG to the right coronary artery (SVG-RCA) The 3-dimensional whole-heart reconstruction of the whole heart is shown at left and the 2-dimensional reconstruction of the SVG-LADD is shown at right.
Figure 2.
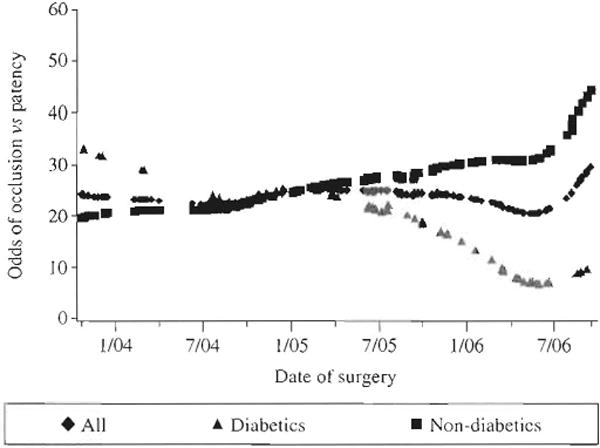
Six-month SVG outcome. (A) Patency status of 611 SVGs implanted in 291 patients. (B) Proportion of occluded SVGs per patient in 210 patients with implantation of ≥2 SVGs.
Predictors of SVG occlusion
Univariate analysis was performed for the outcome of odds of SVG occlusion versus patency. Potential risk factors for SVG occlusion that were investigated included patient-specific demographic and clinical factors (Table II), operative/perioperative factors (Table III) and graft/target vessel-specific factors (Table IV). SVG occlusion was more likely to occur in females (OR 2.08, P=0.03), Hispanics (OR 4.83, P=0.05) and in patients with current tobacco use (OR 2.09, P=0.03). Unadjusted analysis yielded no significant associations between the odds of early SVG occlusion and use of modern pharmacologic therapies such as statins or thienopyridine antiplatelet agents, use of cardiopulmonary bypass, administration of blood products or hemostatic agents such as aprotinin or with method of vein graft harvest. Several anatomic factors, however, did associate with SVG occlusion, including a target vessel diameter ≤1.5 mm (OR 2.78, P=0.001), increasing SVG-target vessel diameter ratio (OR 1.66, P=0.02) and calcification in the target vessel at the site of the distal anastomosis (OR 2.15, P=0.03). Compared to SVGs with single distal anastomosis, skip-grafts were associated with a lower odds of occlusion (OR 0.40, P=0.03).
Table II.
Univariate analysis of patient-specific factors associated with odd of SVG occlusion versus patency.
| Variable | Odds of occlusion | P value |
|---|---|---|
| Gender† | ||
| Male | Reference | |
| Female | 2.08 | 0.04 |
| Age* | 0.99 | 0.59 |
| Race† | ||
| White | Reference | |
| African-American | 2.16 | 0.09 |
| Asian | 2.63 | 0.28 |
| Native-American | 12.82 | 0.14 |
| Ethnicity† | ||
| Non-Hispanic | Reference | |
| Hispanic | 4.83 | 0.05 |
| Operative urgency | ||
| Elective | Reference | |
| Urgent | 0.87 | 0.65 |
| Emergent | 2.27 | 0.55 |
| Body mass index, kg/m2* | 0.998 | 0.93 |
| EuroSCORE* | 1.05 | 0.42 |
| Hypertension† | 0.55 | 0.12 |
| Diabetes mellitus† | 0.61 | 0.12 |
| Hemoglobin A1C, %* | 0.98 | 0.88 |
| Hyperlipidemia | 0.62 | 0.22 |
| Tobacco use† | ||
| Current use | 2.09 | 0.03 |
| Former use | 1.90 | 0.06 |
| Peripheral arterial disease | 1.32 | 0.53 |
| Cerebrovascular disease | 0.76 | 0.63 |
| Atrial fibrillation | 1.07 | 0.93 |
| Chronic obstructive pulmonary disease | 0.82 | 0.73 |
| Congestive heart failure | 0.85 | 0.73 |
| Preoperative ejection fraction | ||
| ≥50% | Reference | |
| 30–50% | 0.85 | 0.61 |
| <30% | 0.78 | 0.67 |
| Creatinine, mg/dL* | 1.85 | 0.40 |
| Prior myocardial infarction | 1.42 | 0.24 |
| Myocardial infarction <90 days | 1.37 | 0.33 |
| Prior percutaneous coronary intervention | 0.88 | 0.73 |
| Left main stenosis >50% | 0.92 | 0.77 |
| Number of coronary arteries with ≥50% stenosis* | 0.95 | 0.90 |
Odds of occlusion for every variable unit increase
Included in rile multivariate analysis.
Table III.
Univariate analysis of operative/perioperative factors associated with odd of SVG occlusion versus patency.
| Variable | Odds of occlusion | P value |
|---|---|---|
| Date of surgery* | 1.00 | 0.77 |
| Cardiopulmonary bypass | ||
| On-pump | Reference | |
| Off-pump | 2.99 | 0.41 |
| Cardiopulmonary bypass rime, minutes* | 1.006 | 0.30 |
| Protamine use | 1.55 | 0.75 |
| Aprotinin use | 0.88 | 0.75 |
| Aminocaproic acid use | 1.11 | 0.78 |
| Cryoglobulin use | 1.39 | 0.75 |
| Fresh frozen plasma use | 1.39 | 0.32 |
| Red blood cell transfusion† | 1.63 | 0.11 |
| Units of red cells transfused* | 0.96 | 0.67 |
| Platelet transfusion | 1.04 | 0.91 |
| Medications at discharge | ||
| Thienopyridine | 1.30 | 0.31 |
| Warfarin | 1.03 | 0.97 |
| Betablocker | 1.37 | 0.87 |
| Lipid lowering therapy | 0.60 | 0.54 |
| ACE/ARB | 1.11 | 0.71 |
ACF: angiotensin convening enzyme; ARB: angiotensin receptor blocker.
Odds of occlusion for every variable unit increase;
included in the mulrivariate analysis.
Table IV.
Univariate analysis of graft/target/vessel-specific factors associated with odd of SVG occlusion versus patency
| Variable | Odds of occlusion | P value |
|---|---|---|
| Number of implanted SVGs per patient* | 1.04 | 0.76 |
| SVG type† | ||
| Single distal anastomosis | Reference | |
| Multiple distal anastomosis (skip-graft† | 0.40 | 0.03 |
| Y-graft† | 3–05 | 1.11 |
| Target vessel | ||
| Left anterior descending artery | Reference | |
| Left anterior descending diagonal | 1.25 | 0.77 |
| Ramus intermedius | 1.25 | 0.78 |
| Circumflex obtuse marginal artery | 0.46 | 0.30 |
| Right coronary artery | 0.55 | 0.45 |
| Posterior descending artery (right or left) | 0.58 | 0.49 |
| Posterolateral branch (right or left) | 0.55 | 0.58 |
| Percent proximal native artery stenosis* | 1.005 | 0.51 |
| Target vessel diameter ≤1.5 mm† | 2.78 | 0.001 |
| SVG diameter, mm* | 0.99 | 0.95 |
| SVG-target vessel diameter ratio*† | 1.66 | 0.02 |
| Endoscopic SVG harvest | 0.75 | 0.32 |
| SVG quality at implantation† | ||
| Normal | Reference | |
| Thin or aneurysmal | 1.87 | 0.17 |
| Thickened or sclerotic | 0.62 | 0.34 |
| Aortic anastomosis clamp technique | ||
| Side-biting clamp† | Reference | |
| Cross-clamp | 0.57 | 0.06 |
| Distal anastomosis type† | ||
| End-to-side | Reference | |
| Side-to-side | 0.43 | 0.10 |
| Target vessel quality at distal anastomosis† | ||
| No intimai disease | Reference | |
| Mild intimai disease | 0.88 | 0.73 |
| Moderate intimai disease | 0.63 | 0.26 |
| Severe intimai disease | 1.40 | 0.41 |
| Calcification at distal anastomosis† | 2.15 | 0.03 |
| Target vessel endarterectomy† | 3.62 | 0.15 |
Abbreviations: SVG, saphenous vein graft.
Odds of occlusion for every variable unit increase
included in the multivariate analysis.
Variables with P values <0.2 in the univariate analysis were included in the initial multivariate model. Age was also included in the model, given its established role in the pathogenesis of atherothrombosis, as was date of surgery given that surgeons were provided results of the CTA and could have modified their practice. Serial testing for effect modification of variables by study time period was conducted, after dividing into two groups by median date of surgery. The interaction term between the effect of diabetes and date of surgery was highly significant (P=0.01) and suggested an effect of diabetes on SVG occlusion that differed between the first and second half of the study. Because lowess smoothing functions revealed that the odds of SVG occlusion decreased for diabetics during the course of the RIGOR study (Figure 3), a time-dependent interaction term was therefore included in the model. The final random intercept model for the odds of SVG occlusion versus patency (Table V) revealed the strongest predictors of SVG occlusion to be a target vessel diameter ≤1.5 mm (OR 2.37, P=0.003) and female gender (OR 2.46, P=0.01). Calcification in the target artery at the site of the distal anastomosis (OR 2.09, P=0.05) and former tobacco use (OR 1.87, P=0.06) trended toward positive associations and the use of skip-grafts (OR 0.48, P=0.08) trended toward a negative association with SVG occlusion, respectively.
Figure 3.
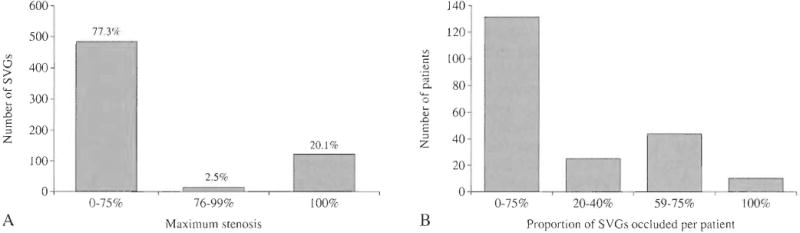
Odds of 6-month SVG occlusion over time in diabetic versus non-diabetic patients.
Table V.
Final multivariate random intercept model for odds of SVG occlusion versus patency.
| Variable | Odds of occlusion | P value | 95% Confidence interval | |
|---|---|---|---|---|
| Target vessel size ≤1.5 mm | 2.37 | 0.003 | 1.34 | 4.21 |
| Female gender | 2.46 | 0.01 | 1.24 | 4.93 |
| Diabetes × Date of operation* | 0.19 | 0.01 | 0.05 | 0.70 |
| Calcification at distal anastomosis | 2.09 | 0.05 | 1.00 | 4.39 |
| Former tobacco use | 1.87 | 0.06 | 1.00 | 3.61 |
| Multiple distal anastomoses (skip-graft) | 0.48 | 0.08 | 0.21 | 1.09 |
| Date of operation* | 1.62 | 0.18 | 0.81 | 3.26 |
| Y-graft | 2.22 | 0.27 | 0.55 | 9.01 |
| Diabetes | 1.06 | 0.89 | 0.47 | 2.35 |
Date of operation: August 12, 2005–September 18, 2006 versus October 24, 2003 versus August 11, 2005.
Though not mandated in the study protocol, measurement of graft blood flow was performed in 164 panems (354 SVGs) at the discretion of the surgeon. The average mean intraoperative blood flow was modestly lower in SVGs that occluded within 6 months compared to those that remained patent (60 versus 89 mL/min, P<0.001). Multivariate analysis of this patiem subgroup revealed that mean flow, when analyzed as a continuous variable, significantly correlated with SVG occlusion (OR 0.986, 95% CI 0.980–1.00, P=0006) along with target vessel diameter ≤1.5 mm (OR 2.63, 95% CI 1.27–5.47, P=0.009). This translated into a 14% increase in the odds of SVG occlusion for every 10 mL/min decrease in flow. However, when analyzed as a clinically-relevant dichotomous variable, mean blood flow <40 mL/min was not significantly associated with an increased risk of early SVG occlusion (adjusted OR 1.86, 95% CI 0.919–3.69, P=0.08) in comrast to target vessel diameter ≤1.5 mm (adjusted OR 3.07, 95% CI 1.50–6.27, P=0.002).
Discussion
The major findings of this study are: 1) The rate of SVG failure within the first 6 months after CABG surgery remains substantial; 2) The predominantly all-or-none-pattern of occlusion is consistent with thrombosis, rather than atherosclerosis, being the predominate underlying cause of early SVG failure, and; 3) Native target vessel diameter ≤1.5 mm, female gender and lower intraoperative graft blood flow were significantly associated with early SVG thrombosis.
In the RIGOR study population, target artery diameter ≤1.5 mm was a strong predictor of SVG occlusion. This has been a consistent finding in several previous studies and is thought to be due to impaired distal run-off compared to larger target vessels7–12, 14, 15 In contrast to previous reports7, 9, 11, 14, 15 we also found that female gender was associated with more than twice the odds of SVG occlusion. Importantly, this effect of gender was independent of body mass index and target vessel size. Though women in our study were more likely to be diabetic, dyslipidemic and have peripheral arterial disease, presence of these risk factors also failed to explain gender differences in SVG patency. Our data mirror gender differences in outcomes following acute myocardial infarction and percutaneous coronary interventions where women have a higher risk of death than men18, 19 and suggest that there may be unknown factors that predispose women to SVG thrombosis early after CABG surgery which merits further investigation.
Though is not routinely performed by many surgeons, quantification of mean blood flow in bypass grafts at the time of implantation has been shown to be useful at detecting technical errors that can potentially be corrected during surgery.20 Several prior studies found that low SVG blood flow, especially <40 mL/min, is associated with an increased risk of SVG failure.16, 21, 22 In a recent prospective angiographic study, mean blood flow in both SVGs and arterial grafts correlated so strongly with target vessel location and diameter that the authors concluded that measuring this parameter alone was insufficient to predict graft outcome.23 While not mandated in the RIGOR study protocol, measurement of SVG blood flow was performed in slightly more than half of enrolled patients at the discretion of the surgeon. Although this introduces an obvious potential for bias that limits interpretation of the data, we did nonetheless find that both mean intra-operative graft blood flow and target vessel diameter were independent predictors of SVG outcome. Further studies are needed to determine what additional therapy other than surgical revision might improve the outcome of SVGs with low blood flow.
A unique predictor of SVG occlusion in our study population was the presence of calcium at the distal anastomosis, assessed by the surgeon at the time of graft implantation. We are unaware of any previous report linking coronary calcification and SVG patency outcome. It is possible that coronary calcification, like small target vessel diameter, might impair SVG blood flow. Alternatively, calcification could predispose to technical complications during construction of the distal anastomosis. Because CTA is exceptional at localizing and quantifying coronary artery calcification, some have advocated its use prior to surgery to guide revascularization.24
An intriguing finding of our study was the time-dependent association between diabetes and six-month graft occlusion rate. While diabetes is associated with worse outcome after both surgical and percutaneous revascularization, prior studies have failed to identify a specific association between diabetes and early SVG failure.9, 11, 25 As risk factors for SVG occlusion remained constant over time in our analysis, the improved patency over time in diabetics may reflect the recognition by referring cardiologists of a better outcome in diabetics with CABG compared to percutaneous coronary interventions and subsequent referral bias. It is also possible that institution of protocols to achieve tighter glycemic control in the perioperative period improved graft outcomes in diabetic patients over time in our study cohort. Several studies published after enrollment in the RIGOR study began in 2003 demonstrated improve clinical outcomes in diabetics maintained on insulin infusions compared to standard intermittent insulin therapy.26, 27 Most intriguing was the observation that perioperative insulin infusions were associated with significantly improved survival, fewer ischemic events and reduced angina burden over the ensuing two years of follow up, raising the possibility that tighter glycemic control and/or antiglycemic medications may improve SVG patency.27
We failed to find a correlation between the method of vein harvest and six-month SVG occlusion. Recent analysis of outcome data from the large PREVENT 4 trial found that endoscopic harvesting of vein segments increased both the odds of SVG occlusion and failure (containing a ≥75% diameter stenosis) 12–18 months after surgery approximately 1.5-fold (P<0.001).28 Notably, however, individual SVG outcome was not adjusted for target vessel diameter in this study but only for the quality of the worst grafted target vessel in that patient (graded good, fair or poor by the surgeon at the time of implantation). The study also found that endoscopic vein harvesting was associated with a modestly higher MACE rate that became manifest between one and three years after surgery (HR 1.22, P=0.04). This suggests that endoscopic harvesting might be more apt to promote accelerated graft atherosclerosis than thrombosis, which would be expected to have a greater impact on late (>1 year) rather than early (<6 month) SVG patency.
A novel aspect of the RIGOR study was the use of CTA to assess the SVG patency after CABG surgery. SVGs are particularly well-suited for imaging by CTA, even by early generation scanners, given their large lumenal diameters, limited motion during the cardiac cycle and paucity of calcification. In a recent meta-analysis of comparative angiographic studies utilizing 8-, 16- and 64-slice scanning protocols, the pooled sensitivity anel specificity was 85.2% and 97.2%, respectively, for detecting a significant SVG stenosis and 99.1% and 100%, respectively, for detecting total SVG occlusion.29 Given that SVG failure in the first six months after CABG surgery is essentially an all-or-none phenomenon and the high sensitivity and specificity for distinguishing patent from occluded SVGs, CTA is an ideal non-invasive method for assessing SVG patency in clinical trials testing therapeutic strategies to improve outcome after CABG surgery.
Conclusions
Small target vessel diameter, female gender and low mean graft blood flow are significant risk factors for SVG thrombosis within six months of CABG surgery in patients on postoperative aspirin therapy. These first two factors are readily identifiable preoperatively and may be useful at guiding revascularization strategies in selected patients.
Acknowledgments
Funding. This study was supported by the Johns Hopkins Institute for Clinical and Translational Research (funded by UL1 RR025005 from the Narional Cemer for Research Resources, National Institutes of Health. Bethesda, MD, USA), The Medicines Company, Parsippany, NJ and a grant from the Flight Arrendanr Ivledical Resea rch Foundation. Miami, FLA. USA (ro JJR).
References
- 1.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein grafl failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 2.Widimsky P, Straka Z, Slros P, Jirasek K, Dvorak J, Votava J, et al. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of lhe PRAGUE-4 trial. Circulalion. 2004;110:3418–23. doi: 10.1161/01.CIR.0000148139.79580.36. [DOI] [PubMed] [Google Scholar]
- 3.FilzGibbons GM, Kafka HP, Leach AJ, Keon WJ, Hoopel GD, Burton JR. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperalion in 1,388 palienrs during 25 years. J Am Coll Cardiol. 1996;28:616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 4.Halabi AR, Alexander JH, Shaw LK, Lorenz TJ, Liao L, Kong DF, et al. Relation of early saphenous vein graft failure 10 outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96:1254–9. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 5.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease. Circulalion. 1998;97:916–3l. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 6.Antiplatelet Trialists Collaboration. Collaboralive overview of ranclomised trials of antiplatelet therapy-lI:Mainlenance of vascular graft or arterial palency by antiplatelet therapy. BMJ. 1994;308:168. [PMC free article] [PubMed] [Google Scholar]
- 7.Bjork VO, Ekestrom S, Henze A, Ivert T, Landou C. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–2l. doi: 10.3109/14017438109101020. [DOI] [PubMed] [Google Scholar]
- 8.Roth JA, Cukingnan RA, Brown BG, Gocka E, Carey JS. Factors influencing patency of saphenous vein grafts. Ann Thorac Surg. 1979;28:176–83. doi: 10.1016/s0003-4975(10)63777-0. [DOI] [PubMed] [Google Scholar]
- 9.Cataldo G, Braga M, Pirotta N, Lavezzari M, Rovelli F, Marubini E. Factors influencing 1-year patency of coronary artery saphenous vein grafts. Studio Indobufene nel Bypass Aortocororico (SINBA) Circulation. 1993;88:1193–118. [PubMed] [Google Scholar]
- 10.Higginhotham M, Hunt D, Stuckey J, Sloman G. Prospective angiographic assessment of factors affecting early patency of saphenous vein-coronary artery bypass grafts. Aust N Z J Med. 1980;10:295–99. doi: 10.1111/j.1445-5994.1980.tb04073.x. [DOI] [PubMed] [Google Scholar]
- 11.Paz MA, Lupon J, Bosch X, Pomar JL, Sanz G. Predictors of early saphenous vein aortocorona bypass graft occlusion. The GESIC Study Group. Ann Thorac Surg. 1993;56:1101–6. doi: 10.1016/0003-4975(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 12.Cho KR, Kim JS, Choi JS, Kim KB. Serial angiographic follow-up of grafts one year and five years after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:511–6. doi: 10.1016/j.ejcts.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Kim KB, Cho KR, Jeong DS. Miclterm angiographic follow-up after off-pump coronary artery bypass: serial comparison using early, 1-year, and 5-year postoperative angiugrams. J Thorac Cardiovasc Surg. 2008;135:300–7. doi: 10.1016/j.jtcvs.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O’Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–5. doi: 10.1161/01.cir.93.4.652. [DOI] [PubMed] [Google Scholar]
- 15.Shah PJ, Gordon I, Fulkr J, Seevanayagam S, Rosalion A, Tatoulis J, et al. Factors affecting saphenous vein graft patency: clinical and angiographic srudy in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–7. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 16.Chesebro JH, Clements JP, Fuster V, Elveback LR, Smith HC, Bardsley WT, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med. 1982;307:73–8. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman TJ, Segal JB, Schulman SP, Shapiro EP, Kickier TS, Prechel MM, et al. Effect of anti-platelet factor-4/heparin antibody induction on early saphenous vein graft occlusion after coronary artery bypass surgery. J Thromb Haemost. 2009;7:1457–64. doi: 10.1111/j.1538-7836.2009.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de WF, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–32. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 19.Anderson HV, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, et al. A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) J Am Coll Cardiol. 2002;59:1096–103. doi: 10.1016/s0735-1097(02)01733-3. [DOI] [PubMed] [Google Scholar]
- 20.Leong DK, Ashok V, Nishkantha A, Shan YH, Sim EK. Transit-time flow measurement is essential in coronary artery bypass grafting. Ann Thorac Surg. 2005;79:854–7. doi: 10.1016/j.athoracsur.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Marco JD, Barner HB, Kaiser GC, Codd JE, Mudd JG, Willman V. Operative flow measurements and coronary bypass graft patency. J Thorac Carcliovasc Surg. 1976:71545–7. [PubMed] [Google Scholar]
- 22.Cetin SM, Massoudy P, Thielmann M, Yildirim C, Schmermund A, Erbel R, et al. Inraoperative coronary graft flow determination–does it have a prognostic value for midterm graft patency? Eur J Med Res. 2006:11267–72. [PubMed] [Google Scholar]
- 23.Hirotani T, Kameda T, Shirota S, Nakao Y. An evaluation of the intraoperative transit time measurements of coronary bypass flow. Eur J Cardiothorac Surg. 2001;19:848–5. 2. doi: 10.1016/s1010-7940(01)00700-x. [DOI] [PubMed] [Google Scholar]
- 24.Caimmi PP, Fossaceca R, Lanfranchi M, Kapetanakis El, Verde A, Panella A, et al. Cardiac angio-CT Scan for planning MIDCAB. Heart Surg Forum. 2004;7:E113–E6. doi: 10.1532/HSF98.200328101. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz L, Kip KE, Frye RL, Alderman EL, Schaff HV, Detre KM. Coronary bypass graft patency in patients with diabetes in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2652–8. doi: 10.1161/01.cir.0000038885.94771.43. [DOI] [PubMed] [Google Scholar]
- 26.Furnary AP, Gao G, Grunkermeier Gl, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortaliry in patients with diabetes undergoing coronary artery bypass grafting. J Tilorac Cardiovasc Surg. 2003;125:1007–21. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 27.Lazar HL, Chipkin SR, Firzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves rerioperative outcomes and decreases recurrent ischemic evems. Circulation. 2004;109:J497–502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 28.Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009:561235–44. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 29.Jones CM, Athanasiou T, Dunne I, Kirby J, Aziz O, Haq A, et al. Mutri-detector computed tomography in coronary artery bypass graft assessment: a meta-analysis. Ann Thorac Surg. 2007;83:341–8. doi: 10.1016/j.athoracsur.2006.08.018. [DOI] [PubMed] [Google Scholar]


