Abstract
Background:
The optimal postoperative analgesia after primary total hip arthroplasty remains in question. This randomized, double-blind, placebo-controlled study compared the use of patient-controlled epidural analgesia (PCEA) with use of a multimodal pain regimen including periarticular injection (PAI). We hypothesized that PAI would lead to earlier readiness for discharge, decreased opioid consumption, and lower pain scores.
Methods:
Forty-one patients received PAI, and forty-three patients received PCEA. Preoperatively, both groups were administered dexamethasone (6 mg, orally). The PAI group received a clonidine patch and sustained-release oxycodone (10 mg), while the PCEA group had placebo. Both groups received combined spinal-epidural anesthesia and used an epidural pain pump postoperatively; the PAI group had normal saline solution, while the PCEA group had bupivacaine and hydromorphone. The primary outcome, readiness for discharge, required the discontinuation of the epidural, a pain score of <4 (numeric rating scale) without parenteral narcotics, normal eating, minimal nausea, urination without a catheter, a dry surgical wound, no acute medical problems, and the ability to independently transfer and walk 12.2 m (40 ft).
Results:
The mean time to readiness for discharge (and standard deviation) was 2.4 ± 0.7 days (PAI) compared with 2.3 ± 0.8 days (PCEA) (p = 0.86). The mean length of stay was 3.0 ± 0.8 days (PAI) compared with 3.1 ± 0.7 days (PCEA) (p = 0.46). A significant mean difference in pain score of 0.74 with ambulation (p = 0.01; 95% confidence interval [CI], 0.18 to 1.31) and 0.80 during physical therapy (p = 0.03; 95% CI, 0.09 to 1.51) favored the PCEA group. The mean opioid consumption (oral morphine equivalents in milligrams) was significantly higher in the PAI group on postoperative day 0 (43 ± 21 compared with 28 ± 23; p = 0.002) and postoperative days 0 through 2 (136 ± 59 compared with 90 ± 79; p = 0.004). Opioid-Related Symptom Distress Scale (ORSDS) composite scores for severity and bothersomeness as well as scores for nausea, vomiting, and itchiness were significantly higher in the PCEA group (p < 0.05). Quality of Recovery-40 scores and patient satisfaction were similar.
Conclusions:
PAI did not decrease the time to discharge and was associated with higher pain scores and greater opioid consumption but lower ORSDS scores compared with PCEA. The choice for analgesic regimen may depend on a particular patient’s threshold for pain and the potential side effects.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
By 2030, the demand for primary hip arthroplasty is estimated to increase by 174%, to 572,000 annually in the U.S.1. Presently, the need for adequate postoperative pain control continues to be an issue2,3.
Pain control and comfort level are linked to earlier mobilization, better range of motion, decreased length of hospital stay, and lower medical cost4-6. Consequently, much interest in an effective analgesic regimen while minimizing side effects has been generated. Patient-controlled epidural analgesia (PCEA) has been used as an effective form of postoperative analgesia but can be associated with urinary retention, hypotension, pruritus, and motor block7-9. It may also lead to a delay in hospital discharge due to nausea and vomiting. This has led to other modes of pain management, such as the use of periarticular injection (PAI) of local anesthetic, morphine, corticosteroids, and, at times, nonsteroidal anti-inflammatory drugs (NSAIDs) along with a multimodal pain regimen10-12. The goal is to target different pain pathways in a synergistic fashion by using specific agents with analgesic and/or anti-inflammatory properties. A preemptive approach is also achieved by giving medications preoperatively.
In view of the controversy associated with the use of PCEA and the potential advantage of PAI in reducing side effects, we undertook this double-blind, placebo-controlled study comparing the two techniques. We hypothesized that PAI would lead to faster recovery with earlier hospital discharge, lower pain scores, and decreased opioid utilization.
Materials and Methods
After institutional review board approval and informed consent were obtained, ninety patients were enrolled in this double-blind, placebo-controlled study (from February 2012 to January 2013) at a university-affiliated orthopaedic teaching hospital. The study was registered with ClinicalTrials.gov, identifier NCT01658072. Patients were consecutively randomized (1:1 allocation, parallel trial design) to either the PAI or the PCEA group. The group to which patients were assigned was indicated in numbered sealed envelopes, which were prepared by an independent researcher and opened by the anesthesiologist assigned to the procedure after enrollment.
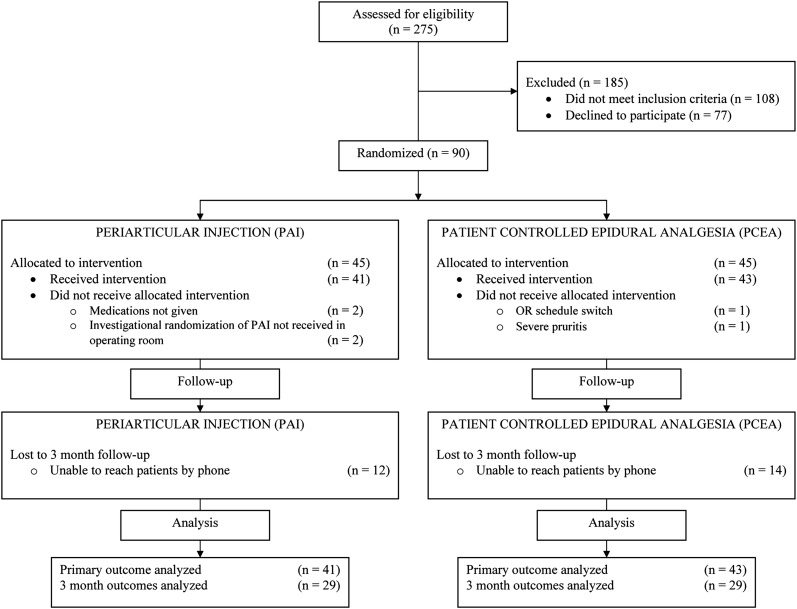
Patients who were fifty to eighty years old were eligible if they had a diagnosis of osteoarthritis and were scheduled for primary unilateral total hip replacement. Surgery was performed by one of five surgeons using a standard posterolateral approach and without the use of cement. Exclusion criteria included an allergy or intolerance to one of the study medications, an American Society of Anesthesiologists (ASA) physical status classification score of IV13, hepatic or renal failure, and chronic opioid use (more than three months) (Fig. 1). The characteristics of the patients in the two groups were similar (Table I).
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram showing the flow of patients through the protocol.
TABLE I.
Demographic Characteristics
| Periarticular Injection | Patient-Controlled Epidural Analgesia | P Value | |
| Patients (no.) | 41 | 43 | |
| Mean age ± SD (yr) | 63.7 ± 8.5 | 64.8 ± 7.1 | 0.49 |
| Male sex (no. [%]) | 20 (49%) | 24 (56%) | 0.52 |
| Mean BMI ± SD (kg/m2) | 28.0 ± 5.4 | 27.9 ± 6.2 | 0.90 |
| BMI (no. [%]) | 0.43 | ||
| Normal (<25 kg/m2) | 14 (34%) | 11 (26%) | |
| Overweight (25 to <30 kg/m2) | 13 (32%) | 21 (49%) | |
| Obese (30 to <40 kg/m2) | 13 (32%) | 10 (23%) | |
| Morbidly obese (≥40 kg/m2) | 1 (2%) | 1 (2%) | |
| Race (no. [%]) | 0.68 | ||
| White | 37 (90%) | 38 (88%) | |
| Black | 3 (7%) | 2 (5%) | |
| Asian | 0 (0%) | 1 (2%) | |
| Not specified | 0 (0%) | 2 (5%) | |
| Other | 1 (3%) | 0 (0%) | |
| ASA classification (no. [%]) | 0.61 | ||
| I | 9 (22%) | 6 (14%) | |
| II | 30 (73%) | 34 (79%) | |
| III | 2 (5%) | 3 (7%) | |
| Indication for surgery (no.) | |||
| Osteoarthritis | 40 | 43 | |
| Osteonecrosis of head and neck of femur | 1 | 0 |
All patients received dexamethasone (6 mg, orally) thirty to sixty minutes before transfer to the operating room as part of our standard antiemetic regimen. In addition, the PAI group received sustained-release oxycodone (10 mg) and a clonidine patch (100 mg/24 hr). The PCEA group received a placebo sustained-release oxycodone pill and a placebo clonidine patch. Every patient also had combined spinal-epidural anesthesia with 0.5% bupivacaine (10 mg, 12.5 mg, or 15 mg). Both groups received intraoperative intravenous sedation with midazolam and propofol and 15 mg of ketorolac intravenously.
At the end of surgery, a surgeon who had received video training performed the local infiltration in the PAI group; this consisted of a deep injection into the anterior and posterior capsule, the periosteum, the gluteus maximus, and the abductor muscles and fascia lata. The deep injection consisted of 0.5% bupivacaine with epinephrine, 30 mL; morphine, 1 mL of 8 mg/mL; methylprednisolone, 1 mL of 40 mg/mL; and cefazolin, 500 mg in 10 mL in normal saline solution, 42 mL. Epinephrine was used to prolong the action of the local anesthetic by decreasing the reabsorption rate. In addition, 40 mL of 0.25% bupivacaine was injected into subcutaneous tissue prior to wound closure.
On arrival in the postanesthesia care unit, both groups received urinary catheters, which were subsequently removed at the same time as the epidural catheters. Postoperative epidural with patient-controlled infusion pump (0.06% bupivacaine and hydromorphone, 10 μg/mL) was started at 2 mL/hr with a 4 mL bolus with 10-min lockout and with a 20 mL hourly maximum in the PCEA group. Using a pump with the same settings, the PAI group received epidural infusion with normal saline solution. The basal rate was reduced to 0 mL/hr at 6:59 a.m. on the first postoperative day, and the epidural catheter was removed at noon. In addition to an epidural for patient-controlled anesthesia, both the PAI and the PCEA groups received acetaminophen, 1000 mg every eight hours; ketorolac, 30 mg (15 mg if sixty-five years of age or older) every six hours intravenously until postoperative day 1, for a total of four doses; oxycodone every three hours as needed (5 mg for mild pain; 10 mg for moderate to severe pain); and meloxicam once the ketorolac was discontinued. The PAI group also received sustained-release oxycodone, 10 mg every twelve hours for forty-eight hours, for a total of four doses; the PCEA group had the placebo.
Assessments were made using various questionnaires including patient satisfaction, Quality of Recovery (QoR)-4014, and Opioid-Related Symptom Distress Scale (ORSDS)15,16 on postoperative days 1, 2, and 3 as well as at three months. During the first three postoperative days, patients were interviewed twice daily by a research assistant to assess readiness for discharge (the primary outcome), pain, activity, and analgesic use. Patients rated their preoperative pain at rest and with movement on a numeric rating scale (NRS), with 0 representing no pain and 10, the worst pain imaginable. They were contacted by telephone at three months to identify neuropathic pain (using the Leeds Assessment of Neuropathic Symptoms and Signs [LANSS], which was also administered before surgery17).
Discharge readiness was calculated from the time of leaving the operating room to the time the discharge criteria were met.
Patients were judged ready to be discharged when (1) the patient-controlled infusion pump (if present) was discontinued (the epidural catheter was removed at noon on postoperative day 1); (2) they were experiencing only mild pain (an NRS score of <4); (3) they had required no parenteral narcotics within the last four hours; (4) they had minimal nausea within the last four hours; and (5) they were eating normally. They were also required to have no urinary catheter, a dry surgical wound, no acute medical problems, and stable vital signs during physical therapy as well as the ability to transfer independently to and from a chair as well as from the bed to a standing position. Additional criteria for readiness for discharge included the ability to walk 12.2 m (40 ft) with only a walker or crutch, if needed.
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools18. REDCap is a secure, web-based application designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources.
Statistical Methods
The primary outcome was readiness for discharge. We hypothesized that the periarticular injection would reduce the time until patients were ready for discharge by 0.5 day. We considered a reduction of 0.5 day to be the minimal clinically important difference on the basis of preliminary data indicating a mean time to readiness for discharge after total hip replacement (and standard deviation [SD]) of 3.5 ± 1.2 days. A power analysis with two-sided hypothesis testing and α = 0.05 indicated that forty-five patients in each group were needed in order to identify the minimal clinically important difference of 0.5 day with 80% power. Continuous variables are presented as the mean (and standard deviation) and compared using a two-sample t test or Mann-Whitney-Wilcoxon rank test. Discrete variables are presented using proportions and were analyzed with a chi-square test or Fisher exact test. Cox proportional-hazards regression was performed to compare readiness for discharge while adjusting for patient demographics (sex, body mass index [BMI], age, race, and ASA classification). Linear regression analysis was conducted to compare length of stay while adjusting for the same set of patient demographics. NRS pain scores were analyzed using generalized estimating equations (GEEs), a technique that is better suited for repeated measurements of pain scores from a group of individuals19. Significance was set at a p value of <0.05.
Source of Funding
The study was funded by Hospital for Special Surgery Anesthesiology Department Research and Education Fund and the Adult Reconstruction and Joint Replacement (ARJR) Research & Innovation Committee Research Grant. The REDCap electronic data capture tools were funded by Clinical Translational Science Center (CTSC) grant UL1 TR000457-06 from the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Yan Ma’s contribution to this project was supported in part by AHRQ (Agency for Healthcare Research and Quality) grant R01 HS021734 and CTSC grant UL1 RR024996 (from the National Institutes of Health).
Results
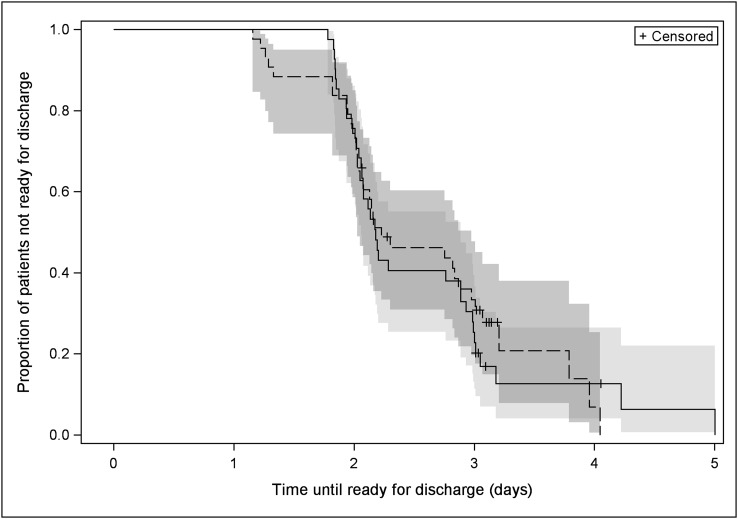
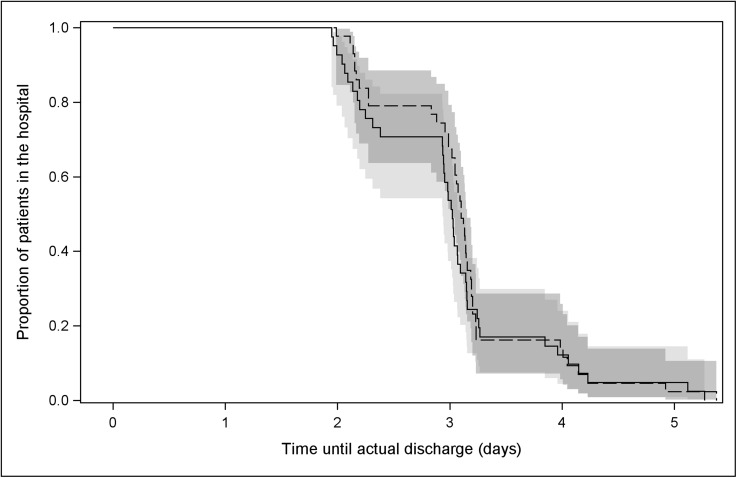
Forty-one patients in the PAI group and forty-three patients in the PCEA group received the allocated intervention. Both groups had similar demographic characteristics (Table I). The time to readiness for discharge and length of stay were the same in both groups (Figs. 2 and 3). The mean length of stay was 3.0 ± 0.8 days for the PAI group compared with 3.1 ± 0.7 days for the PCEA group (p = 0.46). Thirty-three patients in the PAI group were discharged to home and eight, to a rehabilitation center; thirty-one patients in the PCEA group were discharged to home and twelve, to a rehabilitation center. The mean time to readiness for discharge was 2.4 ± 0.7 days for the PAI group compared with 2.3 ± 0.8 days for the PCEA group (hazard ratio [HR], 1.05; 95% confidence interval [CI], 0.63 to 1.74; p = 0.86) (Table II). The difference in pain at rest between groups was significant only on postoperative day 1 (p.m., p = 0.03). An overall mean difference of 0.74 for pain with ambulation (95% CI, 0.18 to 1.31; p = 0.01) and 0.80 for pain during physical therapy (95% CI, 0.09 to 1.51; p = 0.03) favored the PCEA group (Table III).
Fig. 2.
Kaplan-Meier survival curves of time (days) to readiness for discharge. Time 0 = time out of the operating room. The solid line represents the periarticular injection (PAI) group, and the dashed line represents the patient-controlled epidural analgesia (PCEA) group.
Fig. 3.
Kaplan-Meier survival curves of time (days) to actual discharge. Time 0 = time out of the operating room. The solid line represents the periarticular injection (PAI) group, and the dashed line represents the patient-controlled epidural analgesia (PCEA) group.
TABLE II.
Readiness for Discharge and Length of Stay
| Periarticular Injection | Patient-Controlled Epidural Analgesia | P Value | |
| Readiness for discharge* (d) | 2.4 ± 0.7 | 2.3 ± 0.8 | 0.86† |
| Length of stay* (d) | 3.0 ± 0.8 | 3.1 ± 0.7 | 0.46† |
| Discharge | 0.37 | ||
| To rehabilitation center (no.) | 8 | 12 | |
| To home (no.) | 33 | 31 |
Values presented as the mean and standard deviation.
Adjusted for other variables including sex, age, BMI, race, and ASA physical status classification.
TABLE III.
Numeric Rating Scale (NRS) Pain Scores*
| Periarticular Injection | Patient-Controlled Epidural Analgesia | Overall Difference Between Groups | P Value† | |
| NRS at rest | ||||
| Preoperative | 2.7 ± 2.9 (2.0) | 2.8 ± 2.5 (2.0) | 0.52 | |
| POD 1 (a.m.) | 1.0 ± 1.5 (0) | 0.7 ± 1.4 (0) | 0.33 | |
| POD 1 (p.m.) | 1.3 ± 1.7 (0) | 0.5 ± 0.8 (0) | 0.03 | |
| POD 2 (a.m.) | 1.3 ± 1.3 (1.0) | 1.5 ± 1.9 (1.0) | 0.86 | |
| POD 2 (p.m.) | 1.2 ± 2.0 (0) | 0.9 ± 1.6 (0) | 0.47 | |
| POD 3 (a.m.) | 0.9 ± 1.3 (0) | 1.0 ± 1.4 (0) | 0.8 | |
| POD 3 (p.m.) | 1.8 ± 2.3 (1.0) | 1.0 ± 0.9 (1.0) | 0.74 | |
| 3 months | 0.6 ± 1.1 (0) | 0.6 ± 1.2 (0) | 0.66 | |
| NRS with ambulation | ||||
| Preoperative | 6.9 ± 2.4 (7.5) | 6.1 ± 2.8 (6.0) | 0.24 | |
| POD 1 (a.m.) | 3.0 ± 2.4 (3.0) | 1.5 ± 1.3 (2.0) | 0.002 | |
| POD 1 (p.m.) | 2.8 ± 2.1 (2.0) | 1.9 ± 1.7 (2.0) | 0.07 | |
| POD 2 (a.m.) | 3.1 ± 2.4 (3.0) | 2.4 ± 2.4 (2.0) | 0.13 | |
| POD 2 (p.m.) | 2.8 ± 2.4 (2.0) | 2.1 ± 2.0 (2.0) | 0.22 | |
| POD 3 (a.m.) | 2.6 ± 2.3 (2.5) | 2.2 ± 1.7 (2.0) | 0.65 | |
| POD 3 (p.m.) | 4.2 ± 3.4 (3.0) | 2.3 ± 0.8 (2.5) | 0.29 | |
| 3 months | 1.3 ± 1.4 (1.0) | 1.0 ± 1.4 (0) | 0.25 | |
| NRS during physical therapy | ||||
| POD 1 (a.m.) | 2.5 ± 2.0 (2.5) | 1.5 ± 1.4 (2.0) | 0.02 | |
| POD 1 (p.m.) | 2.5 ± 2.0 (2.0) | 1.9 ± 1.7 (2.0) | 0.18 | |
| POD 2 (a.m.) | 2.7 ± 2.3 (2.0) | 2.1 ± 2.4 (1.0) | 0.1 | |
| POD 2 (p.m.) | 2.7 ± 2.3 (2.0) | 2.0 ± 2.1 (2.0) | 0.25 | |
| POD 3 (a.m.) | 3.1 ± 2.4 (3.0) | 2.0 ± 1.9 (2.0) | 0.08 | |
| POD 3 (p.m.) | 3.8 ± 3.8 (3.0) | 2.8 ± 1.0 (2.5) | 0.9 | |
| Pain at rest | 0.0441 (0.21) | 0.83 | ||
| Pain with ambulation | 0.7445 (0.29) | 0.01 | ||
| Pain during physical therapy | 0.7995 (0.36) | 0.03 |
NRS scores are presented as the mean and standard deviation with the median in parentheses. Differences between groups are presented as the mean with the standard error in parentheses. POD = postoperative day.
P values for the NRS at the individual time points are from unadjusted comparisons using Mann-Whitney-Wilcoxon rank tests. P values for the difference between groups are from adjusted comparisons using regression analyses based on the generalized estimating equations [GEE] method (adjusted for other variables including sex, age, BMI, race, and ASA physical status classification).
GEE analysis of pain scores over time included age, race, BMI, ASA classification, and sex. Females experienced higher pain at rest (p = 0.03) and with ambulation (p = 0.01). Overweight/obese patients had lower pain scores at rest (p = 0.04) and with ambulation (p = 0.04).
The mean total opioid (epidural and oral) consumption (converted to oral morphine equivalents in milligrams20-22) was significantly higher in the PAI group on postoperative day 0 (43 ± 21 compared with 28 ± 23; p = 0.002), on postoperative day 1 (57 ± 26 compared with 29 ± 39; p = 0.0002), and for postoperative days 0 through 2 (136 ± 59 compared with 90 ± 79; p = 0.004) (Table IV). Oral opioid consumption was higher in the PAI group on postoperative days 0 and 1 (p < 0.0001). One patient from the control group (PAI) converted to intravenous patient-controlled analgesia because of severity of pain. However, the composite ORSDS scores were significantly higher in the PCEA group in terms of severity and bothersomeness (p < 0.05) (Table V). The notable symptoms included nausea (p = 0.01), vomiting (p = 0.04), and itchiness (p = 0.01). There was a higher prevalence of headache in the PCEA group. That group also had a higher likelihood of dizziness (p = 0.05). QoR-40 and LANSS scores, along with patient satisfaction, were similar between the groups (Tables V and VI).
TABLE IV.
Opioid Usage*
| Outcome | Periarticular Injection, N = 41 | Patient-Controlled Epidural Analgesia, N = 43 | P Value |
| Total oral opioid usage | |||
| POD 0 | 42 ± 20 | 11 ± 14 | <0.0001 |
| POD 1 | 57 ± 26 | 20 ± 22 | <0.0001 |
| POD 2 | 35 ± 29 | 33 ± 32 | 0.7228 |
| POD 3 | 18 ± 21 | 15 ± 17 | 0.4468 |
| Total epidural usage | |||
| POD 0 | 0 ± 0 | 17 ± 13 | <0.0001 |
| POD 1 | 0 ± 0 | 9 ± 20 | 0.0105 |
| Total oral + epidural usage | |||
| POD 0 | 43 ± 21 | 28 ± 23 | 0.002 |
| POD 1 | 57 ± 26 | 29 ± 39 | 0.0002 |
| POD 2 | 35 ± 29 | 33 ± 32 | 0.7661 |
| POD 3 | 18 ± 21 | 15 ± 17 | 0.4468 |
| POD 0-2 | 136 ± 59 | 90 ± 79 | 0.004 |
Opioid usage converted to oral morphine equivalents in milligrams. Values are presented as the mean and standard deviation. POD = postoperative day.
TABLE V.
Opioid-Related Symptom Distress Scale (ORSDS) and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Scores
| Outcome | Periarticular Injection* | Patient-Controlled Epidural Analgesia* | P Value |
| ORSDS | |||
| Composite | 0.36 ± 0.35 | 0.58 ± 0.50 | 0.03 |
| Frequency | 0.41 ± 0.37 | 0.62 ± 0.54 | 0.05 |
| Severity | 0.33 ± 0.34 | 0.51 ± 0.45 | 0.04 |
| Bothersomeness | 0.35 ± 0.40 | 0.61 ± 0.55 | 0.02 |
| Nausea overall | 0.3 ± 0.73 | 0.95 ± 1.37 | 0.01 |
| Vomiting overall | 0.06 ± 0.38 | 0.42 ± 0.97 | 0.04 |
| Itchiness overall | 0.42 ± 0.85 | 1.07 ± 1.31 | 0.01 |
| Constipation overall | 0.7 ± 1.06 | 0.32 ± 0.75 | 0.07 |
| Difficulty passing urine overall | 0.05 ± 0.30 | 0.19 ± 0.71 | 0.25 |
| Difficulty concentrating overall | 0.24 ± 0.66 | 0.3 ± 0.77 | 0.72 |
| Drowsiness or difficulty staying awake overall | 0.65 ± 0.98 | 0.64 ± 1.10 | 0.97 |
| Feeling lightheaded or dizzy overall | 0.49 ± 0.84 | 0.95 ± 1.18 | 0.05 |
| Feeling confused overall | 0.08 ± 0.33 | 0.05 ± 0.34 | 0.77 |
| Feelings of general fatigue or weakness overall | 0.38 ± 0.82 | 0.63 ± 0.92 | 0.21 |
| Dry mouth overall | 1.02 ± 1.14 | 1.13 ± 1.12 | 0.65 |
| Headache overall | 0 ± 0 | 0.3 ± 0.84 | 0.03 |
| LANSS | 1.21 ± 2.25 | 1.93 ± 3.68 | 0.38 |
Values are presented as the mean and standard deviation.
TABLE VI.
Quality of Recovery (QoR)-40 and Patient Satisfaction Scores
| Periarticular Injection | Patient-Controlled Epidural Analgesia | P Value | |
| POD 1* | |||
| No. of patients | 40 | 41 | |
| QoR-40† | |||
| Global | 178.4 ± 9.99 | 177.1 ± 17.44 | 0.67 |
| Emotional state | 42.1 ± 3.49 | 41.3 ± 5.80 | 0.48 |
| Physical comfort | 53.5 ± 4.45 | 52 ± 7.25 | 0.27 |
| Psychological support | 29.4 ± 1.30 | 29.5 ± 1.31 | 0.83 |
| Physical independence | 20.1 ± 2.43 | 20.8 ± 2.33 | 0.16 |
| Pain | 33.4 ± 2.01 | 33.5 ± 3.90 | 0.90 |
| Patient satisfaction | 9.4 ± 0.92 | 9.3 ± 1.77 | 0.92 |
| POD 3* | |||
| No. of patients | 36 | 36 | |
| QoR-40† | |||
| Global | 181.9 ± 8.56 | 179.9 ± 9.17 | 0.35 |
| Emotional state | 42.2 ± 2.75 | 41.8 ± 3.58 | 0.61 |
| Physical comfort | 55.6 ± 4.70 | 54.9 ± 4.43 | 0.49 |
| Psychological support | 29.4 ± 1.32 | 28.8 ± 3.08 | 0.26 |
| Physical independence | 21.2 ± 1.81 | 21.1 ± 1.62 | 0.84 |
| Pain | 33.4 ± 1.42 | 33.3 ± 1.93 | 0.78 |
| Patient satisfaction | 9.4 ± 1.18 | 8.8 ± 2.00 | 0.12 |
POD = postoperative day.
Values are presented as the mean and standard deviation.
Discussion
In this study comparing PCEA with PAI among patients undergoing total hip arthroplasty, we found no difference between groups in time to readiness for discharge or length of stay. Readiness for discharge was used as the main outcome because it accounts for many aspects of recovery, including level of pain, nausea, medical status, and rehabilitation milestones. Length of stay may depend on additional factors, such as the availability of a bed at a rehabilitation center, and some patients may be discharged to that center without meeting the milestones. There was no major difference between the PAI group and the PCEA group in terms of the number of patients who went home versus to a rehabilitation center. In addition, in actual practice, patients receiving PAI with a multimodal pain regimen do not have an epidural catheter or a urinary catheter inserted. Whether the presence of these two interventions could have contributed to our results is unknown. However, this was a requirement given the double-blind, placebo-controlled design of the study. Of note, the epidural catheter was removed by noon on postoperative day 1, and the likelihood of it having affected the time to discharge is low.
Few total hip arthroplasty studies have compared PCEA with PAI. Andersen et al. found a shorter length of stay with PAI (a mixture of ropivacaine, ketorolac, and epinephrine) compared with PCEA, but the analgesic regimen was different between the two groups (the use of ketorolac was not uniform)23. Pandazi et al. studied sixty-eight total hip arthroplasty patients divided among three groups24. One group had intravenous patient-controlled analgesia and was compared with a group who had PAI (ropivacaine, morphine, cefuroxime, epinephrine, methylprednisolone, and clonidine) and with a group using PCEA. Morphine consumption and pain scores were lower in the PAI group, but there was no difference between the PAI and epidural groups. Of note, our study is, to our knowledge, the first double-blind, placebo-controlled study in total hip arthroplasty surgery looking at the use of PCEA and PAI. Two double-blind, randomized trials in total hip arthroplasty have assessed the use of PAI with ropivacaine with or without additives compared with normal saline solution; decreased narcotic use, along with less pain, was found in one, but the other did not show any difference in opioid consumption, pain scores, or length of stay25,26. In the former, the analgesic regimen was not equivalent in both groups, while in the latter, the same multimodal regimen was used.
Another double-blind, randomized study looking at PAI compared levobupivacaine with normal saline solution and found similar pain-intensity scores but with less morphine consumption for the first twelve hours in the levobupivacaine group27. In a randomized clinical trial of ninety-six patients that compared the use of levobupivacaine and epinephrine with the use of no injection, no difference in morphine consumption, rehabilitation milestones, or earlier mobilization was found28. Busch et al. randomized sixty-four patients to receive either a periarticular injection of ropivacaine, epinephrine, and morphine or no injection, with all patients receiving patient-controlled analgesia29. The PAI group used less patient-controlled analgesia at six hours postoperatively and at twenty-four hours after surgery. The PAI group also had lower pain scores on activity in the postanesthesia care unit. However, there was no effect on patient satisfaction or length of stay. Two other randomized studies using either preemptive analgesia and/or a multimodal pain regimen found different results. Lee et al. compared the use of regional anesthesia and PAI (morphine, methylprednisolone, and ropivacaine) with the use of general anesthesia and patient-controlled analgesia and found reduced pain scores in the PAI group but without any difference in length of hospital stay30. Utilizing a comparative cohort design, Parvataneni et al. found lower pain scores and length of stay (3.2 compared with 4.2 days) in the group who received PAI compared with the group who received intravenous patient-controlled analgesia12. A meta-analysis by Kehlet and Andersen concluded that the previously published studies assessing the use of PAI in hip replacement surgery lacked proper design and did not support the use of PAI31. Hence, we undertook this double-blind, placebo-controlled study comparing the use of a multimodal analgesic regimen consisting of sustained-release oxycodone, a clonidine patch, and PAI with the use of an epidural analgesic regimen of bupivacaine and hydromorphone, both protocols currently used at our institution. Both groups, in addition, received acetaminophen, NSAIDs, and oxycodone as needed. It is possible that different results may be obtained with different versions of either the PCEA or PAI protocols.
Although the primary outcome was similar in both groups, overall, better analgesia was achieved in the PCEA group both with ambulation and with physical therapy (ambulation, p = 0.01; and physical therapy, p = 0.03). Interestingly, women had higher pain scores both at rest and with ambulation. From previous studies, females tend to report higher pain scores32-34. Patients with a BMI of ≥30 kg/m2 had less pain, although in another study of patients who underwent hip arthroplasty, obesity was not a factor in pain experienced postoperatively35. There was higher analgesic consumption in the PAI group; however, the ORSDS scores were greater in the PCEA group, with nausea, vomiting, dizziness, and pruritus being the most common symptoms. This was an interesting finding because neuraxial opioids may protect against nausea and vomiting36,37. A systematic review by Choi et al. found no difference between epidural and systemic analgesia in the frequency of nausea and vomiting8. Of note, the PAI group received 40 mg of methylprednisolone as part of the intervention. This may serve as a depot of corticosteroids. The use of corticosteroids has been proven as an effective method of decreasing emesis38,39. Similar QoR-40 and LANSS scores were found in both groups. Patient satisfaction was also similar.
In conclusion, both types of analgesic regimens were associated with similar functional recovery scores and patient satisfaction. PAI did not decrease the time to discharge; it was associated with higher pain scores and greater opioid consumption but lower ORSDS scores and, hence, fewer side effects. The decision to choose one regimen over the other should not be made on the basis of readiness for discharge, but mostly on patient characteristics and preference. In the narcotic-naive or nausea-prone patient, PAI may be preferable; however, in the patient with chronic pain, PCEA may be more effective.
Footnotes
Investigation performed at the Departments of Anesthesiology and Orthopedic Surgery, Hospital for Special Surgery, New York, New York
A commentary by John Brian Meding, MD, is linked to the online version of this article at jbjs.org.
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007April;89(4):780-5. [DOI] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003August;97(2):534-40. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Bloomfield MR. Multimodal pain management in orthopedics: implications for joint arthroplasty surgery. Orthopedics. 2013February;36(2)(Suppl):7-14. [DOI] [PubMed] [Google Scholar]
- 4.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998July;87(1):88-92. [DOI] [PubMed] [Google Scholar]
- 5.Kroll MA, Otis JC, Sculco TP, Lee AC, Paget SA, Bruckenstein R, Jensen DA. The relationship of stride characteristics to pain before and after total knee arthroplasty. Clin Orthop Relat Res. 1989February;239:191-5. [PubMed] [Google Scholar]
- 6.Ryu J, Saito S, Yamamoto K, Sano S. Factors influencing the postoperative range of motion in total knee arthroplasty. Bull Hosp Jt Dis. 1993Summer;53(3):35-40. [PubMed] [Google Scholar]
- 7.Tang R, Evans H, Chaput A, Kim C. Multimodal analgesia for hip arthroplasty. Orthop Clin North Am. 2009July;40(3):377-87. [DOI] [PubMed] [Google Scholar]
- 8.Choi PT, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev. 2003;3:CD003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharrock NE, Beckman JD, Inda EC, Savarese JJ. Anesthesia for orthopedic surgery. In: Miller RD, Fleisher LA, Johns RA, Savarese JJ, Wiener-Kronish JP, Young WL, editors. Miller’s anesthesia. 6th ed Philadelphia: Elsevier; 2005. p 2409-34. [Google Scholar]
- 10.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006February;88(2):282-9. [DOI] [PubMed] [Google Scholar]
- 11.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006May;88(5):959-63. [DOI] [PubMed] [Google Scholar]
- 12.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007September;22(6)(Suppl 2):33-8 Epub 2007 Jul 26. [DOI] [PubMed] [Google Scholar]
- 13.Cullen DJ, Apolone G, Greenfield S, Guadagnoli E, Cleary P. ASA physical status and age predict morbidity after three surgical procedures. Ann Surg. 1994July;220(1):3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000January;84(1):11-5. [DOI] [PubMed] [Google Scholar]
- 15.Apfelbaum JL, Gan TJ, Zhao S, Hanna DB, Chen C. Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg. 2004September;99(3):699-709, table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Yadeau JT1, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg. 2011August;113(2):369-77 Epub 2011 Apr 27. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005March;6(3):149-58. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009April;42(2):377-81 Epub 2008 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med. 2012Jan-Feb;37(1):99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Carpenter RL, Mulroy MF, Weissman RM, McGill TJ, Rupp SM, Allen HW. Intravenous versus epidural administration of hydromorphone. Effects on analgesia and recovery after radical retropubic prostatectomy. Anesthesiology. 1995March;82(3):682-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu CL. Acute postoperative pain. In: Miller RD, editor. Miller’s anesthesia. 6th ed Philadelphia: Elsevier; 2005. p 2745-7. [Google Scholar]
- 22.Gordon DB, Stevenson KK, Griffie J, Muchka S, Rapp C, Ford-Roberts K. Opioid equianalgesic calculations. J Palliat Med. 1999Summer;2(2):209-18. [DOI] [PubMed] [Google Scholar]
- 23.Andersen KV, Pfeiffer-Jensen M, Haraldsted V, Søballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: a randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop. 2007April;78(2):180-6. [DOI] [PubMed] [Google Scholar]
- 24.Pandazi A, Kanellopoulos I, Kalimeris K, Batistaki C, Nikolakopoulos N, Matsota P, Babis GC, Kostopanagiotou G. Periarticular infiltration for pain relief after total hip arthroplasty: a comparison with epidural and PCA analgesia. Arch Orthop Trauma Surg. 2013November;133(11):1607-12 Epub 2013 Sep 14. [DOI] [PubMed] [Google Scholar]
- 25.Andersen LJ, Poulsen T, Krogh B, Nielsen T. Postoperative analgesia in total hip arthroplasty: a randomized double-blinded, placebo-controlled study on peroperative and postoperative ropivacaine, ketorolac, and adrenaline wound infiltration. Acta Orthop. 2007April;78(2):187-92. [DOI] [PubMed] [Google Scholar]
- 26.Lunn TH, Husted H, Solgaard S, Kristensen BB, Otte KS, Kjersgaard AG, Gaarn-Larsen L, Kehlet H. Intraoperative local infiltration analgesia for early analgesia after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2011Sep-Oct;36(5):424-9. [DOI] [PubMed] [Google Scholar]
- 27.Murphy TP, Byrne DP, Curtin P, Baker JF, Mulhall KJ. Can a periarticular levobupivacaine injection reduce postoperative opiate consumption during primary hip arthroplasty? Clin Orthop Relat Res. 2012April;470(4):1151-7 Epub 2011 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobie I, Bennett D, Spence DJ, Murray JM, Beverland DE. Periarticular local anesthesia does not improve pain or mobility after THA. Clin Orthop Relat Res. 2012July;470(7):1958-65 Epub 2012 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch CA, Whitehouse MR, Shore BJ, MacDonald SJ, McCalden RW, Bourne RB. The efficacy of periarticular multimodal drug infiltration in total hip arthroplasty. Clin Orthop Relat Res. 2010August;468(8):2152-9 Epub 2009 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KJ, Min BW, Bae KC, Cho CH, Kwon DH. Efficacy of multimodal pain control protocol in the setting of total hip arthroplasty. Clin Orthop Surg. 2009September;1(3):155-60 Epub 2009 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kehlet H, Andersen LO. Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiol Scand. 2011August;55(7):778-84 Epub 2011 Apr 4. [DOI] [PubMed] [Google Scholar]
- 32.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012March;13(3):228-34 Epub 2012 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas T, Robinson C, Champion D, McKell M, Pell M. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain. 1998April;75(2-3):177-85. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama K, Kawai M, Tani M, Ueno M, Hama T, Yamaue H. Gender differences in postoperative pain after laparoscopic cholecystectomy. Surg Endosc. 2006March;20(3):448-51 Epub 2006 Jan 21. [DOI] [PubMed] [Google Scholar]
- 35.Motaghedi R, Bae JJ, Memtsoudis SG, Kim DH, Beathe JC, Paroli L, YaDeau JT, Gordon MA, Maalouf DB, Lin Y, Ma Y, Cunningham-Rundles S, Liu SS. Association of obesity with inflammation and pain after total hip arthroplasty. Clin Orthop Relat Res. 2014May;472(5):1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997December;85(6):1288-93. [DOI] [PubMed] [Google Scholar]
- 37.Cooper DW, Lindsay SL, Ryall DM, Kokri MS, Eldabe SS, Lear GA. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br J Anaesth. 1997March;78(3):311-3. [DOI] [PubMed] [Google Scholar]
- 38.De Oliveira GS Jr, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013January;116(1):58-74 Epub 2012 Dec 7. [DOI] [PubMed] [Google Scholar]
- 39.Shaikh S, Verma H, Yadav N, Jauhari M, Bullangowda J. Applications of steroid in clinical practice: a review. ISRN Anesthesiology. 2012;2012. [Google Scholar]