Abstract
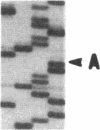
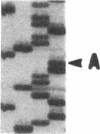
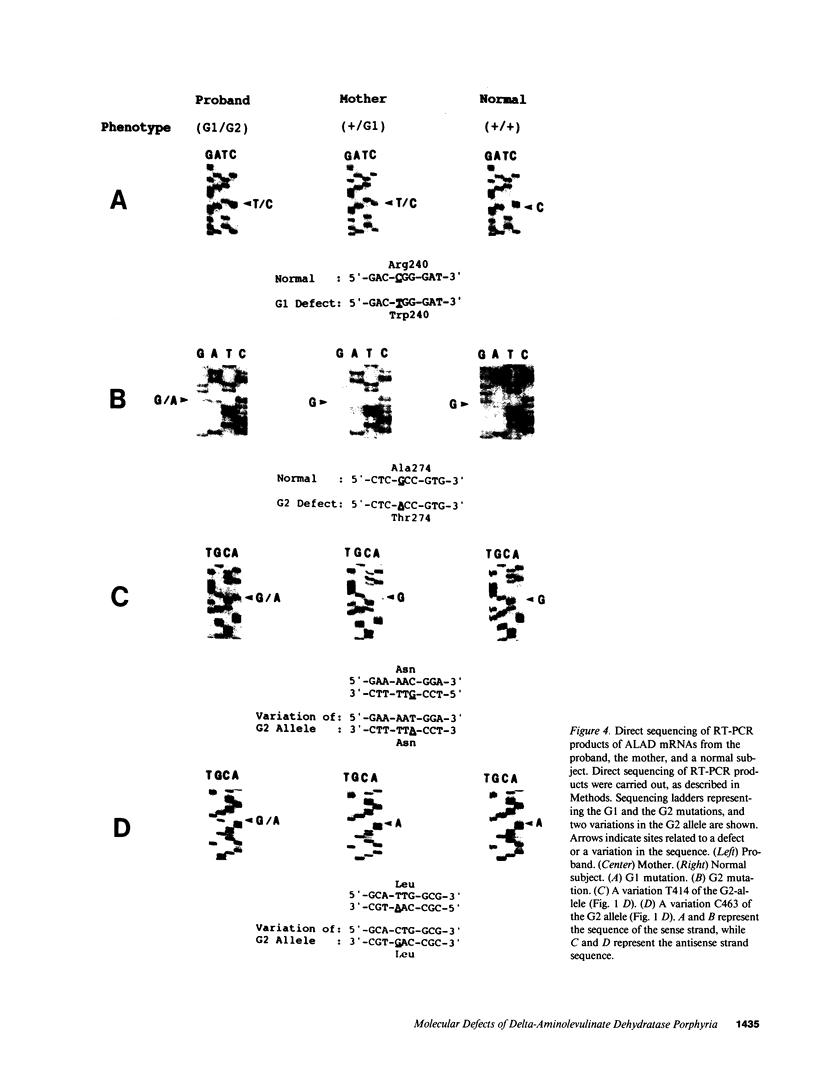
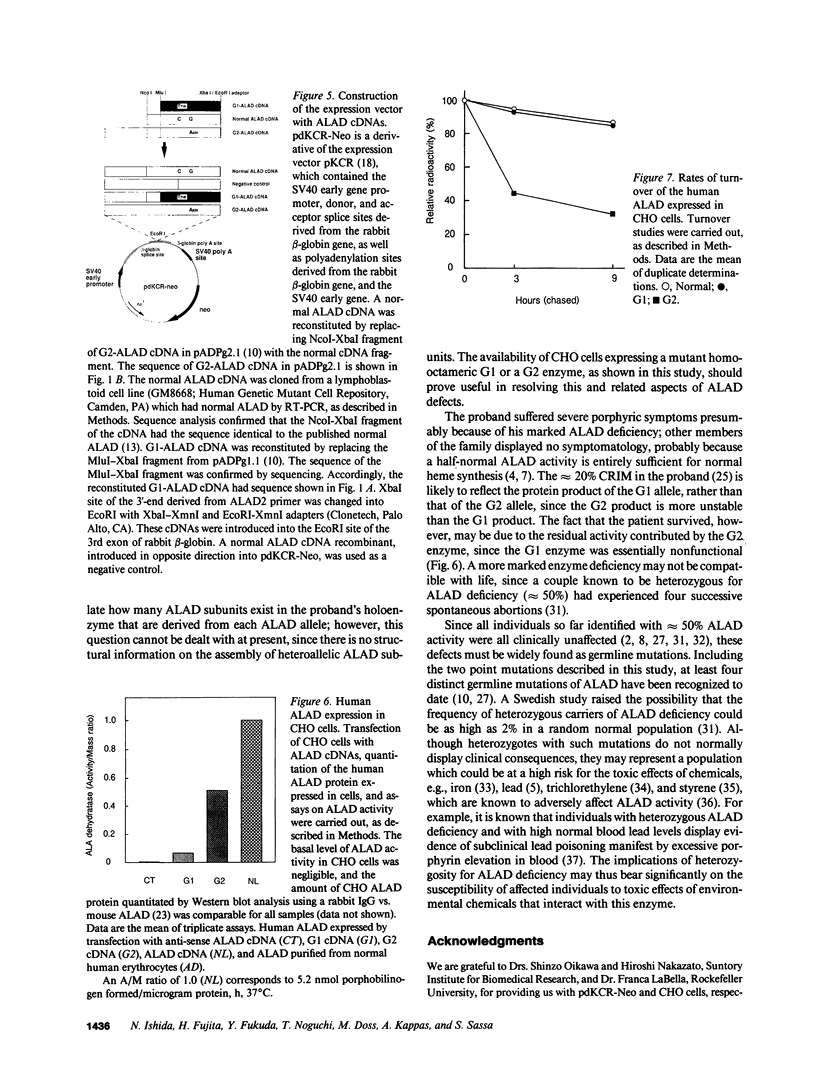
Cloning and expression of the defective genes for delta-aminolevulinate dehydratase (ALAD) from a patient with inherited ALAD deficiency porphyria (ADP) were carried out. Cloning of cDNAs for the defective ALAD were performed from EBV-transformed lymphoblastoid cells of the proband, and nucleotide sequences were determined. Two separate point mutations resulting in a single amino acid change in each ALAD allele were identified. One, C718----T, termed 'G1', occurred in the allele within the substrate-binding site, producing an Arg240----Trp substitution; the other, G820----A, termed 'G2', occurred downstream of this site in the other allele, resulting in an Ala274----Thr substitution. Using the reverse transcription-polymerase chain reaction, the mother, the brother, and the sister were shown to have the G1 defect. Expression of the G1 cDNA in Chinese hamster ovary cells produced ALAD protein with little activity; the G2 cDNA produced the enzyme with approximately 50% normal activity. Pulse-labeling studies demonstrated that the G1 enzyme had a normal half life, while the G2 enzyme had a markedly decreased half life. These data thus define the separate point mutations in each ALAD allele, as well as the altered properties of the two enzymic proteins encoded by the mutant genes in a patient with ADP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. D., Hamernyik P., Nutter J. Y., Labbe R. F. Inherited deficiency of delta-aminolevulinic acid dehydratase. Am J Hum Genet. 1979 Nov;31(6):662–668. [PMC free article] [PubMed] [Google Scholar]
- Bonkovsky H. L., Healey J. F., Lincoln B., Bacon B. R., Bishop D. F., Elder G. H. Hepatic heme synthesis in a new model of experimental hemochromatosis: studies in rats fed finely divided elemental iron. Hepatology. 1987 Nov-Dec;7(6):1195–1203. doi: 10.1002/hep.1840070605. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Sassa S., Doyle D. An immunological study of delta-aminolevulinic acid dehydratase specificity consistent with the phylogeny of species. Biochim Biophys Acta. 1984 Mar 1;797(3):297–301. doi: 10.1016/0304-4165(84)90249-6. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Sassa S. delta-Aminolevulinate dehydratase in human erythroleukemia cells: an immunologically distinct enzyme. Blood. 1985 Apr;65(4):939–944. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Doss M., Benkmann H. G., Goedde H. W. delta-Aminolevulinic acid dehydrase (porphobilinogen synthase) in two families with inherited enzyme deficiency. Clin Genet. 1986 Sep;30(3):191–198. doi: 10.1111/j.1399-0004.1986.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Doss M., Laubenthal F., Stoeppler M. Lead poisoning in inherited delta-aminolevulinic acid dehydratase deficiency. Int Arch Occup Environ Health. 1984;54(1):55–63. doi: 10.1007/BF00378728. [DOI] [PubMed] [Google Scholar]
- Fujita H., Koizumi A., Hayashi N., Ikeda M. Reduced synthesis of 5-aminolevulinate dehydratase in styrene-treated rats. Biochim Biophys Acta. 1986 Jun 20;867(3):89–96. doi: 10.1016/0167-4781(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Fujita H., Koizumi A., Yamamoto M., Kumai M., Sadamoto T., Ikeda M. Inhibition of delta-aminolevulinate dehydratase in trichloroethylene-exposed rats, and the effects on heme regulation. Biochim Biophys Acta. 1984 Jul 16;800(1):1–10. doi: 10.1016/0304-4165(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Fujita H., Sassa S., Lundgren J., Holmberg L., Thunell S., Kappas A. Enzymatic defect in a child with hereditary hepatic porphyria due to homozygous delta-aminolevulinic acid dehydratase deficiency: immunochemical studies. Pediatrics. 1987 Dec;80(6):880–885. [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Fujita H., Noguchi T., Doss M., Kappas A., Sassa S. Message amplification phenotyping of an inherited delta-aminolevulinate dehydratase deficiency in a family with acute hepatic porphyria. Biochem Biophys Res Commun. 1990 Oct 15;172(1):237–242. doi: 10.1016/s0006-291x(05)80199-8. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt S., Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Matsuoka Y., Kosaki G., Nakazato H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: homophilic and heterophilic adhesion. Biochem Biophys Res Commun. 1989 Oct 16;164(1):39–45. doi: 10.1016/0006-291x(89)91679-3. [DOI] [PubMed] [Google Scholar]
- Plewinska M., Thunell S., Holmberg L., Wetmur J. G., Desnick R. J. delta-Aminolevulinate dehydratase deficient porphyria: identification of the molecular lesions in a severely affected homozygote. Am J Hum Genet. 1991 Jul;49(1):167–174. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28(2-3):133–145. doi: 10.1159/000459097. [DOI] [PubMed] [Google Scholar]
- Sassa S., Fujita H., Doss M., Hassoun A., Verstraeten L., Mercelis R., Kappas A. Hereditary hepatic porphyria due to homozygous delta-aminolevulinic acid dehydratase deficiency: studies in lymphocytes and erythrocytes. Eur J Clin Invest. 1991 Apr;21(2):244–248. doi: 10.1111/j.1365-2362.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Kappas A. Effect of lead and genetic factors on heme biosynthesis in the human red cell. Ann N Y Acad Sci. 1975 Apr 15;244:419–440. doi: 10.1111/j.1749-6632.1975.tb41546.x. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983 Mar;71(3):625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Effect of pyrophosphorolysis and metal ions. J Biol Chem. 1990 May 15;265(14):8322–8328. [PubMed] [Google Scholar]
- Thunell S., Holmberg L., Lundgren J. Aminolaevulinate dehydratase porphyria in infancy. A clinical and biochemical study. J Clin Chem Clin Biochem. 1987 Jan;25(1):5–14. doi: 10.1515/cclm.1987.25.1.5. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Adachi H., Kodama S., Tsuruoka N., Yamada Y., Tanaka S., Mita S., Takatsu K. Purification and characterization of recombinant human interleukin 5 expressed in Chinese hamster ovary cells. J Biochem. 1989 Jul;106(1):23–28. doi: 10.1093/oxfordjournals.jbchem.a122812. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Cantelmo C., Desnick R. J. Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7703–7707. doi: 10.1073/pnas.83.20.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Ostasiewicz L., Desnick R. J. Molecular cloning of a cDNA for human delta-aminolevulinate dehydratase. Gene. 1986;43(1-2):123–130. doi: 10.1016/0378-1119(86)90015-6. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Doss M., Brusco N., Beaumont C., Nordmann Y. Hereditary hepatic porphyria with delta aminolevulinate dehydrase deficiency: immunologic characterization of the non-catalytic enzyme. Hum Genet. 1985;69(2):174–177. doi: 10.1007/BF00293292. [DOI] [PubMed] [Google Scholar]