Abstract
The Stigonemataceae family of cyanobacteria produces a class of biogenetically related indole natural products that include hapalindoles and ambiguines. In this full account, a practical route to the tetracyclic hapalindole family is presented by way of an eight-step, enantiospecific, protecting-group-free total synthesis of (−)-hapalindole U that features an oxidative indole–enolate coupling. With gram-scale access to hapalindole U, the first total synthesis of an ambiguine alkaloid, (+)-ambiguine H, was completed via an isonitrile-assisted prenylation of an indole followed by a photofragmentation cascade.
Keywords: Total synthesis, Alkaloid, Terpene, Indole, Protecting-group-free
1. Introduction: Isolation and Structures of Complex Cyanobacteria-derived Indole Alkaloids
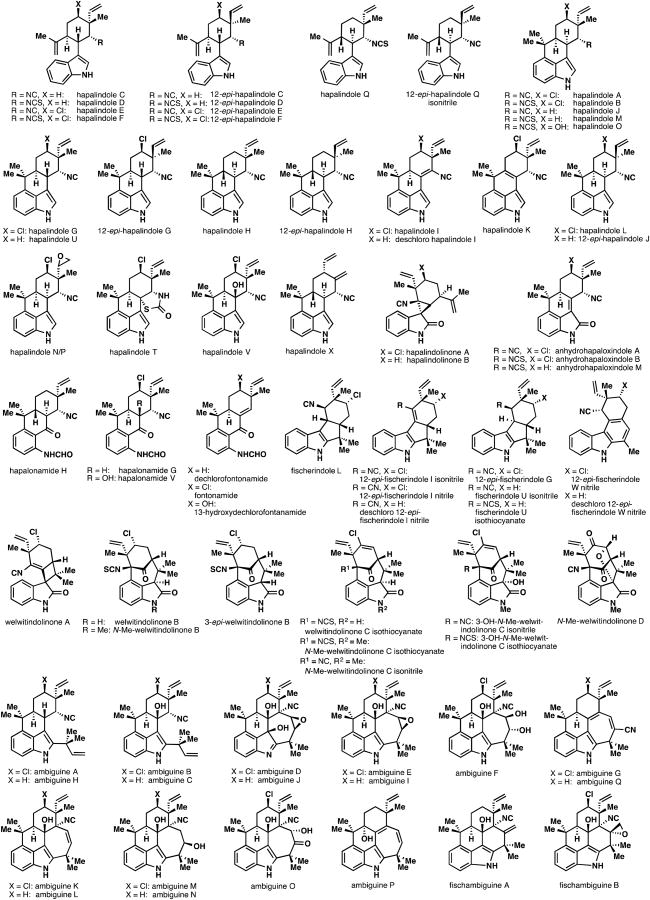
The Stigonemataceae family of cyanobacteria produces a plethora of biogenetically related and structurally fascinating indole natural products. Comprising over 70 members, these compounds form the basis of the hapalindole,1 fischerindole,2 welwitindolinone,3 ambiguine,4 and related alkaloid classes.5–7 In 1984, isolation efforts by Moore and co-workers opened an exciting new door in marine natural products chemistry.1a Isolated from soil samples found all over the world (e.g., Marshall Islands,1a Everglades,5b Australia,2b Micronesia,2b,3b Papua New Guinea,1c Israel4c), many of these natural products exhibit a broad range of biological profiles. In particular, numerous hapalindole,1 welwitindolinone,3 and ambiguine4 alkaloids have shown insecticidal,1d,2b antialgal,1a antimycotic,1a,1c,2b,4a,4c or antibacterial4c,8 properties. In addition, the hapalindolinones have been found to inhibit arginine vasopression binding.5b Finally, the welwitindolinones show anticancer activity against multiple drug resistant ovarian cancer cell lines.9
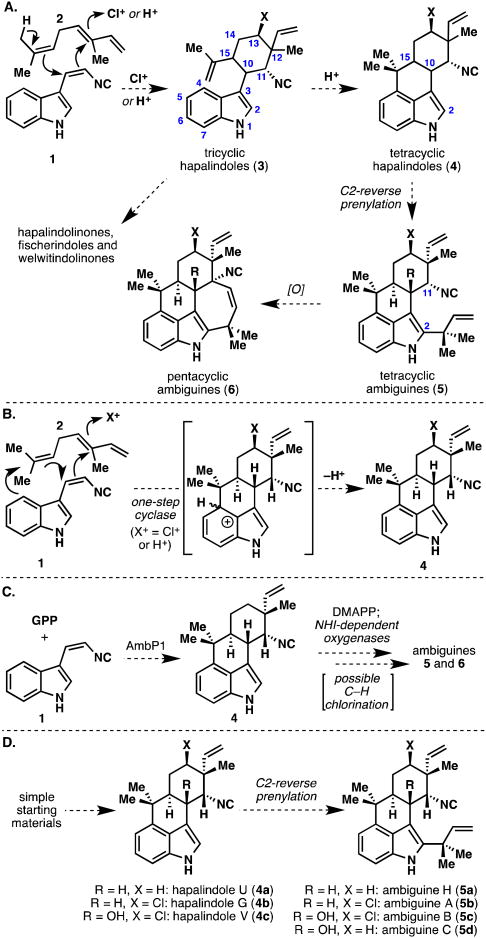
Although the biological activities of many of these complex alkaloids are noteworthy, it is truly their molecular structures that piqued our interest as targets for total synthesis.6,10,11 All compounds shown in Figure 1 are related by the presence of an indole (or indole-derived) subunit merged to a monoterpene fragment. In addition, a rather unusual isonitrile or isothiocyanate group is present in nearly all members. Finally, many of these natural products contain an asymmetric chlorine atom as well as multiple sites of further oxygenation. Moore has proposed that the entire conserved unit of these intriguing natural products (i.e., the tricyclic hapalindole core 3) arises from an exotic chloronium (or proton) induced cyclization of tryptophan-derived isonitrile 1 with the monoterpene β-ocimene (2) (Figure 2A).2a,2b This putative reaction forms the hallmark five continuous stereocenters unique to this alkaloid family. It should be noted, however, that nature makes many permutations of this stereochemical array (both with and without a halogen), an observation that could be attributed to imperfections in the biosynthetic machinery.4c From 3, an oxidative C–C bond formation between C3 of the indole ring with the isonitrile-bearing carbon (C11) leads to the spirocyclopropyl hapalindolinone framework, a cyclization between the isopropylidene group and C2 of the indole ring furnishes the fischerindole family, and further oxidative rearrangements furnish the welwitindolinones in Moore's biosynthetic hypothesis. The tetracyclic hapalindole nucleus 4 (which nature makes in both cis- and trans-fused forms across the C10–C15 bond) is presumably formed via cyclization of the isopropylidene onto the indole C4 position.6 Further “reverse” prenylation of the tetracyclic hapalindoles leads to the basic ambiguine framework (5). Only trans-fused hapalindoles appear to be substrates for processing into ambiguines. Finally, an enzymatic cyclization between the isonitrile- bearing carbon (C11) and the terminus of the reverse prenyl group leads to the pentacyclic ambiguine skeleton (6), which represents the pinnacle of complexity in this natural product family. The recently isolated fischambiguines show divergent regiochemical preference in this late-stage cyclization, affording 6-membered rings as opposed to the usual 7-membered ambiguine carbocycles.
Figure 1.
Isolated members of the hapalindole family of alkaloids.
Figure 2.
Evolution of biosynthetic hypotheses regarding the origins of the hapalindoles and ambiguines: A. Moore's classic halonium (or proton) induced cyclization of β-ocimene (2). B. Carmeli's alternative one-step enzymatic cyclization to the tetracyclic hapalindole core (4). C. Liu's recent biosynthetic studies. D. Tetracyclic hapalindoles (4) and ambiguines (5) of interest in this synthesis campaign. GPP = geranyl pyrophosphate; DMAPP = dimethylallyl pyrophosphate; NHI = non-heme iron.
Despite Moore's unifying biosynthetic hypothesis, it should be noted that Carmeli and co-workers have suggested that the entire tetracyclic hapalindole framework could also be formed in a single biosynthetic step rather than from the tricyclic hapalindole series (Figure 2B).4c Recent biosynthetic studies by Liu and co-workers, which have identified the ambiguine biosynthetic gene cluster in Fischerella ambigua, point to geranyl pyrophosphate (GPP) as the likely origin of the terpene framework in the hapalindole core and not β-ocimene (2) (Figure 2C).12 Furthermore, Liu's group has also suggested that non-heme-iron (NHI) dependent oxygenases are responsible for late-stage C–H activation events including the introduction of the chlorine atom,12c which many members possess.
With so many unique structural types found in this natural product family, it is not surprising that many syntheses have been attempted.13–17 Synthetic approaches toward hapalindoles,13 as well as total syntheses of hapalindoles A,14k G,14h,14k H,14e,14mJ, 14b,14c,14d,14l K,14k M,14b,14c,14d O,14g,14n Q, 10a,14f,14i,14j,14m and U11,14e,14l have been reported. Very recently, the total synthesis of hapalonamide H and some fischerindoles have been completed as well.14m There have been many reports of approaches toward the welwitindolinones,15 however, total syntheses from our laboratory16a and the Wood group16b,16c were the only synthetic routes to these challenging molecules at the time of the first communication of this research campaign.11 (Thereafter, the Rawal16d,16f and Garg16e,16g,16h,16i groups have reported elegant total syntheses in this area.) At the beginning of this work, there was not a single report of an approach toward an ambiguine alkaloid, however, since then, a few reports have surfaced.17 A simple yet flexible entry to the hapalindole and ambiguine families was accomplished through the efficient synthesis of hapalindole U (4a) and ambiguine H (5a), which was reported briefly in an earlier article11 and is described herein as a full account (Figure 2D).
2. Results and Discussion
2.1. Retrosynthetic analysis: Oxidative enolate coupling
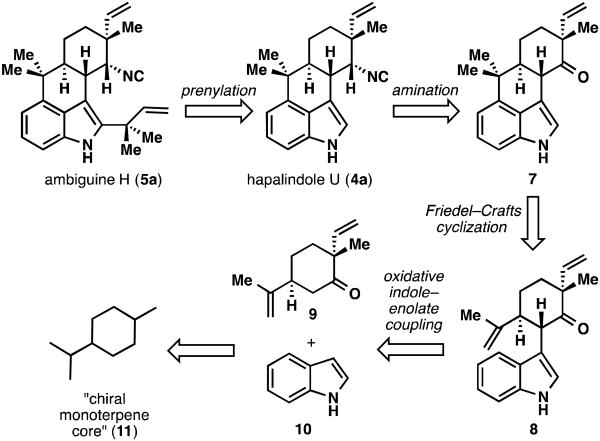
The direct indole–enolate coupling reaction initially employed for the synthesis of hapalindole Q and 12-epi-fischerindole U isothiocyanate stands as the key retrosynthetic disconnection to form the core of all of the structural types found in this family.10a Treatment of ketone, ester, or amide enolates with indole anion followed by the addition of a single-electron oxidant (Cu(II) 2-ethylhexanoate is optimal) allows for single-step access to a number of interesting heterocyclic structures that would be difficult to synthesize by other means.18 This powerful reaction allowed for exceedingly concise syntheses of the hapalindole, fischerindole and welwitindolinone natural products.6,10,11 Ambiguine H (5a), the simplest ambiguine natural product, was targeted for total synthesis and a retrosynthetic blueprint was developed (Figure 3).11 We planned to proceed via the tetracyclic hapalindole family, namely hapalindole U (4a), by way of a late-stage reverse prenylation. Hapalindole U (4a), in turn, could be traced back to tetracyclic ketone 7. We had hoped to form the tetracyclic core of 7 by a biomimetic-type cyclization of compound 8, which would be the product of an oxidative indole–enolate coupling reaction. Ketone 9 could then be generated by manipulation of the chiral terpene pool.
Figure 3.
Initial retro synthetic analysis of the tetracyclic ambiguine and hapalindole alkaloids as exemplified by the targets ambiguine H (5a) and hapalindole U (4a).
2.2. Development of a gram-scale route to (−)-hapalindole U
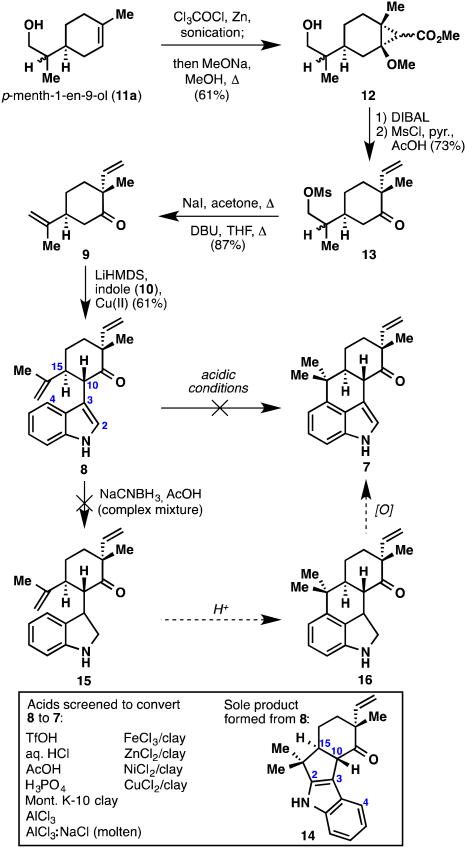
Initial forays into the hapalindoles began by procuring large amounts of ketone 9 (Figure 4). It should be noted that the chlorinated version of this ketone had already been prepared by Fukuyama and co-workers in their elegant synthesis of hapalindole G (4b).14h Chemistry developed by Mehta19 was amenable to solve the problem at hand. Starting with the chiral terpene, p-menth-1-en-9-ol (11a), a dichloroketene [2+2] cycloaddition (Cl3COCl, Zn) followed by sodium methoxide-induced rearrangement led to cyclopropane 12 in 61% yield as an inconsequential mixture of four diastereomers. DIBAL reduction, mesylation, and cyclopropane fragmentation then furnished mesylate 13 in good yield (73% over two steps, again as an inconsequential mixture of diastereomers). Displacement of the mesylate with iodide, followed by E2 elimination, furnished ketone 9.
Figure 4.
Synthesis of ketone 9, oxidative coupling with indole (10), and attempts at forging the tetracyclic core of the hapalindole family.
With compound 9 in hand, work on the key ring-forming reactions was initiated. Oxidative coupling of 9 with indole (10) proceeded smoothly and provided coupled product 8 as a single diastereomer in 61% yield on gram scale. As mentioned earlier, the simplest solution to the tetracyclic hapalindole skeleton would proceed via a Friedel–Crafts cyclization at the indole C4 position. As the locus of reactivity resides in the indole C2,C3 π-bond, we recognized that this bond formation would be difficult, if not impossible. Furthermore, the undesired cyclization at the C2 position had already been documented by Fukuyama and co-workers,14h as well as in our laboratory,10 as this is one of the key steps in the fischerindole synthesis. Nevertheless, we had hoped that a certain combination of acids/temperatures might lead to at least some amount of the desired cyclization product. We briefly screened an assortment of Lewis acids and unfortunately never observed any of the desired compound 7. Not surprisingly, only C2-cyclized ketone 14 was observed when cyclization occurred.
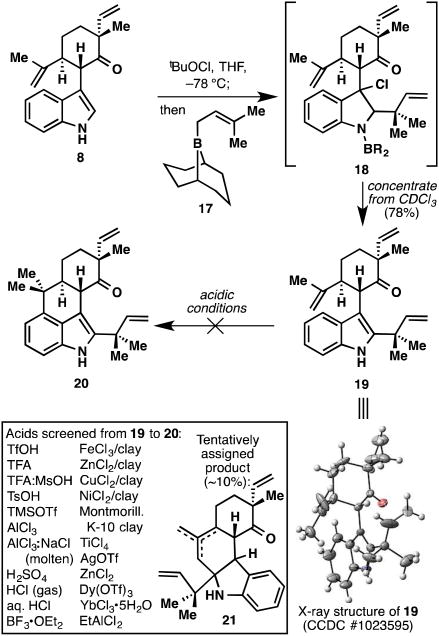
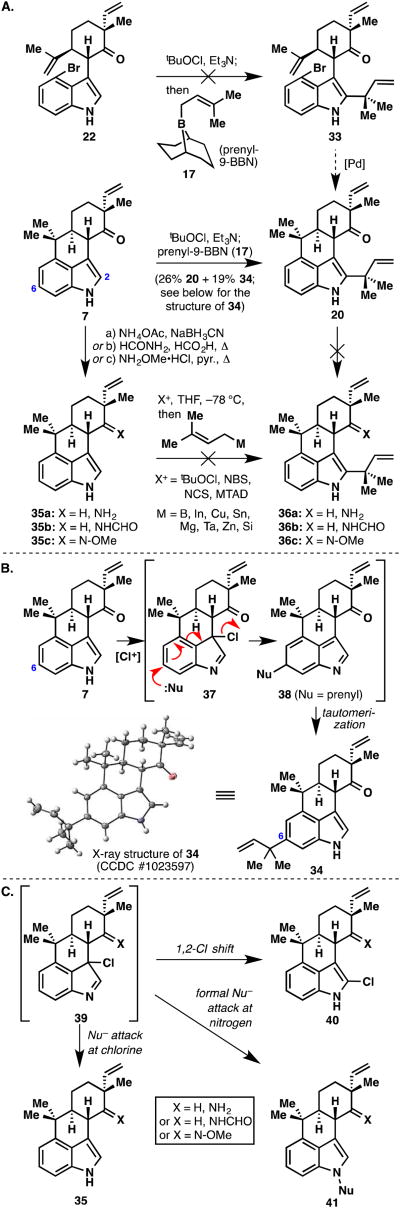
A logical solution to this problem was to remove the indole C2,C3 π-bond completely, thus arriving at indoline 15 (Figure 4). Such a compound, if found to cyclize to structure 16, could then be oxidized back to the indole nucleus. Unfortunately, compound 15 proved troublesome to prepare via Gribble-type reduction.20 This fact, coupled with an inelegant oxidation state fluctuation, led us to move on to a more direct solution. A more attractive idea was to block the indole C2,C3 π-bond with the bulky reverse prenyl moiety, thereby forcing bond formation to occur at the C4 position (Figure 5). Although this strategy would rule out direct access to the tetracyclic hapalindoles, it would offer an extremely expedient entry into the ambiguine carbon skeleton. To this end, indole 8 was subjected to Danishefsky's reverse prenylation protocol21 and furnished prenylated indole 19 in 78% yield (structure verified by X-ray crystallographic analysis). Interestingly, the compound first isolated after column chromatography appeared to still have the boron attached and was presumably the highly non-polar compound 18. After dissolution in CDCl3 for NMR analysis, a new, much more polar compound formed quantitatively, which turned out to be desired product 19.
Figure 5.
Synthesis of reverse-prenylated product 19, and attempts at forging the tetracyclic core of the ambiguine family.
Despite significant experimentation and a fairly extensive Lewis acid screen, we were unfortunately not able to realize the cyclization from 19 to 20. Under most conditions, only starting material and decomposition were observed. In a few instances, we observed very small amounts (∼10%) of compounds tentatively appearing to be olefinic mixtures resembling structures 21. These compounds were presumably formed via initial protonation of the electron-rich indole ring, which is then trapped in Prins-type fashion followed by proton loss. It should be noted that this undesired mode of cyclization was also observed in our laboratory's previous studies toward the fischerindoles.6 Once again, the electron-rich nature of the indole C2,C3 π-bond thwarted attempts at C4 cyclization.
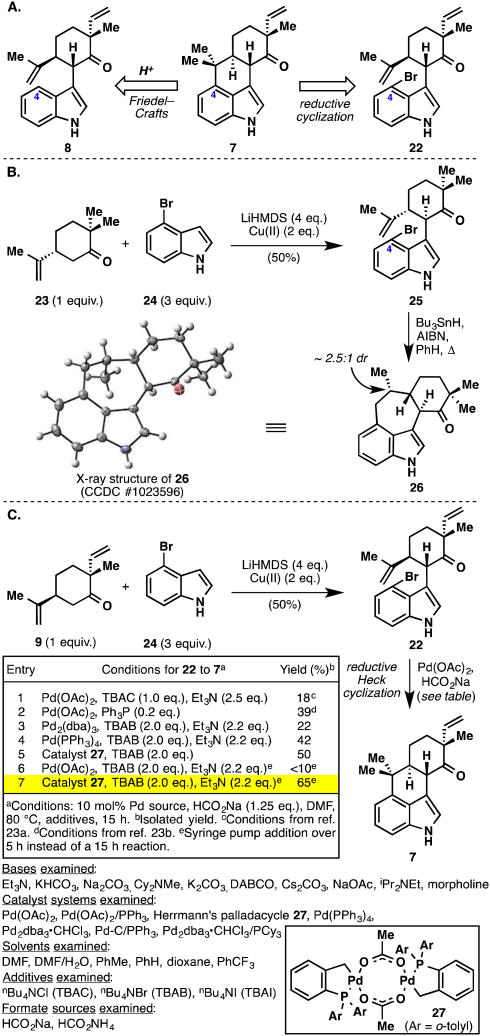
At this juncture, the retrosynthesis needed to be revised to one in which the indole C4 position would possess greater reactivity (Figure 6A). A logical solution would be to place a halogen at this position and thereby switch the reaction pathways from ones involving acid-catalyzed cyclizations to those involving radicals or transition metals. As a model study, we prepared brominated indole 25, which results from the oxidative coupling of 4-bromoindole (24) with simplified ketone 23 (Figure 6B). Significant optimization was required to coax the electron-deficient indole into coupling; in the end, it was discovered that 3 equivalents of the brominated indole and 2 equivalents of Cu(II) 2-ethylhexanoate oxidant were required to obtain synthetically useful yields of product (50%). With 25 in hand, a standard radical-based cyclization22 (Bu3SnH, AIBN, refluxing benzene) was attempted. Unfortunately, the reaction underwent 7-endo closure rather than the desired 6-exo pathway, producing compound 26 as an approximate 2.5:1 mixture of diastereomers (major isomer verified by X-ray crystallographic analysis). Molecular models suggest that the terminus of the isopropylidene group is probably closer to the indole C4 position. Coupled with the fact that a more stable tertiary radical is formed during the 7-endo cyclization, this observed result is not entirely surprising. We next turned our attention to palladium-based methods in the hope to elicit a reductive Heck cyclization23 forming the desired 6-membered ring. We returned to the real system bearing a vinyl group and prepared compound 22 (again in 50% yield) via oxidative coupling of 4-bromoindole (24) and ketone 9 (Figure 6C). The first attempt using literature conditions (Pd(OAc)2, HCO2Na, Et3N, TBAC, DMF) were encouraging and formed tetracycle 7 in 25% isolated yield. Although the yield was modest, this was the first time that the desired tetracyclic ring system was observed and optimization of this reaction was then undertaken. It took extensive screening of reaction parameters (over 80 experiments performed) to optimize this reaction (22 to 7) to synthetically useful yields (Figure 6C, table). Catalyst destruction in the highly reducing formate environment as well as debromination without cyclization proved to be significant challenges. A wide variety of bases were screened and found to have a minimal impact on the outcome of this transformation, thus the use of Et3N was continued. Catalyst screening showed the palladacycle of Herrmann and co-workers (27)24 to be particularly robust. DMF and toluene emerged as the optimal solvents, and sodium formate as the ideal formate source. The additive tetrabutylammonium bromide (TBAB) gave higher yields than its chloro (TBAC) and iodo (TBAI) counterparts. Even with many of these parameters somewhat optimized, we were still faced with poor catalyst turnover. Thus, while catalyst 27 was ideal at minimizing the amount of debrominated material, the reactions never reached completion even with generous catalyst loadings (i.e., 10% Pd). Perhaps the most important finding in the entire screening process was a slow addition experiment wherein a DMF solution of 27 (5 mol%) was added over 5 hours to the heated reaction mixture. To our satisfaction, when the addition was complete, all of the starting material had been consumed and tetracycle 7 was obtained in 65% isolated yield. Furthermore, this reaction was robust and could be conducted on a gram-scale.
Figure 6.
A. Revised retrosynthesis of key intermediate 7 that invokes a reductive cyclization pathway. B. Model radical cyclization. C. Successful reductive Heck cyclization and reaction optimization.
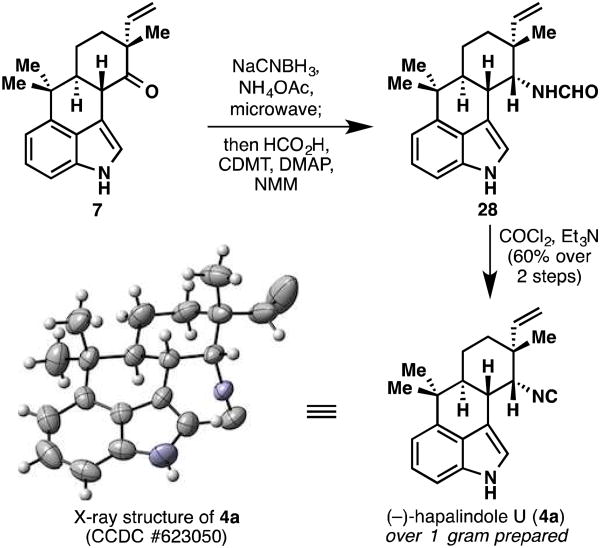
With ketone 7 in hand, the completion of hapalindole U (4a) was rather straightforward (Figure 7).11 A stereoselective microwave-assisted reductive amination followed by formylation of the resulting amine furnished compound 28 as a mixture of formamide rotamers. It should be noted that diastereocontrol in this reaction without microwave assistance is poor (∼2.5:1 dr).14e Dehydration of formamide 28 with COCl2 then provided (−)-hapalindole U (4a) in 62% overall yield from 7. Pleasingly, this natural product was amenable to single crystal X-ray diffraction analysis. The described route allowed for gram quantities of the natural product to be synthesized, which was fortunate because the seemingly simple task of attaching the reverse prenyl group proved to be extremely challenging.
Figure 7.
Completion of a gram-scale, enantiospecific synthesis of (−)-hapalindole U (4a).
2.3. Total synthesis of (+)-ambiguine H
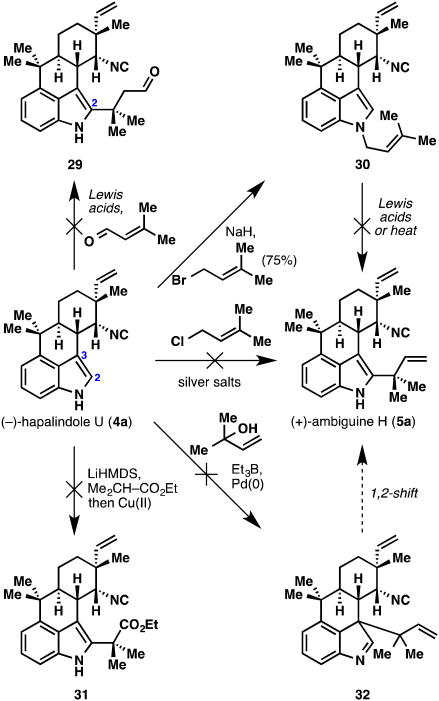
All that remained to gain entry into the ambiguine alkaloid family was the attachment of the reverse prenyl group at the indole C2 position. Many methods to install a reverse prenyl group, or its equivalent, were attempted on hapalindole U (4a), and a small summary of failures are shown in Figure 8. Friedel–Crafts reactions of 4a with prenyl chloride in the presence of silver salts failed to furnish even trace quantities of ambiguine H (5a). Michael-type reaction of 4a with dimethylacrolein also failed to furnish C2-substituted product 29. N-Prenylated indoles are known to undergo acid-mediated rearrangement to mixtures of C2-reverse prenylated indoles and C2-prenylated indoles,25 and therefore, conversion of 4a to N-prenyl compound 30 was achieved with NaH and prenyl bromide (75% yield). Unfortunately, this compound failed to undergo the rearrangement to ambiguine H (5a) under thermal or Lewis acidic conditions. Furthermore, an oxidative enolate coupling between 4a and ethyl 2-methylpropanoate was unsuccessful at providing 31. Although the oxidative indole–enolate coupling at the C2 position has never been observed in this laboratory, we had hoped that the relatively electron-rich indole nucleus in 4a might allow the reaction to take place. The methods of Trost26 and Tamaru27 for indole C3 reverse prenylation also failed to afford compound 32, which we had hoped to rearrange to ambiguine (5a).
Figure 8.
Failed attempts to introduce the reverse prenyl group onto (−)-hapalindole U (4a).
Given the early success of Danishefsky's protocol21 in delivering a C2 reverse prenylated indole (see transformation from 8 to 19 in Figure 5), we returned to ketone intermediate 22 with plans of prenylation then Pd-catalyzed cyclization to ketone 20 (Figure 9A). Unfortunately, 22 was largely resistant to this methodology, perhaps due to the mildly electron-withdrawing character of bromine, thus inhibiting initial chloroindolenine formation at low temperature. This setback turned out to be insignificant because the Danishefsky reaction worked satisfactorily on cyclized ketone 7. Surprisingly, the desired compound (20) was formed in nearly equal quantities with its C6 isomer (34), which is presumably formed via the mechanism shown in Figure 9B. With ketone 20 in hand, we were poised to complete the first ambiguine synthesis. Unfortunately, we were never able to incorporate a nitrogen source into 20 via reductive amination or even oxime formation to give 36a–36c. Molecular modeling suggested that the terminus of the reverse prenyl group lies directly over the ketone moiety, thus inhibiting the approach of the amine nucleophile. At this juncture, we realized that the nitrogen functionality would have to be incorporated prior to the reverse prenyl group. Taking a variety of nitrogen-containing intermediates 35a–35c from the hapalindole U synthesis and subjecting them to the Danishefsky protocol failed to afford any C2 reverse prenylated product 36a–36c. A variety of prenyl-based metal nucleophiles were screened as well as the standard prenyl-9-BBN reagent (17). In addition to the standard tBuOCl conditions, several “X+” activating reagents known to react with indoles were screened (NBS, NCS, MTAD28). While disappointing, we did gain some intelligence into shortcomings of this reaction. Analysis of the crude reaction mixtures indicated mixtures of C2-chlorinated indole, recovered starting material, and at times even N-prenylated indoles (Figure 9C). The recovered starting material was suspicious since TLC analysis indicated that it had been completely consumed after addition of tBuOCl. Thus, we believed that we were forming the putative 3-chloroindolenine species (39) cleanly, however, it was either: a) rapidly undergoing a 1,2-chloro shift (to afford structures resembling 40), or b) acting as a chlorinating agent to the prenyl nucleophile thus returning starting material. This latter pathway would also produce prenyl chloride, which could alkylate the indole ring leading to N-prenylated compound 41 in what is formally a substitution reaction at nitrogen.
Figure 9.
A. Attempts at prenylating various indole-containing intermediates. B. Mechanism of the prenylation reaction of 7, leading to side product 34. C. Observed and undesired pathways from chlorinated intermediate 39.
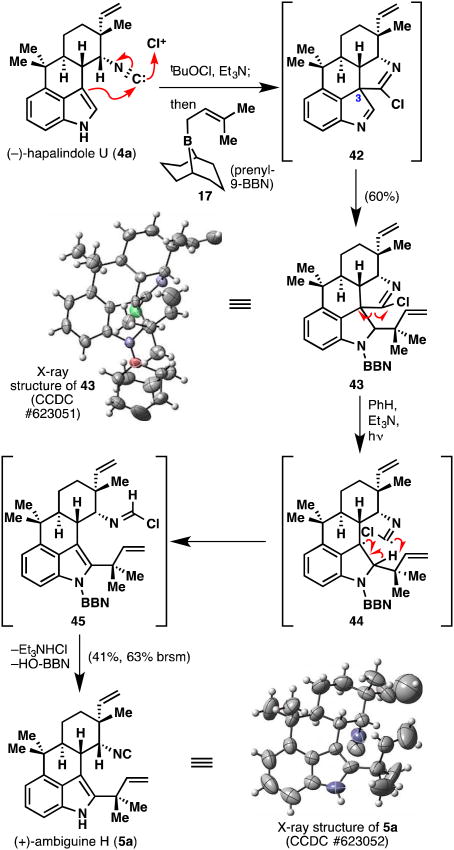
Despite some of the failures in adapting Danishefsky's protocol to hapalindole intermediates, we attempted the reverse prenylation with the natural product, hapalindole U (4a), resulting in an unexpected yet interesting transformation (Figure 10). The chlorinating reagent reacted with the isonitrile moiety forming a highly reactive electrophile, which was trapped by the neighboring indole π-bond, presumably leading to intermediate imine 42. This compound then reacted with the prenyl-9-BBN reagent (17) affording pentacycle 43, which showed the entire incorporation of 17 via X-ray analysis. This fortuitous discovery avoided the problems encountered earlier since: a) intermediate 42 cannot undergo the 1,2-shift reminiscent of the C3-chloro species; b) nucleophiles cannot attack at nitrogen since there is no leaving group at the C3 position, and c) 42 cannot act as a halogenating source to quench the prenyl nucleophile. It is of note that the 9-BBN group is strongly bound to the indoline nitrogen in 43 and typical conditions for boron removal could not cleave it (vide infra).
Figure 10.
A novel isonitrile-assisted reverse prenylation of an indole followed by a light-induced fragmentation cascade to furnish (+)-ambiguine H (5a). brsm = based on recovered starting material.
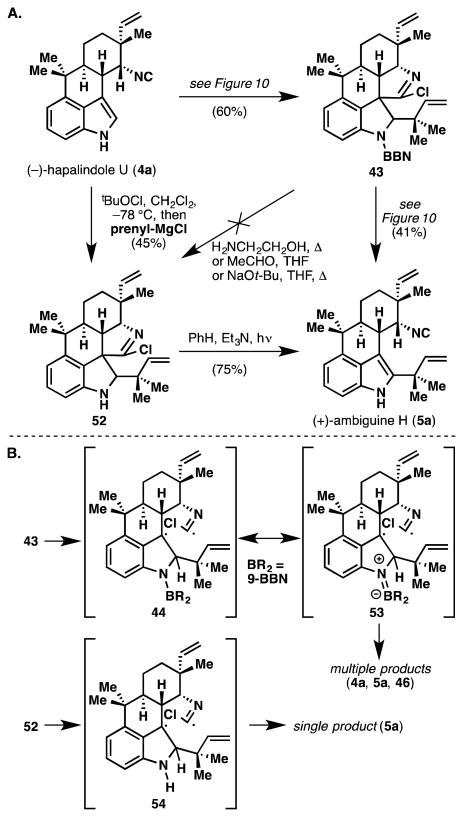
Although 43 incorporates the desired reverse prenyl group, it also contains an unwanted quaternary C–C bond and is missing the key isonitrile group. We had hoped that it might be possible to cleave the unwanted C–C bond by using photochemistry somewhat reminiscent of the venerable Norrish type I process (albeit with an iminyl chloride rather than a ketone chromophore).29 Gratifyingly, when 43 was irradiated in benzene with Et3N, ambiguine H (5a) was formed directly (Figure 10).
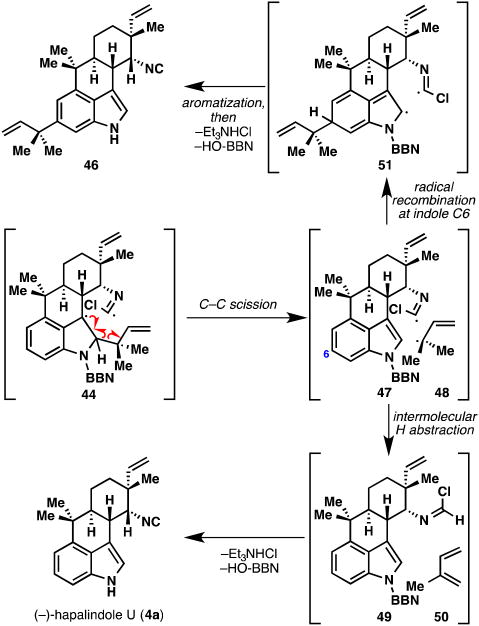
A plausible mechanism is shown in Figure 10 and supported by the side products shown in Figure 11, namely hapalindole U (4a) and C6 reverse prenylated compound 46. The initial benzylic radical intermediate (44), formed after excitation and C–C cleavage, can undergo homolytic C–C scission, thus restoring aromaticity to the indole group (47) and liberating tertiary allylic radical 48. This tertiary radical can serve as a hydrogen source leading to hapalindole U (4a) via 49, and isoprene (50). Radical 48 can also recombine with the electron-rich indole ring to give intermediate 51, affording 46 after rearomatization and losses of HCl and the boron group respectively. As in the production of compound 34 (see Figure 9B), the hapalindole aromatic nucleus displays significant reactivity at the unexpected C6 position. It should be mentioned that thermal heating of 43 (temperatures of 180 to 250 °C) fails to produce any ambiguine H (5a). To probe the role of the boron atom in the fragmentation, we endeavored to prepare non-boronated compound 52 (Figure 12A). As mentioned earlier, the 9-BBN group could not be removed in compound 43, so an alternative procedure was developed. By switching the nucleophile in the Danishefsky prenylation reaction from prenyl-9-BBN (17) to prenylmagnesium chloride, we were able to prepare 52 in 45% from hapalindole U (4a). Subjecting 52 to the photofragmentation conditions smoothly formed ambiguine H (5a) in higher chemical yield and conversion (75%), and more interestingly, without the formation of C6 reverse prenylated compound 46 and hapalindole U (4a). Assuming that both intermediates 44 and 54 are formed in the reactions of 43 and 52, respectively (Figure 12B), it is interesting that intermediate 44 leads to 3 products (4a, 5a, and 46) while 54 produces only ambiguine H (5a). It is tempting to consider that resonance contributor 53 could play a role in increasing the lifetime of the radical intermediate, thereby allowing for alternative pathways to compete with hydrogen radical abstraction. Alternatively, relief of unfavorable steric congestion between the large 9-BBN group and the reverse prenyl moiety in 44 may provide an additional driving force for C–C bond cleavage.
Figure 11.
Side products 51 and (−)-hapalindole U (4a) formed during the photofragmentation reaction: involvement of benzylic radical intermediates.
Figure 12.
A. Two ways to access (+)-ambiguine H (5a) from (−)-hapalindole U (4a), as well as the unusual heterolytic stability of the N–B bond in compound 43. B. Boron substituent influencing reaction pathways.
3. Conclusion: A Strategic Perspective
In conclusion, we developed concise, enantiospecific syntheses of the alkaloids hapalindole U (4a) and ambiguine H (5a) without resorting to protecting group manipulations.11,30 It is interesting to note that the first disclosure of these syntheses11 and a review in 200930b have led to an explosive increase in the number of protecting-group-free syntheses.30c While the avoidance of protecting groups is obviously beneficial in terms of streamlining these syntheses (both by step count and atom economy),11 one could argue that, in this work, the real benefit to their exclusion was in the arena of discovery. Novel intermediates and cascade reactions would likely have not been observed had typical bond constructions with protected heteroatoms been performed. These endeavors also highlight the role serendipity plays in natural product synthesis, as one could argue that the final synthetic routes are more interesting than the original, more concise, retrosynthesis of ambiguine H.
4. Experimental Section
4.1. General
All reactions were carried out under a nitrogen atmosphere with dry solvents under anhydrous conditions, unless otherwise noted. Dry tetrahydrofuran (THF), triethylamine (Et3N), dichloromethane (DCM), methanol (MeOH), dimethylformamide (DMF), diethyl ether (Et2O) and benzene were obtained by passing commercially available pre-dried, oxygen-free formulations through activated alumina columns. Tetra-n-butyl ammonium bromide (TBAB), sodium formate, ammonium acetate, sodium iodide, and copper(II) 2-ethylhexanoate were dried and kept stored under high vacuum prior to use. Herrmann's catalyst 27 [Pd(P(o-tol)3)OAc]2 was freshly prepared according to standard procedures.24 Yields refer to chromatographically and spectroscopically (1H NMR) homogeneous materials, unless otherwise stated. Reagents were purchased at the highest commercial quality and used without further purification, unless otherwise stated. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-254) using UV light as visualizing agent and p-anisaldehyde in ethanol/aqueous H2SO4/CH3CO2H and heat as developing agents. NMR spectra were recorded on a Bruker DRX 600, DRX 500, or AMX 400 spectrometer and were calibrated using residual undeuterated solvent as an internal reference. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, b = broad. IR spectra were recorded on a Perkin-Elmer Spetrum BX spectrometer. High-resolution mass spectra (HRMS) were recorded on an Agilent mass spectrometer using ESI-TOF (electrospray ionization-time-of-flight) or a ThermoFinnigan mass spectrometer using FAB (fast atom bombardment) or EI (electron impact). Low-resolution mass spectra (LRMS) were recorded on an Agilent or ThermoFinnigan mass spectrometer. Photochemical reactions were conducted using a 450-watt Hanovia lamp with a quartz filter. Melting points (m.p.) are uncorrected and were recorded on a Fisher-Johns 12-144 melting point apparatus. Optical rotations were obtained on a Perkin-Elmer 431 polarimeter. All microwave reactions were performed in a Biotage initiator microwave. Sonications were carried out in a Fisher Scientific FS30H ultrasonic cleaning bath. Azeotroping refers to dissolving the compound to be dried in benzene and removing the solvent by rotary evaporation.
4.2. Experimental procedures and data of synthetic intermediates
4.2.1. Cyclopropane 12
Step 1: A flame-dried flask was charged with p-menth-1-en-9-ol (11a: 6.79 g, 44.1 mmol, 1.0 equiv., inseparable but inconsequential mixture of diastereomers), Zn dust (11.5 g, 176.0 mmol, 4.0 equiv.), and Et2O (400 mL). The flask was placed in an ultrasound bath, and freshly distilled trichloroacetyl chloride (19.5 mL, 174.7 mmol, 4.0 equiv.) in Et2O (200 mL) was added dropwise to the sonicating solution over the course of 1 h at 25 °C. Sonication was continued for 6 h while maintaining a bath temperature of 25–30 °C by the periodic addition of ice. The reaction mixture was filtered through a plug of Celite® and concentrated in vacuo. The dark red oil was partitioned between Et2O (350 mL) and water (350 mL). The aqueous layer was extracted with Et2O (100 mL, 4X). The combined organic layers were washed with saturated NaHCO3 (400 mL, 2X) then brine (400 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo to give a dark red oil. Flash column chromatography (silica gel, gradient from 2:1 to 1:1 hexanes:DCM) gave a yellow oil (10.9 g, 66%). [NOTE: the intermediate cyclobutanone was prone to slight decomposition on silica gel. Using crude material for the next step gives a similar overall yield.] Step 2: To a flame-dried flask was added the aforementioned cyclobutanone (1.06 g, 2.82 mmol, 1.0 equiv.), NaOMe (765 mg, 13.4 mmol, 4.8 equiv.), and anhydrous MeOH (28 mL). The mixture was placed into a pre-heated oil bath at 65 °C and heated for 30 min. Upon cooling, the reaction mixture was poured into 1 N HCl (100 mL) and extracted with EtOAc (100 mL, 3X). The combined organic layers were washed with 1 N HCl (200 mL, 2X) then brine (200 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo to give a red oil. Flash column chromatography (silica gel, gradient from 2:1 to 1:1 hexanes:Et2O) yielded a yellow oil (671 mg, 61% yield over 2 operations) as an inseparable but inconsequential mixture of four diastereomers (two at the ester bearing carbon, each of which is a mixture of two diastereomers at the α-hydroxy bearing carbon).
4.2.2. Mesylate 13
To a solution of cyclopropane 12 (767 mg, 2.99 mmol, mixture of diastereomers) in DCM (30 mL) was added a solution of DIBAL (1.5 M in toluene, 10.0 mL, 15.0 mmol, 5.0 equiv.) dropwise at −78 °C. The reaction mixture was stirred for 30 min at −78 °C, then quenched by the dropwise addition of MeOH (5 mL). The reaction mixture was warmed to room temperature, diluted with EtOAc (200 mL) and saturated Rochelle's salt solution (200 mL) and vigorously stirred overnight. The layers were separated and the aqueous layer was extracted with EtOAc (100 mL, 4X). The combined organic layers were dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, gradient from 1:1 to 3:2 to 1:0 EtOAc:hexanes) furnished the corresponding diol (560 mg, 82%, inseparable but inconsequential mixture of diastereomers). The diol (540 mg, 2.36 mmol, mixture of diastereomers) was dissolved in anhydrous pyridine (25 mL) and cooled to 0 °C. Methanesulfonyl chloride (0.55 mL, 7.10 mmol, 3.0 equiv.) was slowly added dropwise to the solution at 0 °C and stirring was continued for 75 min at this temperature. The reaction mixture was then warmed to room temperature and stirred for an additional 30 min before pouring into a mixture of 1 N HCl (100 mL) and Et2O (200 mL). The aqueous layer was extracted with Et2O (100 mL, 4X). The combined organic extracts were washed with 1 N HCl (200 mL, 2X) and brine (200 mL). The solvent was removed in vacuo to give an oil, which was then dissolved in 4:1 AcOH:H2O (35 mL) and stirred for 2.5 h at 23 °C (to hydrolyze any enol ethers that are formed). The reaction mixture was diluted with Et2O (150 mL) and H2O (150 mL) and the aqueous layer was extracted with Et2O (100 mL, 4X). The combined organic layers were washed with saturated NaHCO3 (500 mL, 4X, carefully) then brine (250 mL, 2X), then dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, gradient from 1:1 to 2:1 Et2O:hexanes) gave 13 as a clear oil (576 mg, 89%, mixture of two diastereomers).
4.2.3. Ketone (−)-9
Mesylate 13 (485 mg, 1.76 mmol) was dissolved in acetone (10 mL). Dry sodium iodide (2.63 g, 17.6 mmol, 10.0 equiv.) was added and the reaction mixture was heated at reflux for 15 h. Upon cooling to 23 °C, the reaction was partitioned between Et2O (100 mL) and H2O (100 mL) and the aqueous layer was extracted with Et2O (75 mL, 3X). The combined organic layers were washed with saturated Na2S2O3 (200 mL, 2X) then brine (200 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo to give an oil, which was azeotropically dried with benzene, then dissolved in THF (10 mL). To this solution was added DBU (1.33 mL, 8.82 mmol, 5.0 equiv.) and the reaction was degassed by bubbling argon through the mixture for 5 min in an ultrasound bath. The reaction mixture was then heated for 3 h at 65 °C under argon atmosphere. Upon cooling to 23 °C, the mixture was partitioned between 1 N HCl (50 mL) and Et2O (100 mL) and the aqueous layer was extracted with Et2O (50 mL, 4X). The combined organic layers were washed with 1 N HCl (200 mL, 2X) then brine (200 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo to give a yellow liquid. Flash column chromatography (silica gel, 15:1 hexanes:Et2O) gave (−)-9 (272 mg, 87%) as a clear liquid. TLC: Rf = 0.66 (silica gel, hexanes:Et2O, 2:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −24.9 (c = 2.4 g•cm–3 in DCM); 1H NMR (400 MHz, CDCl3): δ 6.04 (dd, J = 10.9, 17.6 Hz, 1 H), 5.09 (d, J = 10.9 Hz, 1 H), 4.98 (d, J = 18.2 Hz, 1 H), 4.77 (s, 1 H), 4.67 (s, 1 H), 2.54–2.40 (m, 3 H), 1.88–1.72 (m, 4 H), 1.70 (s, 3 H), 1.20 (s 3 H); 13C NMR (126 MHz, CDCl3, APT): δ 213.1, 146.9, 142.6, 113.3, 110.6, 50.6, 44.8, 42.9, 35.9, 25.4, 22.8, 20.9; IR (film): y= 3082, 2932, 1707, 1644, 1453, 1311, 1246, 1097, 1000, 896 cm–1; HRMS (m/z): [M+H]+ calcd for C12H18O + H+, 179.1430; found, 179.1422.
4.2.4. Coupled product 8
Indole (10: 1.00 g, 8.54 mmol, 1.9 equiv) was azeotropically dried with benzene (2X) and the residual solvent was removed under high vacuum. ent-9 (792 mg, 4.44 mmol, 1.0 equiv.) and THF (25 mL) were added, and the mixture was cooled to −78 °C under an atmosphere of dry nitrogen. Freshly prepared LiHMDS (1.0 M in THF, 15 mL, 15 mmol, 3.4 equiv.) was added dropwise to the solution at −78 °C and the reaction was stirred for 30 min at that temperature. Copper(II) 2-ethylhexanoate (0.2 M solution in THF, 33 mL, 6.6 mmol, 1.5 equiv.) was added rapidly via syringe. The mixture was stirred for 5 min at −78 °C then warmed to 23 °C and immediately poured into 1 N HCl (150 mL) and EtOAc (150 mL). The aqueous layer was extracted with EtOAc (100 mL, 3X). The combined organic layers were washed with 1 N HCl (500 mL), 1 N NaOH (500 mL) then brine (500 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, gradient from 10:1 to 5:1 hexanes:EtOAc) to give the coupled product ent-8 (794 mg, 61%) as a white solid. [Note: this compound was prepared with ent-compound 9, i.e., whose absolute configuration is opposite to the ones depicted in the figures.] m.p.: 151–153 °C; TLC: Rf = 0.33 (silica gel, hexanes:Et2O, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +47.4 (c = 3.65 g•cm–3 in DCM); 1H NMR (500 MHz, CDCl3): δ 8.14 (bs, 1 H), 7.37 (d, J = 7.8 Hz, 1 H), 7.16 (d, J = 7.9 Hz, 1 H), 7.12 (t, J = 6.8 Hz, 1 H), 7.07 (t, J = 7.3 Hz, 1 H), 6.61 (d, J = 2.35 Hz, 1 H), 6.35 (dd, J = 11.0, 17.7 Hz, 1 H), 5.18 (d, J = 11.0 Hz, 1 H), 5.14 (d, J = 17.7 Hz, 1 H), 4.64 (s, 1 H), 4.57 (s, 1 H), 4.23 (d, J = 12.4 Hz, 1 H), 2.97 (td, J = 3.9, 12.0 Hz, 1 H), 2.27–2.18 (m, 1 H), 2.11 (td, J = 3.8, 13.4 Hz, 1 H), 2.02 (dt, J = 3.5, 13.5 Hz, 1 H), 1.96–1.91 (m, 1 H), 1.60 (s, 3 H), 1.57 (s, 3 H); 13C NMR (126 MHz, CDCl3): δ 212.2, 146.4, 143.0, 136.0, 127.1, 123.6, 121.2, 118.8, 118.7, 112.2, 112.0, 111.3, 110.8, 52.3, 50.6, 47.9, 36.7, 27.5, 22.9, 18.5; IR (film): y= 3369, 2931, 1701, 1642, 1457, 1373, 1340, 1247, 1099, 1011, 914, 893 cm–1; HRMS (m/z): [M+H]+ calcd for C20H23NO + H+, 294.1852; found, 294.1848.
4.2.5. C2-cyclized side product 14
Representative procedure for undesired cyclization: To a flame-dried flask was added coupled product ent-8 (11.4 mg, 0.039 mmol, 1 equiv.), DCM (0.75 mL), and MeOH (4.7 μl, 0.12 mmol, 3.0 equiv.). The flask was cooled to 0 °C and TMSOTf (23 μl, 0.12 mmol, 3.0 equiv.) was added dropwise. The reaction mixture was stirred at 0 °C for 1 h, then quenched with saturated aqueous sodium bicarbonate (1 mL) at 0 °C. DCM (5 mL) was added and the layers separated. The aqueous layer was extracted with DCM (5 mL, 3X). The combined organic layers were washed with brine (25 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by preparative thin-layer silica gel chromatography (1:1 hexanes:Et2O) to give ent-14 (2.0 mg, 18%) as a white foam and recovered starting material (8.4 mg, 74%). [Note: this compound was prepared with ent-8, i.e., whose absolute configuration is opposite to the ones depicted in the figures.] TLC: Rf = 0.32 (silica gel, hexanes:Et2O, 2:1 v/v); [α ]D20 (deg•cm3•g–1•dm–1): −109.3 (c = 4.0 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 7.86 (bs, 1 H), 7.76 (m, 1 H), 7.31–7.28 (m, 1 H), 7.12–7.10 (m, 2 H), 6.24 (dd, J = 12, 18 Hz, 1 H), 5.17 (d, J = 12 Hz, 1 H), 5.14 (d, J = 18 Hz, 1 H), 4.05 (d, J = 12 Hz, 1 H), 2.44–2.39 (m, 1 H), 2.03–1.91 (m, 3 H), 1.87–1.83 (m, 1 H), 1.47 (s, 3 H), 1.38 (s, 3 H), 1.16 (s, 3 H); 13C NMR (126 MHz, CDC13): δ 211.9, 151.2, 143.0, 139.8, 124.6, 121.2, 120.5, 120.4, 113.2, 113.0, 111.5, 63.1, 51.6, 51.3, 41.2, 39.0, 25.3, 23.8, 21.4, 20.6; IR (film): y= 3393, 2958, 2927, 2864, 1705, 1449, 1386, 1297, 1245, 1164, 1109, 1033, 1008, 918, 743 cm–1; HRMS (m/z): [M+H]+ calcd for C20H23NO + H+, 294.1852; found, 294.1847.
4.2.6. C2-reverse prenylated indole 19
ent-8 (26 mg, 0.09 mmol, 1 equiv.) was azeotropically dried with benzene. THF (0.85 mL) and Et3N (15 μL, 0.11 mmol, 1.2 equiv.) were added and the solution was cooled to −78 °C. Freshly prepared tBuOCl (13 μL, 0.11 mmol, 1.3 equiv) was added and the reaction mixture was stirred for 30 min at −78 °C. Prenyl-9-BBN (17: 1.0 M solution in THF, 0.18 mL, 0.18 mmol, 2 equiv.) was added dropwise at −78 °C and the mixture was stirred for 45 min at this temperature, then warmed to 23 °C. The mixture was poured into saturated aqueous NaHCO3 (5 mL) and EtOAc (5 mL). The aqueous layer was extracted with EtOAc (5 mL, 3X). The combined organic layers were washed with water (25 mL) then brine (25 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, 15:1 hexanes:EtOAc) to give tentative intermediate 18. This compound was allowed to stand in CDCl3 for 30 min then concentrated in vacuo and dried under high vacuum. The material was re-purified by flash column chromatography (silica gel, 10:1 hexanes:EtOAc) to give ent-19 (25.1 mg, 78%) as a white crystalline solid. [Note: this compound was prepared with ent-compound 8, i.e., whose absolute configuration is opposite to the ones depicted in the figures.] Recrystallization from EtOAc gave slightly yellow cubes that were suitable for X-ray diffraction (CCDC# 1023595); m.p.: 170–173 °C; TLC: Rf = 0.23 (silica gel, hexanes:Et2O, 2:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +13.6 (c = 4.2 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 7.85 (bs, 1 H), 7.30 (d, J = 6 Hz, 1 H), 7.25 (d, J = 6 Hz, 1 H), 7.06 (t, J = 6 Hz, 1 H), 6.97 (t, J = 6 Hz, 1 H), 6.32 (dd, J = 12, 18 Hz, 1 H), 6.10 (dd, J = 6, 12 Hz, 1 H), 5.20 (dd, J = 18 Hz, 1 H), 5.11 (dd, J = 12 Hz, 2 H), 5.07 (dd, J = 18 Hz, 1 H), 4.53 (d, J = 24 Hz, 2 H), 4.48 (d, J = 12 Hz, 1 H), 3.26 (dt, J = 6, 12 Hz, 1 H), 2.18–2.05 (m, 2 H), 1.96–1.94 (m, 1 H), 1.91–1.87 (m, 1 H), 1.47 (s, 3 H), 1.46 (s, 3 H), 1.42 (s, 3 H), 1.41 (s, 3 H); 13C NMR (126 MHz, CDC13): δ 211.3, 146.8, 146.5, 143.6, 140.5, 134.7, 128.5, 121.0, 120.9, 119.0, 112.3, 112.1, 111.5, 110.7, 108.4, 50.5, 50.0, 49.8, 38.9, 36.8, 28.3, 27.7, 27.4, 23.0, 21.2; IR (film): y= 3407, 2920, 2850, 1701, 1460, 1375, 1012, 912, 739 cm–1; HRMS (m/z): [M+H] calcd for C25H31NO + H+, 362.2478; found, 362.2475.
4.2.7. Model compound 25
4-Bromoindole (24: 1.65 g, 8.4 mmol, 3.0 equiv.) was azeotropically dried with benzene (2X) and the residual solvent removed under high vacuum. Ketone 23 (465 mg, 2.80 mmol, 1.0 equiv.) and THF (2.8 mL) were then added, and the mixture was cooled to −78 °C under a dry nitrogen atmosphere. LiHMDS (1.0 M in THF, 12.3 mL, 12.3 mmol, 4.4 equiv.) was added dropwise at −78 °C and stirring was continued for 30 min. The rubber septum was quickly removed and solid copper(II) 2-ethylhexanoate (1.96 g, 5.6 mmol, 2.0 equiv.) was added rapidly in one portion at −78 °C followed by immediate replacement of the rubber septum. [Note: rapid stirring is essential and brief exposure of the reaction mixture to the atmosphere had a negligible effect on the overall outcome of the reaction]. The reaction was stirred for 5 min at −78 °C, then warmed to 23 °C and immediately poured into 1 N HCl (50 mL) and EtOAc (50 mL). The aqueous layer was extracted with EtOAc (50 mL, 3X). The combined organic layers were washed with 1 N HCl (250 mL), 1 N NaOH (250 mL) then brine (250 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, gradient from 7:1 to 5:1 hexanes:EtOAc) to give the title compound (498 mg, 50%) as a white solid; m.p.: 185–187 °C; TLC: Rf = 0.22 (silica gel, hexanes:Et2O, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +50.9 (c = 4.3 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 8.43 (bs, 1 H), 7.15 (d, J = 12 Hz, 1 H), 7.06 (t, J = 16 Hz, 1 H), 6.90 (m, 1 H), 6.86 (dt, J = 2, 8 Hz, 1 H), 5.25 (d, J = 12 Hz, 1 H), 4.76 (s, 1 H), 4.65 (s, 1 H), 2.81 (dt, J = 3, 12 Hz, 1 H), 2.26–2.18 (m, 1 H), 1.93–1.90 (m, 1 H), 1.82–1.75 (m, 2 H), 1.63 (s, 3 H), 1.49 (s, 3 H), 1.12 (s, 3 H); 13C NMR (126 MHz, CDC13): δ 214.9, 147.4, 137.4, 125.6, 125.1, 124.0, 122.4, 113.6, 112.4, 112.2, 110.9, 53.5, 47.2, 45.5, 40.8, 28.9, 26.0, 25.3, 18.7; IR (film): y= 3340, 2967, 2932, 1701, 1645, 1471, 1337, 1187, 1075, 910, 756 cm–1; HRMS (m/z): [M+H]+ calcd for C19H22BrNO + H+, 360.0957; found, 360.0957.
4.2.8. 7-Endo products 26 (major) and 26' (minor)
To a sealable vial was added compound 25 (15 mg, 0.04 mmol, 1 equiv.) and AIBN (5 mg, 0.04 mmol, 1.1 equiv.). The flask was then evacuated and back-filled with argon. Dry, degassed benzene (0.85 ml) and Bu3SnH (28 μl, 0.10 mmol, 2.5 equiv.) were added and the sealed vial was placed into a 100 °C oil bath for 1 h. After cooling to 23 °C, the volatiles were removed in vacuo and the crude material was purified by preparative thin-layer silica gel chromatography (2:1 hexanes:Et2O) to yield “upper diastereomer” 26' (2.6 mg, 22%) as a white crystalline solid and “lower diastereomer” 26 (6.2 mg, 53%) as a white crystalline solid as well.
The lower (major) diastereomer was recrystallized from cyclohexane/EtOAc to yield colorless needles suitable for X-ray diffraction (CCDC# 1023596). Data for “lower diastereomer” (major product) 26: m.p.: 185 °C; TLC: Rf = 0.24 (silica gel, hexanes:Et2O, 2:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −190 (c = 6.2 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 8.24 (bs, 1 H), 7.18 (d, J = 6 Hz, 1 H), 7.15 (s, 1 H), 7.05 (t, J = 12 Hz, 1 H), (d, J = 6 Hz, 1 H), 4.06 (d, J = 12 Hz, 1 H), 3.21 (d, J = 18 Hz, 1 H), 3.13 (dd, J = 6, 18 Hz, 1 H), 2.23–2.10 (m, 3 H), 1.90–1.87 (m, 1 H), 1.75–1.69 (m, 2 H), 1.34 (s, 3 H), 1.15 (s, 3 H), 1.01 (d, J = 6 Hz, 3 H); 13C NMR (126 MHz, CDC13): δ 215.2, 135.7, 132.3, 126.7, 125.0, 121.3, 120.2, 110.4, 108.6, 51.4, 47.3, 44.9, 43.4, 40.6, 37.5, 29.5, 26.2, 25.2, 12.8; IR (film): y= 3383, 2928, 1716, 1458, 1329, 1249, 1123, 1064, 1018, 776, 746 cm–1; HRMS (m/z): [M+H]+ calcd for C19H23NO + H+, 282.1852; found, 282.1846.
The upper (minor) diastereomer is a white crystalline solid that can be recrystallized from cyclohexane/EtOAc to yield colorless needles. Data for “upper diastereomer” (minor product) 26': m.p.: 129–131 °C; TLC: Rf = 0.40 (silica gel, hexanes:Et2O, 2:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +47.8 (c = 3.7 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 7.96 (bs, 1 H), 7.66 (s, 1 H), 7.16 (d, J = 12 Hz, 1 H), 7.03 (t, J = 6 Hz, 1 H), 6.76 (d, J = 12 Hz, 1 H), 4.39 (d, J = 12 Hz, 1 H), 3.67 (dd, J = 3, 15 Hz, 1 H), 2.68 (dd, J = 5, 15 Hz, 1 H), 2.21–2.16 (m, 1 H), 1.92–1.78 (m, 3 H), 1.67–1.62 (m, 1 H), 1.38–1.34 (m, 1 H), 1.27 (s, 3 H), 1.15 (s, 3 H), 0.89 (d, J = 7 Hz, 3 H); 13C NMR (126 MHz, CDCI3): δ 216.6, 135.0, 132.9, 127.5, 122.0, 119.6, 114.4, 108.9, 52.9, 47.6, 45.2, 39.6, 38.9, 38.1, 29.4, 26.3, 25.8, 20.8; IR (turn): y= 3400, 2959, 2923, 1703, 1456, 1338, 1105, 746 cm–1; HRMS (m/z): [M+H]+ calcd. for C19H23NO + H+, 282.1852; found, 282.1847.
4.2.9. Brominated coupled product (−)-22
4-Bromoindole (24: 3.13 g, 16.0 mmol, 2.8 equiv.) was azeotropically dried with benzene (2X) and the residual solvent was removed under high vacuum. Ketone (−)-9 (1.00 g, 5.61 mmol, 1.0 equiv.) and THF (5.6 mL) were then added, and the mixture was cooled to −78 °C under a dry nitrogen atmosphere. LiHMDS (1.0 M in THF, 24.6 mL, 24.6 mmol, 4.4 equiv.) was added dropwise at −78 °C and stirring was continued for 30 min. The rubber septum was quickly removed and solid copper(II) 2-ethylhexanoate (4.0 g, 11.4 mmol, 2.0 equiv.) was added rapidly in one portion at −78 °C followed by immediate replacement of the rubber septum. [Note: rapid stirring is essential and brief exposure of the reaction mixture to the atmosphere had a negligible effect on the overall outcome of the reaction]. The reaction was stirred for 5 min at −78 °C, then warmed to 23 °C and immediately poured into 1 N HCl (200 mL) and EtOAc (200 mL). The aqueous layer was extracted with EtOAc (125 mL, 3X). The combined organic layers were washed with 1 N HCl (600 mL), 1 N NaOH (600 mL) then brine (600 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, gradient from 4:1 to 3:1 to 2.5:1 to 1:1 hexanes:Et2O) to give (−)-22 (1.04 g, 50%) as a white solid [Note: excess 4-bromoindole can also be easily recovered]; m.p.: 130–132 °C; TLC: Rf = 0.59 (silica gel, hexanes:EtOAc, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −19.1 (c = 9.2 g•cm–3 in DCM); 1H NMR (400 MHz, CDC13): δ 8.41 (bs, 1 H), 7.15 (d, J = 7.5 Hz, 1 H), 7.06 (d, J = 7.9 Hz, 1 H), 6.87–6.83 (m, 2 H); 6.28 (dd, J = 11.0, 17.6 Hz, 1 H), 5.30 (d, J = 12.6 Hz, 1 H), 5.11 (d, J = 10.9 Hz, 1 H), 5.06 (d, J = 17.8 Hz, 1 H), 4.77 (s, 1 H), 4.66 (s, 1 H), 2.84 (td, J = 3.8, 12.3 Hz, 1 H), 2.31–2.20 (m, 1 H), 2.05–1.87 (m, 3 H), 1.64 (s, 3 H), 1.61 (s, 3 H); 13C NMR (151 MHz, CDC13): δ 212.8, 147.0, 143.2, 137.2, 125.4, 125.0, 123.9, 122.2, 113.4, 112.4, 112.0, 111.7, 110.7, 52.9, 50.7, 47.2, 37.5, 28.4, 22.6, 18.5; IR (film): y= 3349, 2934, 1699, 1426, 1337, 1186, 1120, 910, 735, 610 cm–1; HRMS (m/z): [M+H]+ calcd for C20H22BrNO + H+, 372.0957; found, 372.0966.
4.2.10. Reductive Heck cyclization product (−)-7
Brominated coupled product (−)-22 (1.12 g, 3.03 mmol, 1.0 equiv.) was azeotropically dried with benzene. Dry sodium formate (258 mg, 3.79 mmol, 1.2 equiv) and dry TBAB (1.96 g, 6.08 mmol, 2.0 equiv.) were added and the flask was evacuated, then backfilled with argon. DMF (30 mL) was then added, followed by Et3N (0.94 mL, 6.74 mmol, 2.2 equiv.). This mixture was degassed by three freeze-pump-thaw iterations and finally back-filled with argon. A solution of Herrmann's catalyst (27: 142 mg, 0.15 mmol, 0.05 equiv.) in DMF (20 mL) was degassed by three freeze-pump-thaw iterations and added dropwise over 5 h (syringe pump) to the substrate at 80 °C. The mixture was heated for an additional 3 h at 80 °C. Upon cooling to 23 °C, the reaction mixture was diluted with Et2O (100 mL) and filtered through a plug of Celite®. The mixture was poured into Et2O (100 mL) and H2O (100 mL) and the aqueous layer was thoroughly extracted with Et2O (100 mL, 5X). The combined organic layers were washed with 1 N HCl (500 mL), 1 N NaOH (500 mL) then brine (500 mL), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, gradient from 8:1 to 5:1 hexanes:Et2O) to give tetracyclic ketone (−)-7 (579 mg, 65%) as white crystals; m.p.: 149–151 °C; TLC: Rf = 0.14 (silica gel, hexanes:Et2O, 3:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −18.1 (c = 1.7 g•cm–3 in DCM); 1H NMR (600 MHz, CDCl3): δ 8.08 (bs, 1 H), 7.49 (t, J = 1.9 Hz, 1 H), 7.19–7.15 (m, 2 H), 7.03 (dd, J = 1.0, 6.5 Hz, 1 H), 6.24 (dd, J = 10.9, 17.6 Hz, 1 H), 5.17 (d, J = 10.9 Hz, 1 H), 5.11 (d, J = 17.7 Hz, 1 H), 3.96 (dd, J = 1.0, 11.5 Hz, 1 H) 2.11–1.92 (m, 5 H), 1.54 (s, 3 H), 1.48 (s, 3 H), 1.24 (s, 3 H); 13C NMR (151 MHz, CDCl3): δ 212.3, 143.0, 139.9, 133.4, 125.2, 122.4, 120.7, 112.7, 112.6, 108.6, 108.2, 51.6, 50.3, 44.4, 38.0, 37.1, 24.7, 24.6, 23.0, 21.3; IR (film): y= 3400, 3058, 2964, 1867, 1698, 1438, 1334, 1175, 1044, 1019, 911, 745 cm–1; HRMS (m/z): [M+H]+ calcd for C20H23NO + H+, 294.1852; found, 294.1847.
4.2.11. Formamide (−)-28
A flame-dried 20 mL Biotage microwave vessel was charged with dry NH4OAc (750 mg, 9.7 mmol, 40.0 equiv.), NaCNBH3 (115 mg, 1.83 mmol, 9.3 equiv.) and dry MeOH (10 mL) under a dry nitrogen atmosphere. Ketone (−)-7 (57.9 mg, 0.20 mmol, 1.0 equiv.) in THF (1 mL) was added to the MeOH solution and the mixture was exposed to microwave irradiation at 150 °C for 2.5 min [caution: high pressures and toxic gases are formed]. Upon cooling and venting the gases in a well-ventilated fume hood, the contents from 10 successive runs were combined, diluted with EtOAc (200 mL), poured into 1 N NaOH (250 mL), and the aqueous layer was thoroughly extracted with EtOAc (100 mL, 5X). The combined organic layers were washed with 1 N NaOH (500 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo and the crude amine was passed through a short plug of silica gel eluting with a gradient from 1:1 EtOAc:hexanes to 100% EtOAc to give a solid (430 mg), which was subsequently dissolved in DCM (20 mL). The following compounds were added sequentially to the mixture: formic acid (0.11 mL, 2.9 mmol, 2.0 equiv.), 2-chloro-4,6-dimethoxy-1,3,5-triazine (565 mg, 3.22 mmol, 2.2 equiv.), 4-(dimethylamino)pyridine (10 mg, 0.08 mmol, 0.056 equiv.), and N-methylmorpholine (0.36 mL, 3.28 mmol, 2.2 equiv.). The resulting slurry was stirred at 23 °C for 2 h, diluted with DCM (100 mL), and poured into saturated NaHCO3 (150 mL). The aqueous layer was thoroughly extracted with DCM (100 mL, 5X). The combined organic layers were washed with 1 N HCl (500 mL, 2X), brine (500 mL, 2X), and dried (Na2SO4). The solvent was removed in vacuo to give a solid, which was purified by flash column chromatography (silica gel, gradient from 2:1 to 4:1 Et2O:hexanes) to give formamide (−)-28 (411 mg, 64 %, mixture of E and Z isomers) as a white solid; m.p.: >250 °C; TLC: Rf = 0.37 (silica gel, Et2O); [α]D20 (deg•cm3•g–1•dm–1): −79.7 (c = 0.77 g•cm–3 in DCM:MeOH 2:1 v/v); 1H NMR (600 MHz, CDCl3): δ 8.16 (bs, 1 H), 7.90 (d, J = 1.9 Hz, 1 H), 7.17–7.15 (m, 2 H), 7.02 (dd, J = 1.8, 6.1 Hz, 1 H) 6.96 (t, 1.86 Hz, 1 H), 5.98 (dd, J = 10.9, 17.5 Hz, 1 H), 5.51 (d, J = 10.7 Hz, 1 H), 5.01 (dd, J = 1.0, 10.9 Hz, 1 H), 4.98 (dd, J = 1.0, 17.5 Hz, 1 H), 4.75 (dd, J = 3.4, 10.9 Hz, 1 H), 3.42 (ddd, J = 1.4, 3.5, 12 Hz, 1 H), 1.98–1.96 (m, 1 H), 1.74–1.62 (m, 4 H), 1.50 (s, 3H), 1.32 (s, 3 H), 1.15 (s, 3 H); 13C NMR (151 MHz, CDCl3): δ 161.4, 146.8, 140.6, 133.9, 125.5, 122.6, 117.3, 112.9, 112.6, 111.4, 108.3, 52.3, 44.7, 40.1, 37.4, 33.6, 30.1, 24.9, 24.5, 23.6, 21.2; IR (film): y= 3402, 2961, 1672, 1517, 1393, 1100, 906, 769, 734, 581 cm–1; HRMS (m/z): [M+H]+ calcd for C21H26N2O + H+, 323.2118; found, 323.2115.
4.2.12. Hapalindole U (−)-4a
A flame-dried flask was charged with formamide (−)-28 (315 mg, 0.98 mmol, 1.0 equiv.) under an atmosphere of dry nitrogen. DCM (60 mL) and Et3N (2.4 mL, 17.2 mmol, 17.6 equiv.) were added, and the mixture was cooled to 0 °C. Phosgene (20 wt% solution in toluene) was carefully added dropwise until TLC analysis showed complete consumption of starting material [caution: phosgene is highly toxic and this reaction should be performed carefully in a well-ventilated fume hood]. The reaction was quenched at 0 °C by the dropwise addition of saturated NaHCO3 (50 mL) and then warmed to 23 °C. The reaction mixture was thoroughly extracted with DCM (75 mL, 5X). The combined organic layers were washed with saturated NaHCO3 (400 mL, 2X) then brine (400 mL, 2X), then dried (Na2SO4). The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, gradient from 3:1 to 2:1 hexanes:Et2O) to give hapalindole U (−)-4a as a white solid (277 mg, 93%). Crystallization from hexanes/Et2O/MeOH yielded white needles of suitable quality for X-ray diffraction (CCDC# 623050); m.p.: 241 °C (decomposition); TLC: Rf = 0.32 (silica gel, hexanes:Et2O, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −2.0 (c = 0.6 g•cm–3 in DCM); 1H NMR (600 MHz, CDCl3): δ 8.00 (bs, 1 H), 7.19–7.18 (m, 2 H), 7.04–7.03 (m, 1 H), 6.90 (bt, 1 H), 6.05 (dd, J = 10.9, 17.5 Hz, 1 H), 5.19 (d, J = 10.9 Hz, 1 H), 5.18 (d, J = 17.4 Hz, 1 H), 4.10 (bd, 1 H), 3.28–3.27 (m, 1 H), 2.03–1.90 (m, 3 H), 1.68–1.59 (m, 2 H), 1.50 (s, 3 H), 1.28 (s, 3 H), 1.15 (s, 3 H); 13C NMR (151 MHz, CDCl3): δ 156.4, 145.4, 140.9, 134.1, 125.6, 123.0, 116.3, 113.3, 112.9, 112.8, 108.2, 63.4, 43.4, 39.3, 37.1, 33.7, 30.0, 25.2, 24.4, 21.6, 21.0; IR (film): y= 3378, 2962, 2142, 1602, 1437, 1334, 1173, 914, 771; HRMS (m/z): [M+Na]+ calcd for C21H24N2 + Na+, 327.1832; found, 327.1846.
4.2.13. N-Prenylated hapalindole 30
Hapalindole U (−)-4a (6.0 mg, 0.02 mmol, 1 equiv.) was azeotropically dried with benzene and the residual solvent was removed under high vacuum. DMF (0.50 mL) was added and the reaction was cooled to 0 °C. NaH (60% dispersion in mineral oil, 2.0 mg, 0.05 mmol, 2.5 equiv.) was added, at which point the mixture became bright yellow. The solution was stirred for 15 min at 0 °C, then prenyl bromide (10 μL, 0.09 mmol, 4.4 equiv.) was added and the solution became clear. After stirring for 7 min at 0 °C, the reaction was quenched with 1 N HCl (1 mL). The mixture was partitioned between saturated NH4Cl (5 mL) and Et2O (2 mL) and the aqueous layer was extracted with Et2O (5 mL, 2X). The combined organic layers were washed with brine (10 ml), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by preparative thin-layer silica gel chromatography (3:1 hexanes:Et2O) to give N-prenylated product 30 (5.5 mg, 75%) as a white solid; m.p.: 115 °C; TLC: Rf = 0.41 (silica gel, hexanes:Et2O, 4:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +184 (c = 4.7 g•cm in CHC3); 1H NMR (600 MHz, CDCl3): δ 7.17 (t, J = 12 Hz, 1 H), 7.11 (d, J = 12 Hz, 1 H), 7.0 (d, J = 12 Hz, 1 H), 6.8 (s, 1 H), 6.05 (dd, J = 12, 18 Hz, 1 H), 5.4 (t, J = 6 Hz, 1 H), 5.18 (d, J = 12 Hz, 1 H), 5.17 (d, J = 18 Hz, 1 H), 4.67 (d, J = 6 Hz, 2 H), 4.07 (s, 1 H), 3.27 (d, J = 6 Hz, 1 H), 2.02–1.88 (m, 3 H), 1.82 (s, 3 H), 1.76 (s, 3 H), 1.65–1.58 (m, 2 H), 1.49 (s, 3 H), 1.26 (s, 3 H), 1.14 (s, 3 H); 13C NMR (151 MHz, CDCl3): δ 155.5, 145.7, 141.1, 136.2, 134.6, 126.3, 122.5, 120.5, 119.7, 113.4, 112.5, 111.5, 107.0, 63.5, 44.5, 43.6, 39.4, 37.3, 33.9, 30.2, 25.8, 25.4, 24.6, 24.6, 21.7, 21.1, 18.2; IR (film): y= 2928, 2137, 1608, 1455, 1363, 1319, 1279, 1165, 1039, 918, 780 cm–1; HRMS (m/z): [M+H]+ calcd for C26H32N2 + H+, 373.2638; found, 373.2635.
4.2.14. C2-Reverse prenylated tetracyclic ketone 20 and C6-reverse prenylated tetracyclic ketone 34
Tetracyclic ketone 7 (29 mg, 0.10 mmol, 1 equiv.) was azeotropically dried with benzene and the residual solvent was removed under high vacuum. THF (2.0 mL) and Et3N (16.5 μL, 0.12 mmol, 1.2 equiv.) were added and the mixture was cooled to −78 °C. Freshly prepared tBuOCl (13.5 μL, 0.12 mmol, 1.2 equiv.) was added to the cooled solution. After 25 min, prenyl-9-BBN (17: 1.0 M solution in THF, 0.20 ml, 2.0 equiv.) was added dropwise over the course of 5 min to the solution at −78 °C. The reaction color turned bright orange. The reaction mixture was stirred for 45 min at −78 °C then warmed to 23 °C and immediately partitioned between 1 N NaOH (5 mL) and EtOAc (5 mL). The reaction mixture was thoroughly extracted with EtOAc (5 mL, 5X). The combined organic layers were washed with 1 N NaOH (25 mL, 2X), 1 N HCl (25 ml) then brine (25 ml), then dried (MgSO4). The solvent was removed in vacuo and the crude material was purified by preparative thin-layer silica gel chromatography (5:1 hexanes:Et2O) to give 20 (9.3 mg, 26%) and 34 (7.0 mg, 19%) as white solids.
Data for C2-reverse prenylated tetracyclic ketone 20: The full compound characterization has not been obtained. Its structure has been assigned by its 1H NMR spectrum and by comparison to the data for 34 (shown below). 1H NMR (500 MHz, acetone-d6): δ 9.70 (bs, 1 H), 7.06 (d, J = 5 Hz, 1 H), 6.96 (t, J = 5 Hz, 1 H), 6.88 (d, J = 5 Hz, 1 H), 6.30 (dd, J = 15, 20 Hz, 1 H), 6.15 (dd, J = 10, 15 Hz, 1 H), 5.10–5.02 (m, 4 H), 4.42 (d, J = 15 Hz, 1 H), 2.12–1.97 (m, 3 H), 1.82–1.73 (m, 2 H), 1.57 (s, 3 H), 1.51 (s, 3 H), 1.48 (s, 3 H), 1.47 (s, 3 H), 1.09 (s, 3 H).
Compound 34 can be recrystallized from EtOAc to yield colorless needles suitable for X-ray diffraction (CCDC# 1023597). Data for C6-reverse prenylated tetracyclic ketone 34: m.p.: 162-165 °C (decomposition); TLC: Rf = 0.30 (silica gel, hexanes:Et2O, 2:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): −7.6 (c = 4.6 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDC13): δ 7.98 (bs, 1 H), 7.43 (s, 1 H), 7.18 (s, 1 H), 7.04 (s, 1 H), 6.22 (dd, J = 6, 18 Hz, 1 H), 6.12 (dd, J = 6, 12 Hz, 1 H), 5.16 (d, J = 6 Hz, 1 H), 5.09 (d, J = 18 Hz, 2 H), 5.04 (d, J = 12 Hz, 1 H), 3.92 (d, J = 12 Hz, 1 H), 2.09–1.88 (m, 5 H), 1.52 (s, 3 H), 1.46 (s, 9 H), 1.22 (s, 3 H); 13C NMR (151 MHz, CDC13): δ 212.5, 149.2, 143.6, 143.2, 139.2, 133.5, 123.7, 120.6, 112.8, 112.1, 110.1, 108.6, 105.7, 51.9, 50.5, 44.6, 41.8, 38.4, 37.2, 29.1, 29.0, 24.8, 24.8 23.2, 21.4; IR (film): y= 3404, 2926, 1707, 1464, 1362, 1011, 918, 862 cm–1; HRMS (m/z): [M+H]+ calcd for C25H31NO + H+, 362.2478; found, 362.2478.
4.2.15. Borono-chlorinated indoline (+)-43
Hapalindole U (−)-4a (100.0 mg, 0.33 mmol, 1.0 equiv.) was azeotropically dried with benzene (2X) and the residual solvent was removed under high vacuum. DCM (5.0 mL) was added, and the solution was cooled to −78 °C under an argon atmosphere. Freshly prepared BuOCl (43 μL, 0.38 mmol, 1.2 equiv.) was added dropwise and the solution was stirred at −78 °C for 12 min. Freshly prepared prenyl-9-BBN (17: 1.12 M solution in DCM, 600 μL, 0.672 mmol, 2.0 equiv.) was slowly added dropwise down the flask walls over the course of 5 min. Stirring was continued for 40 min at −78 °C before the reaction was quenched at low temperature by quickly transferring the contents of the flask to a small plug of silica gel and eluting with EtOAc. The solvent was removed in vacuo and the crude material was purified by flash column chromatography (silica gel, 20:1 hexanes:Et2O) to give borono-chlorinated indoline (+)-43 (104 mg, 60%) as a white solid. Crystallization from Et2O/DCM yielded clear plates of suitable quality for X-ray diffraction (CCDC# 623051). m.p.: 244 °C (decomposition); TLC: Rf = 0.48 (silica gel, hexanes:Et2O, 7:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +46.8 (c = 1.8 g•cm–3 in DCM); 1H NMR (500 MHz, CDC13): δ7.19 (t, J = 7.8 Hz, 1 H), 6.96 (d, J = 7.9 Hz, 1 H), 6.93 (d, J = 7.7 Hz, 1 H), 6.33 (dd, J = 10.9, 17.7 Hz, 1 H), 5.73 (dd, J = 10.7, 17.4 Hz, 1 H), 5.14 (dd, J = 1.3, 10.9 Hz, 1 H), 5.11 (dd, J = 1.3, 17.6 Hz, 1 H), 5.00 (dd, J = 1.0, 17.5 Hz, 1 H), 4.96 (dd, J = 1.0, 10.7 Hz, 1 H), 4.10 (s, 1 H), 3.81 (d, J = 2.1 Hz, 1 H), 2.87 (dd, J = 3.5, 11.4 Hz, 1 H), 2.05–1.87 (m, 9 H), 1.79–1.64 (m, 5 H), 1.57–1.42 (m, 5 H), 1.34 (s, 3 H), 1.14 (s, 3 H), 1.04 (s, 3 H), 0.99 (s, 3 H), 0.60 (s, 3 H); 13C NMR (151 MHz, CDC13): δ 168.1, 150.3, 147.2, 145.8, 145.5, 129.3, 129.1, 119.8, 115.3, 112.2, 111.4, 75.9, 66.7, 44.3, 42.0, 41.2, 38.9, 36.0, 33.6, 33.5, 33.4, 33.3, 32.9, 30.2, 28.0, 27.6, 26.0, 24.6, 24.3, 23.2, 22.9, 21.8, 21.1, 20.7; IR (film): y= 2927, 1596, 1452, 1405, 1340, 1006, 910 cm–1; HRMS (m/z): [M+H]+ calcd for C34H46BClN2 + H+, 529.3515; found, 529.3526.
4.2.16. Ambiguine H (+)-5a and C6-reverse prenylated hapalindole 46
Borono-chlorinated indoline 43 (22.5 mg, 0.04 mmol, 1.0 equiv.) was dissolved in degassed benzene (2.3 mL), and freshly distilled and degassed Et3N (30 μL) was added in a 5-mL Biotage microwave vessel. The mixture was sealed under an atmosphere of argon and irradiated for 5 h, at which point the solvent was decanted (to remove the highly crystalline Et3NHCl that is formed). The crude material was purified by preparative thin-layer chromatography to give recovered starting material (8.2 mg, 36%) along with ambiguine H (+)-5a (6.5 mg, 41%, 63% based on recovered starting material) as a white solid. [Note: the reaction cannot be run to full conversion since the product itself is photoreactive; under similar experimental conditions, de-boronated product 52 cleanly forms ambiguine H (+)-5a in #75% isolated yield in 2.5 h].
Ambiguine H (+)-5a was crystallized from hexanes/Et2O to give off-white plates suitable for X-ray diffraction (CCDC# 623052). Data for (+)-5a: m.p.: 228-231 °C; TLC: Rf = 0.57 (silica gel, hexanes:Et2O, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +20.0 (c = 0.18 g•cm–3 in DCM); 1H NMR (600 MHz, CDC13): δ7.98 (bs, 1 H), 7.13–7.09 (m, 2 H), 6.98 (d, J = 6.5 Hz, 1 H), 6.21 (dd, J = 10.6, 17.5 Hz, 1 H), 5.93 (dd, J = 10.9, 17.5 Hz, 1 H), 5.25 (d, J = 17.5 Hz, 1 H), 5.19 (d, J = 10.6 Hz, 1 H), 5.14 (d, J = 10.9 Hz, 1 H), 5.11 (d, J = 17.5 Hz, 1 H), 4.49 (s, 1 H), 3.18–3.16 (m, 1 H), 2.10–1.90 (m, 3 H), 1.60–1.50 (m, 2 H), 1.58 (s, 3 H), 1.52 (s, 3 H), 1.50 (s, 3 H), 1.23 (s, 3 H), 1.02 (s, 3 H); 13C NMR (151 MHz, CDC13): δ 146.3, 145.8, 140.6, 136.7, 132.2, 127.3, 122.0 113.0, 112.9, 112.4, 107.5, 106.7, 65.1, 43.9, 39.9, 38.7, 36.3, 34.8, 30.6, 29.7, 29.2, 27.8, 24.9, 24.0, 21.71, 21.68; IR (film): y= 3345, 2923, 2362, 2134, 1636, 1444, 1327, 998, 915 cm–1; HRMS (m/z): [M+H]+ calcd for C26H32N2 + H+, 373.2638; found, 373.2636.
Data for C6-reverse prenylated hapalindole 46: white solid; TLC: Rf = 0.25 (silica gel, hexanes:Et2O, 2:1 v/v); [α[D20 (deg•cm3•g–1•dm–1): −10.0 (c = 1.0 g•cm–3 in DCM); 1H NMR (600 MHz, CDC13): δ7.92 (bs, 1 H), 7.18 (d, J = 1 Hz, 1 H), 7.06 (d, J = 1 Hz, 1 H), 6.86 (t, J = 2 Hz, 1 H), 6.12 (dd, J = 12, 18 Hz, 1 H), 6.04 (dd, J = 12, 18 Hz, 1 H), 5.19 (d, J = 6 Hz, 1 H), 5.17 (d, J = 12 Hz, 1 H), 5.10 (dd, J = 1, 18 Hz, 1 H), 5.05 (dd, J = 1, 12 Hz, 1 H), 4.08 (s, 1 H), 3.25 (d, J = 6 Hz, 1 H), 2.01–1.88(m, 4 H), 1.66–1.60 (m, 1 H), 1.48 (s, 3 H), 1.47 (s, 6 H), 1.27 (s, 3 H), 1.14 (s, 3 H); 13C NMR (151 MHz, CDC13): δ 156.6, 145.6, 144.2, 140.2, 134.2, 124.0, 116.2, 113.4, 112.7, 110.2, 105.7, 63.5, 43.8, 41.9, 39.4, 37.5, 33.9, 30.2, 29.0, 29.0, 25.4, 24.5, 21.7, 21.1; IR (film): y= 3411, 2967, 2844, 2136, 1454, 1346, 1054, 1033, 1012, 911 cm–1; HRMS (m/z): [M+H]+ calcd for C26H32N2 + H+, 373.2638; found, 373.2634.
4.2.17. Chlorinated indoline (+)-52
Hapalindole U (−)-4a (10 mg, 0.03 mmol, 1.0 equiv.) was azeotropically dried with benzene and the residual solvent was removed under high vacuum. DCM (0.6 mL) was added, and the solution was cooled to −78 °C under an argon atmosphere. Freshly prepared tBuOCl (4.5 μL, 0.04 mmol, 1.2 equiv.) was added dropwise and the solution was stirred at −78 °C for 15 min. Freshly prepared prenylmagnesium chloride (0.75 M solution in THF, 90 μL, 0.07 mmol, 2.1 equiv) was slowly added dropwise down the flask walls over the course of 2 min. Stirring was continued for 25 min at −78 °C before the reaction was warmed to 0 °C and quickly transferred to a small plug of silica gel and eluted with EtOAc. The solvent was removed in vacuo and the crude material was purified by preparative thin-layer silica gel chromatography (1:1 hexanes:Et2O) to give chlorinated indoline (+)-52 (5.3 mg, 40%) as an oil that slowly solidified to a white solid; TLC: Rf = 0.50 (silica gel, hexanes:Et2O, 1:1 v/v); [α]D20 (deg•cm3•g–1•dm–1): +144 (c 4.6 g•cm–3 in CHCl3); 1H NMR (600 MHz, CDCI3): δ7.09 (t, J = 6 Hz, 1 H), 6.69 (d, J = 6 Hz, 1 H), 6.46 (d, J = 6 Hz, 1 H), 6.33 (dd, J = 12, 18 Hz, 1 H), 5.72 (dd, J = 6, 12 Hz, 1 H), 5.13–5.01 (m, 4 H), 4.03 (bs, 1 H), 3.84 (d, J = 2 Hz, 1 H), 3.42 (s, 1 H), 2.86 (dd, J = 6, 12 Hz, 1 H), 1.76–1.73 (m, 1 H), 1.70–1.65 (m, 1 H), 1.58–1.52 (m, 2 H), 1.39–1.34 (m, 1 H), 1.32 (s, 3 H), 1.16 (s, 3 H), 1.00 (s, 3 H), 0.87 (s, 6 H); 13C NMR (151 MHz, CDC13): δ 169.1, 151.9, 147.2, 145.5, 145.3, 129.9, 122.1, 115.1, 113.3, 111.5, 105.7, 76.7, 75.2, 67.3, 45.1, 42.5, 41.6, 39.1, 36.1, 34.2, 26.8, 24.9, 24.1, 23.4, 22.5, 21.0; IR (film): y= 3378, 2967, 2933, 1601, 1455, 1414, 1363, 1246, 1098, 1064, 1013, 912, 788, 743 cm–1; HRMS (m/z): [M+H]+ calcd for C26H33ClN2 + H+, 409.2405; found, 409.2402.
4.3. X-ray crystallographic data
Crystallographic data for structures 4a, 5a, 19, 26, 34 and 43 have been deposited with the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge from http://www.ccdc.cam.ac.uk/products/csd/request/ (CCDC number 623050 for 4a, 623052 for 5a, 1023595 for 19, 1023596 for 26, 1023597 for 34, and 623051 for 43).
Supplementary Material
Acknowledgments
We thank D.-H. Huang and L. Pasternack for assistance in NMR spectroscopy, G. Siuzdak for assistance in mass spectrometry, and R. Chadha for assistance in X-ray crystallographic analysis. Financial support for this work was provided by the NIH/NIGMS (GM-071498), the Beckman Foundation, the Searle Scholarship Fund, and the Sloan Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Isolation of novel hapalindoles. Hapalindoles A through X, along with some 12-epi variants, are currently known: Moore RE, Cheuk C, Patterson GML. J Am Chem Soc. 1984;106:6456–6457.; Moore RE, Cheuk C, Yang XQG, Patterson GML, Bonjouklian R, Smitka TA, Mynderse JS, Foster RS, Jones ND, Swartzendruber JK, Deeter JB. J Org Chem. 1987;52:1036–1043.; Klein D, Daloze D, Braekman JC, Hoffmann L, Demoulin V. J Nat Prod. 1995;58:1781–1785. doi: 10.1021/np9900324.; Becher PG, Keller S, Jung G, Sussmuth RD, Juttner F. Phytochemistry. 2007;68:2493–2497. doi: 10.1016/j.phytochem.2007.06.024.; Kim H, Lantvit D, Hwang CH, Kroll DJ, Swanson SM, Franzblau SG, Orjala J. Bioorg Med Chem. 2012;20:5290–5295. doi: 10.1016/j.bmc.2012.06.030.
- 2.Isolation of novel fischerindoles. Fischerindoles were named based on their similarity to the hapalindoles, and therefore many alphabets are missing in the nomenclature of the fischerindole alkaloids: Park A, Moore RE, Patterson GML. Tetrahedron Lett. 1992;33:3257–3260.; Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935–9942.; Kim H, Krunic A, Lantvit D, Shen Q, Kroll DJ, Swanson SM, Orjala J. Tetrahedron. 2012;68:3205–3209. doi: 10.1016/j.tet.2012.02.048.
- 3.Isolation of novel welwitindolinones. Welwitindolinones A through D, along with some 3-epi, N-methyl, and isothiocyanate variants are known: (a) ref. 2b; Jimenez JI, Huber U, Moore RE, Patterson GML. J Nat Prod. 1999;62:569–572. doi: 10.1021/np980485t.
- 4.Isolation of novel ambiguines and fischambiguines. Ambiguines A through Q are known: Smitka TA, Bonjouklian R, Doolin L, Jones ND, Deeter JB, Yoshida WY, Prinsep MR, Moore RE, Patterson GML. J Org Chem. 1992;57:857–861.; Huber U, Moore RE, Patterson GML. J Nat Prod. 1998;61:1304–1306. doi: 10.1021/np9801561.; Raveh A, Carmeli S. J Nat Prod. 2007;70:196–201. doi: 10.1021/np060495r.; Shunyan M, Krunic A, Chlipala G, Orjala J. J Nat Prod. 2009;72:894–899. doi: 10.1021/np800751j.; Shunyan M, Krunic A, Santarsiero BD, Franzblau SG, Orjala J. Phytochemistry. 2010;71:2116–2123. doi: 10.1016/j.phytochem.2010.09.004.
- 5.Isolation of hapalindolinones, anhydrohapaloxindoles, and fontonamides: (a) ref. 1e and 4e; Schwartz RE, Hirsch CF, Springer JP, Pettibone DJ, Zink DL. J Org Chem. 1987;52:3704–3706.; Moore RE, Yang XqG, Patterson GML. J Org Chem. 1987;52:3773–3777.; Moore RE, Yang XqG, Patterson GML, Bonjouklian R, Smitka TA. Phytochemistry. 1989;28:1565–1567.
- 6.For a previous tabulation of hapalindole, fischerindole, ambiguine and welwitindolinone natural products, see: Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamás T, Pohjakallio A, Baran PS. J Am Chem Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k.
- 7.For a recent review on the hapalindole, fischerindole, ambiguine and welwitindolinone natural products, see: Bhat V, Dave A, MacKay JA, Rawal VH. In: The Alkaloids. Knölker HJ, editor. Vol. 73. Elsevier; Waltham, MA: 2014. pp. 65–160.
- 8.For examples of investigations regarding the bioactivity of the hapalindole family, see: Doan NT, Rickards RW, Rothschild JM, Smith GD. J App Phycol. 2000;12:409–416.; Doan NT, Stewart PR, Smith GD. FEMS Microbiol Lett. 2001;196:135–139. doi: 10.1111/j.1574-6968.2001.tb10554.x.; Asthana R, Srivastava A, Singh A, Singh S, Nath G, Srivastava R, Srivastava B. J App Phycol. 2006;18:33–39.
- 9.For examples of investigations regarding the bioactivity of the welwitindolinone family, see: Smith CD, Zilfou JT, Stratmann K, Patterson GM, Moore RE. Mol Pharmacol. 1995;47:241–247.; Zhang X, Smith CD. Mol Pharmacol. 1996;49:288–294.
- 10.(a) Baran PS, Richter JM. J Am Chem Soc. 2004;126:7450–7451. doi: 10.1021/ja047874w. [DOI] [PubMed] [Google Scholar]; (b) Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]
- 11.Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 12.For more recent studies in the biosynthesis of hapalindole, fischerindole, ambiguine and welwitindolinone natural products, see: Hillwig ML, Zhu Q, Liu X. ACS Chem Biol. 2014;9:372–377. doi: 10.1021/cb400681n.; Hillwig ML, Fuhrman HA, Ittiamornkul K, Sevco TJ, Kwak DH, Liu X. ChemBioChem. 2014;15:665–669. doi: 10.1002/cbic.201300794.; Hillwig ML, Liu X. Nat Chem Biol. 2014;10:921–923. doi: 10.1038/nchembio.1625.
- 13.Synthesis approaches toward the hapalindole family of natural products: Kinsman AC, Kerr MA. Org Lett. 2000;2:3517–3520. doi: 10.1021/ol0065773.; Brown MA, Kerr MA. Tetrahedron Lett. 2001;42:983–985.; Banwell MG, Ma X, Taylor RM, Willis AC. Org Lett. 2006;8:4959–4961. doi: 10.1021/ol062020x.
- 14.Total synthesis of hapalindole natural products: (a) ref. 6, 10a and 11; Muratake H, Natsume M. Tetrahedron Lett. 1989;30:1815–1818.; Muratake H, Natsume M. Tetrahedron. 1990;46:6331–6342.; Muratake H, Natsume M. Tetrahedron. 1990;46:6343–6350.; Muratake H, Kumagami H, Natsume M. Tetrahedron. 1990;46:6351–6360.; Vaillancourt V, Albizati KF. J Am Chem Soc. 1993;115:3499–3502.; Sakagami M, Muratake H, Natsume M. Chem Pharm Bull. 1994;42:1393–1398.; Fukuyama T, Chen X. J Am Chem Soc. 1994;116:3125–3126.; Kinsman AC, Kerr MA. Org Lett. 2001;3:3189–3191. doi: 10.1021/ol0165138.; Kinsman AC, Kerr MA. J Am Chem Soc. 2003;125:14120–14125. doi: 10.1021/ja036191y.; Chandra A, Johnston JN. Angew Chem Int Ed. 2011;50:7641–7644. doi: 10.1002/anie.201100957.; Rafferty RJ, Williams RM. J Org Chem. 2012;77:519–524. doi: 10.1021/jo202139k.; Lu Z, Yang M, Chen P, Xiong X, Li A. Angew Chem Int Ed. 2014 doi: 10.1002/anie.201406626. Early View.For a formal synthesis, see: Rafferty RJ, Williams RM. Heterocycles. 2012;86:219–231. doi: 10.3987/COM-12-S(N)3.
- 15.Synthetic approaches toward the welwitindolinone family of natural products: Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J Am Chem Soc. 1999;121:6326–6327.; Deng H, Konopelski JP. Org Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r.; Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838.; López-Alvarado P, García-Granda S, Ivarez-Rúa C, Avendaño C. Eur J Org Chem. 2002:1702–1707.; Avendaño C, Menéndez JC. Curr Org Synth. 2004;1:65–82.; Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TV, Wood JL. Angew Chem Int Ed. 2004;43:1270–1272. doi: 10.1002/anie.200353282.; MacKay JA, Bishop RL, Rawal VH. Org Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t.; Baudoux J, Blake AJ, Simpkins NS. Org Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t.; Greshock TJ, Funk RL. Org Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799.; Lauchli R, Shea KJ. Org Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747.; Tian X, Huters AD, Douglas CJ, Garg NK. Org Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684.; Trost BM, McDougall PJ. Org Lett. 2009;11:3782–3785. doi: 10.1021/ol901499b.; Freeman DB, Holubec AA, Weiss MW, Dixon JA, Kakefuda A, Ohtsuka M, Inoue M, Vaswani RG, Ohki H, Doan BD, Reisman SE, Stoltz BM, Day JJ, Tao RN, Dieterich NA, Wood JL. Tetrahedron. 2010;66:6647–6655. doi: 10.1016/j.tet.2010.04.131.; Bhat V, Rawal VH. Chem Commun. 2011;47:9705–9707. doi: 10.1039/c1cc13498a.; Zhang M, Tang W. Org Lett. 2012;14:3756–3759. doi: 10.1021/ol301614v.; Cleary L, Pitzen J, Brailsford JA, Shea KJ. Org Lett. 2014;16:4460–4463. doi: 10.1021/ol5020043.
- 16.Total synthesis of welwitindolinone natural products: (a) ref. 6, 10b and 11; Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s.; Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J Am Chem Soc. 2008;130:2087–2100. doi: 10.1021/ja076663z.; Bhat V, Allan KM, Rawal VH. J Am Chem Soc. 2011;133:5798–5801. doi: 10.1021/ja201834u.; Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J Am Chem Soc. 2011;133:15797–15799. doi: 10.1021/ja206538k.; Allan KM, Kobayashi K, Rawal VH. J Am Chem Soc. 2012;134:1392–1395. doi: 10.1021/ja210793x.; Quasdorf KW, Huters AD, Lodewyk MW, Tantillo DJ, Garg NK. J Am Chem Soc. 2012;134:1396–1399. doi: 10.1021/ja210837b.; Styduhar ED, Huters AD, Weires NA, Garg NK. Angew Chem Int Ed. 2013;52:12422–12425. doi: 10.1002/anie.201307464.; Weires NA, Styduhar ED, Baker EL, Garg NK. J Am Chem Soc. 2014;136:14710–14713. doi: 10.1021/ja5087672.. For a formal synthesis of welwitindolinone C, see: Fu Th, McElroy WT, Shamszad M, Martin SF. Org Lett. 2012;14:3834–3837. doi: 10.1021/ol301424h.
- 17.Synthetic approaches toward the ambiguine family of natural products: (a) ref. 14n; Chandra A, Viswanathan R, Johnston JN. Org Lett. 2007;9:5027–5029. doi: 10.1021/ol702247a.; Rafferty RJ, Williams RM. Tetrahedron Lett. 2011;52:2037–2040. doi: 10.1016/j.tetlet.2010.09.086.
- 18.Richter JM, Whitefield BW, Maimone TJ, Lin DW, Castroviejo MP, Baran PS. J Am Chem Soc. 2007;129:12857–12869. doi: 10.1021/ja074392m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta G, Acharyulu PVR. J Chem Soc Chem Commun. 1994:2759–2760. [Google Scholar]
- 20.Gribble GW, Lord PD, Skotnicki K, Dietz SE, Eaton JT, Johnson JL. J Am Chem Soc. 1974;96:7812–7814. [Google Scholar]
- 21.Schkeryantz JM, Woo JCG, Siliphaivanth P, Depew KM, Danishefsky SJD. J Am Chem Soc. 1999;121:11964–11975. [Google Scholar]
- 22.Schultz AG, Guzzo PR, Nowak DM. J Org Chem. 1995;60:8044–8050. [Google Scholar]
- 23.(a) Larock RC, Babu S. Tetrahedron Lett. 1987;28:5291–5294. [Google Scholar]; (b) Burns B, Grigg R, Ratananukul P, Sridharan V, Stevenson P, Worakun T. Tetrahedron Lett. 1988;29:4329–4332. [Google Scholar]
- 24.Herrmann WA, Brossmer C, Öfele K, Reisinger CP, Priermeier T, Beller M, Fischer H. Angew Chem Int Ed. 1995;34:1844–1848. [Google Scholar]
- 25.Baird KJ, Grundon MF, Harrison DM, Magee MG. Heterocycles. 1981;15:713–717. [Google Scholar]
- 26.Trost BM, Quancard J. J Am Chem Soc. 2006;128:6314–6315. doi: 10.1021/ja0608139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Futamata M, Mukai R, Tamaru Y. J Am Chem Soc. 2005;127:4592–4593. doi: 10.1021/ja0501161. [DOI] [PubMed] [Google Scholar]
- 28.Baran PS, Guerrero CA, Corey EJ. Org Lett. 2003;5:1999–2001. doi: 10.1021/ol034634x. [DOI] [PubMed] [Google Scholar]
- 29.Turro NJ. Modern Molecular Photochemistry. Chapter 13. University Science Books; Sausalito: 1991. [Google Scholar]
- 30.For reviews in protecting-group-free synthesis, see: Hoffmann RW. Synthesis. 2006:3531–3541.; Young IS, Baran PS. Nat Chem. 2009;1:193–205. doi: 10.1038/nchem.216.; Saicic RN. Tetrahedron. 2014;70:8183–8218.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.