Abstract
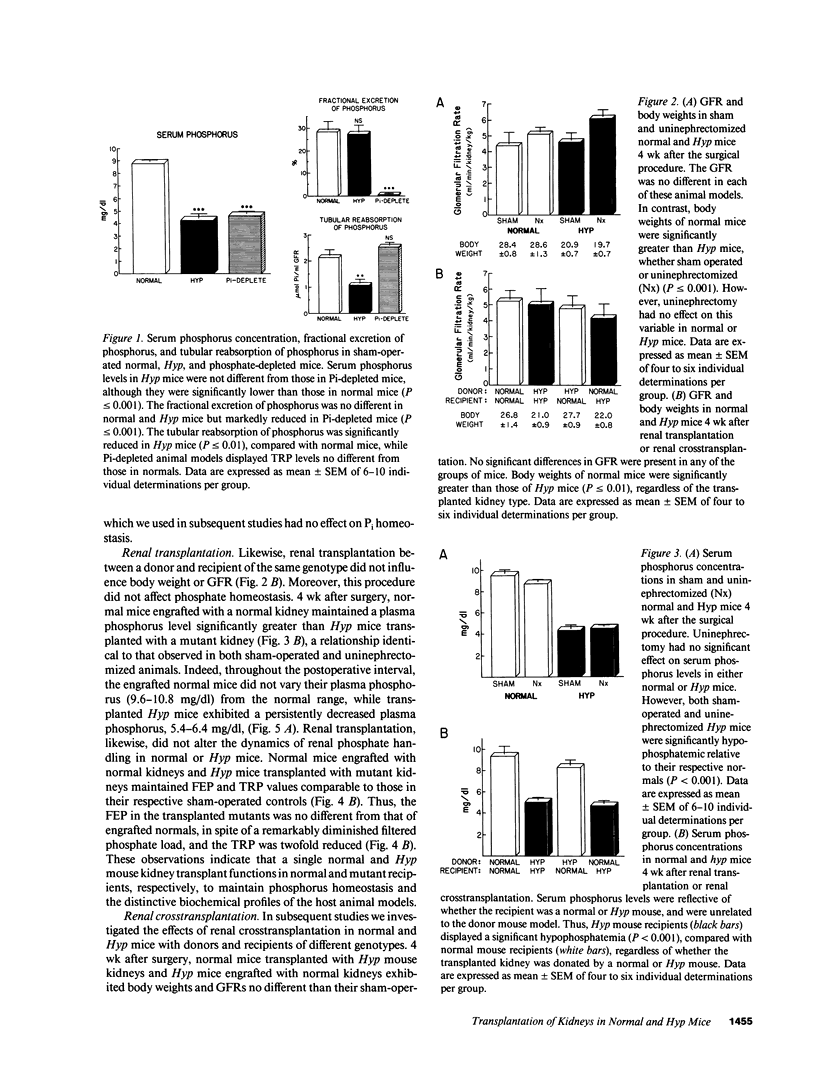
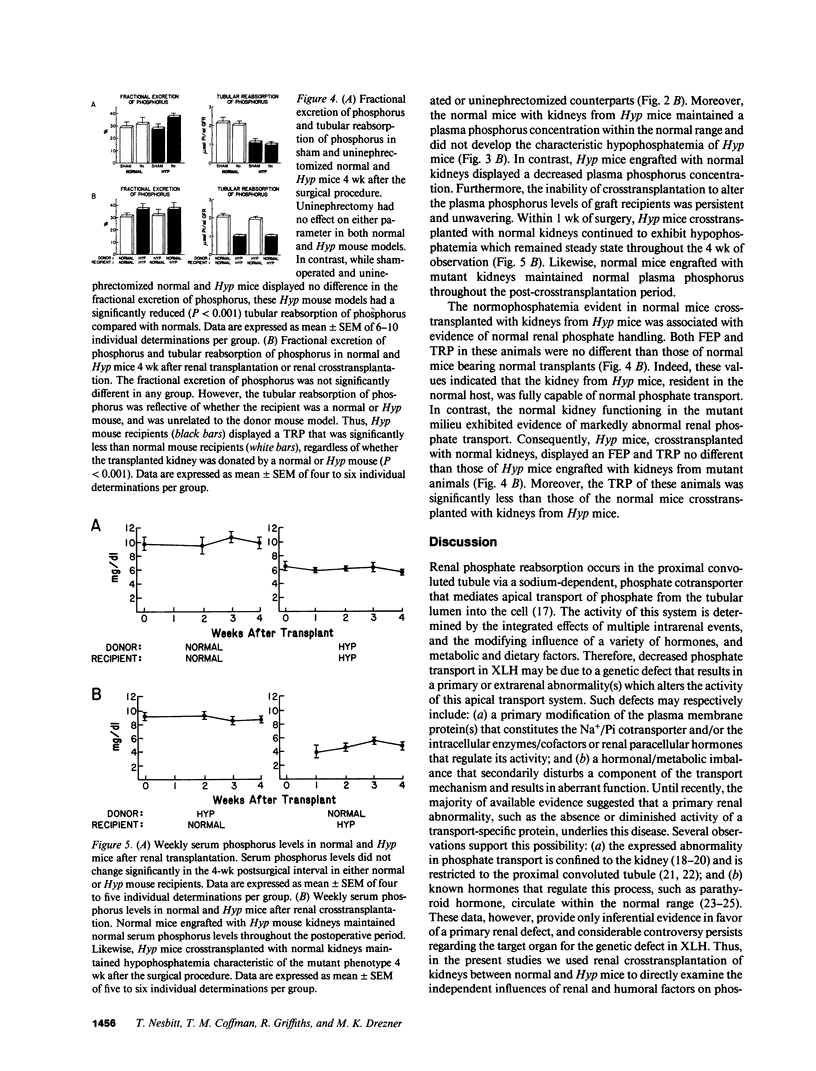
Although deranged phosphate transport is the fundamental abnormality in X-linked hypophosphatemic (XLH) rickets, it remains unknown if this defect is the consequence of an intrinsic kidney abnormality or aberrant production of a humoral factor. To discriminate between these possibilities, we examined phosphate homeostasis in normal and Hyp mice, subjected to renal crosstransplantation. We initially evaluated the effects of uninephrectomy on the indices of phosphate metabolism that identify the mutant biochemical phenotype. No differences were found in the serum phosphorus concentration, fractional excretion of phosphate (FEP), or tubular reabsorption of phosphate per milliliter of glomerular filtrate (TRP) in uninephrectomized normal and Hyp mice, compared with sham-operated controls. Subsequently, single kidneys from normal or Hyp mice were transplanted into normal and Hyp mouse recipients. Normal mice transplanted with normal kidneys and Hyp mice engrafted with mutant kidneys exhibited serum phosphorus, FEP, and TRP no different from those of uninephrectomized normal and Hyp mice, respectively. However, engraftment of normal kidneys in Hyp mice and mutant kidneys in normal mice affected neither serum phosphorus (4.69 +/- 0.31 and 8.25 +/- 0.52 mg/dl, respectively) nor FEP and TRP of the recipients. These data indicate that the Hyp mouse phenotype is neither corrected nor transferred by renal transplantation. Further, they suggest that the phosphate transport defect in Hyp mice, and likely X-linked hypophosphatemia, is the result of a humoral factor, and is not an intrinsic renal abnormality.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud C., Glorieux F., Scriver C. Serum parathyroid hormone in X-linked hypophosphatemia. Science. 1971 Aug 27;173(3999):845–847. doi: 10.1126/science.173.3999.845. [DOI] [PubMed] [Google Scholar]
- Aschinberg L. C., Solomon L. M., Zeis P. M., Justice P., Rosenthal I. M. Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr. 1977 Jul;91(1):56–60. doi: 10.1016/s0022-3476(77)80444-7. [DOI] [PubMed] [Google Scholar]
- Bell C. L., Tenenhouse H. S., Scriver C. R. Primary cultures of renal epithelial cells from X-linked hypophosphatemic (Hyp) mice express defects in phosphate transport and vitamin D metabolism. Am J Hum Genet. 1988 Sep;43(3):293–303. [PMC free article] [PubMed] [Google Scholar]
- Bianchi G., Niutta E., Ferrari P., Salvati P., Salardi S., Cusi D., Colombo R., Cesana B., Tripodi G., Pati P. A possible primary role for the kidney in essential hypertension. Am J Hypertens. 1989 Feb;2(2 Pt 2):2S–6S. doi: 10.1093/ajh/2.2.2s. [DOI] [PubMed] [Google Scholar]
- Coffman T. M., Yarger W. E., Klotman P. E. Functional role of thromboxane production by acutely rejecting renal allografts in rats. J Clin Invest. 1985 Apr;75(4):1242–1248. doi: 10.1172/JCI111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Delzer P. R., Meyer R. A., Jr Normal handling of phosphate in the salivary glands of X-linked hypophosphataemic mice. Arch Oral Biol. 1984;29(12):1009–1013. doi: 10.1016/0003-9969(84)90148-1. [DOI] [PubMed] [Google Scholar]
- Delzer P. R., Meyer R. A., Jr Normal milk composition in lactating X-linked hypophosphatemic mice despite continued hypophosphatemia. Calcif Tissue Int. 1983 Sep;35(6):750–754. doi: 10.1007/BF02405118. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Glorieux F. H., Travers R., Desbarats M., Bouchard F., Hinek A. Defective bone formation by transplanted Hyp mouse bone cells into normal mice. Endocrinology. 1988 Aug;123(2):768–773. doi: 10.1210/endo-123-2-768. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanconi A., Fischer J. A., Prader A. Serum parathyroid hormone concentrations in hypophosphataemic vitamin D resistant rickets. Helv Paediatr Acta. 1974 Aug;29(3):187–194. [PubMed] [Google Scholar]
- Friedel R. O., Schanberg S. M. Incorporation in vivo of intracisternally injected 33 P i into phospholipids of rat brain. J Neurochem. 1971 Nov;18(11):2191–2200. doi: 10.1111/j.1471-4159.1971.tb05077.x. [DOI] [PubMed] [Google Scholar]
- Fukase M., Avioli L. V., Birge S. J., Chase L. R. Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology. 1984 Apr;114(4):1203–1207. doi: 10.1210/endo-114-4-1203. [DOI] [PubMed] [Google Scholar]
- Fuster V., Fass D. N., Kaye M. P., Josa M., Zinsmeister A. R., Bowie E. J. Arteriosclerosis in normal and von Willebrand pigs: long-term prospective study and aortic transplantation study. Circ Res. 1982 Nov;51(5):587–593. doi: 10.1161/01.res.51.5.587. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Hironaka T., Ikari Y., Miyata Y., Morimoto S., Tsunoo A. Transplantation of extensor carpi radialis longus muscle in normal and dystrophic chicks. Exp Neurol. 1984 Feb;83(2):392–402. doi: 10.1016/S0014-4886(84)90107-9. [DOI] [PubMed] [Google Scholar]
- Kinne R., Berner W., Hoffman N., Murer H. Phosphate transport by isolated renal and intestinal plasma membranes. Adv Exp Med Biol. 1977;81:265–277. doi: 10.1007/978-1-4613-4217-5_26. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Fukase M., Nakada M., Fujita T. Defective adaptation to a low phosphate environment by cultured renal tubular cells from X-linked hypophosphatemic (Hyp) mice. Biochem Biophys Res Commun. 1987 Apr 29;144(2):763–769. doi: 10.1016/s0006-291x(87)80030-x. [DOI] [PubMed] [Google Scholar]
- Krohn H. P., Offermann G., Brandis M., Brodehl J., Hanke K., Offner G. Occurrence of hyperparathyroidism in children with X-linked hypophosphatemia under treatment with vitamin D and phsophate. Adv Exp Med Biol. 1977;81:345–351. doi: 10.1007/978-1-4613-4217-5_34. [DOI] [PubMed] [Google Scholar]
- Kuster G., Shorter R. G., Dawson B., Hallenbeck G. A. Uric acid metabolism in dalmatians and other dogs. Role of the liver. Arch Intern Med. 1972 Mar;129(3):492–496. [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Meyer M. H., Gray R. W. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989 Aug;4(4):493–500. doi: 10.1002/jbmr.5650040407. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Tenenhouse H. S., Meyer M. H., Klugerman A. H. The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphatemic mice persists after parathyroidectomy. J Bone Miner Res. 1989 Aug;4(4):523–532. doi: 10.1002/jbmr.5650040411. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Fukase M., Tsutsumi M., Fujita T. Hemangiopericytoma-induced osteomalacia: tumor transplantation in nude mice causes hypophosphatemia and tumor extracts inhibit renal 25-hydroxyvitamin D 1-hydroxylase activity. J Clin Endocrinol Metab. 1988 Jul;67(1):46–53. doi: 10.1210/jcem-67-1-46. [DOI] [PubMed] [Google Scholar]
- Morgan J. M., Hawley W. L., Chenoweth A. I., Retan W. J., Diethelm A. G. Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch Intern Med. 1974 Sep;134(3):549–552. [PubMed] [Google Scholar]
- Nesbitt T., Davidai G. A., Drezner M. K. Abnormal adenosine 3'.5'-monophosphate stimulation of renal 1,25-dihydroxyvitamin D production in hyp mice: evidence that 25-hydroxyvitamin D-1 alpha-hydroxylase dysfunction results from aberrant intracellular function. Endocrinology. 1989 Mar;124(3):1184–1189. doi: 10.1210/endo-124-3-1184. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K. Abnormal parathyroid hormone-related peptide stimulation of renal 25-hydroxyvitamin D-1-hydroxylase in Hyp mice: evidence for a generalized defect of enzyme activity in the proximal convoluted tubule. Endocrinology. 1990 Aug;127(2):843–848. doi: 10.1210/endo-127-2-843. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Lobaugh B., Drezner M. K. Calcitonin stimulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in hypophosphatemic mice. Evidence that the regulation of calcitriol production is not universally abnormal in X-linked hypophosphatemia. J Clin Invest. 1987 Jan;79(1):15–19. doi: 10.1172/JCI112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabico R. C., McKenna B. A., Freeman R. B. Renal function before and after unilateral nephrectomy in renal donors. Kidney Int. 1975 Sep;8(3):166–175. doi: 10.1038/ki.1975.96. [DOI] [PubMed] [Google Scholar]
- Posillico J. T., Lobaugh B., Muhlbaier L. H., Drezner M. K. Abnormal parathyroid function in the X-linked hypophosphatemic mouse. Calcif Tissue Int. 1985 Jul;37(4):418–422. doi: 10.1007/BF02553712. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R. W., Hruska K. A., Korkor A., Anderson C., Slatopolsky E. Decreased phosphate reabsorption after renal transplantation: Evidence for a mechanism independent of calcium and parathyroid hormone. Kidney Int. 1981 Apr;19(4):568–578. doi: 10.1038/ki.1981.54. [DOI] [PubMed] [Google Scholar]
- Spurney R. F., Ruiz P., Pisetsky D. S., Coffman T. M. Enhanced renal leukotriene production in murine lupus: role of lipoxygenase metabolites. Kidney Int. 1991 Jan;39(1):95–102. doi: 10.1038/ki.1991.12. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Effect of 1,25-dihydroxyvitamin D3 on phosphate homeostasis in the X-linked hypophosphatemic (Hyp) mouse. Endocrinology. 1981 Aug;109(2):658–660. doi: 10.1210/endo-109-2-658. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., McInnes R. R., Glorieux F. H. Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse: evidence for a defect in the brush border membrane. Kidney Int. 1978 Sep;14(3):236–244. doi: 10.1038/ki.1978.115. [DOI] [PubMed] [Google Scholar]



