Abstract
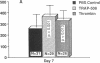
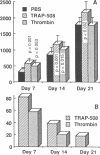
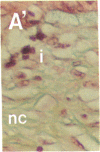
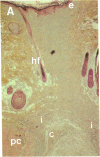
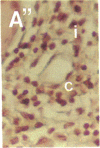
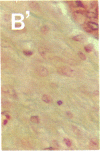
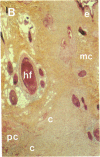
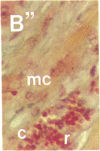
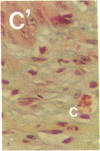
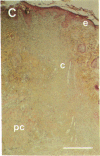
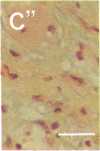
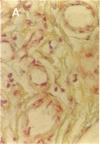
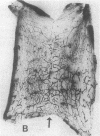
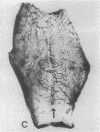
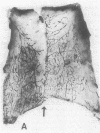
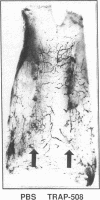
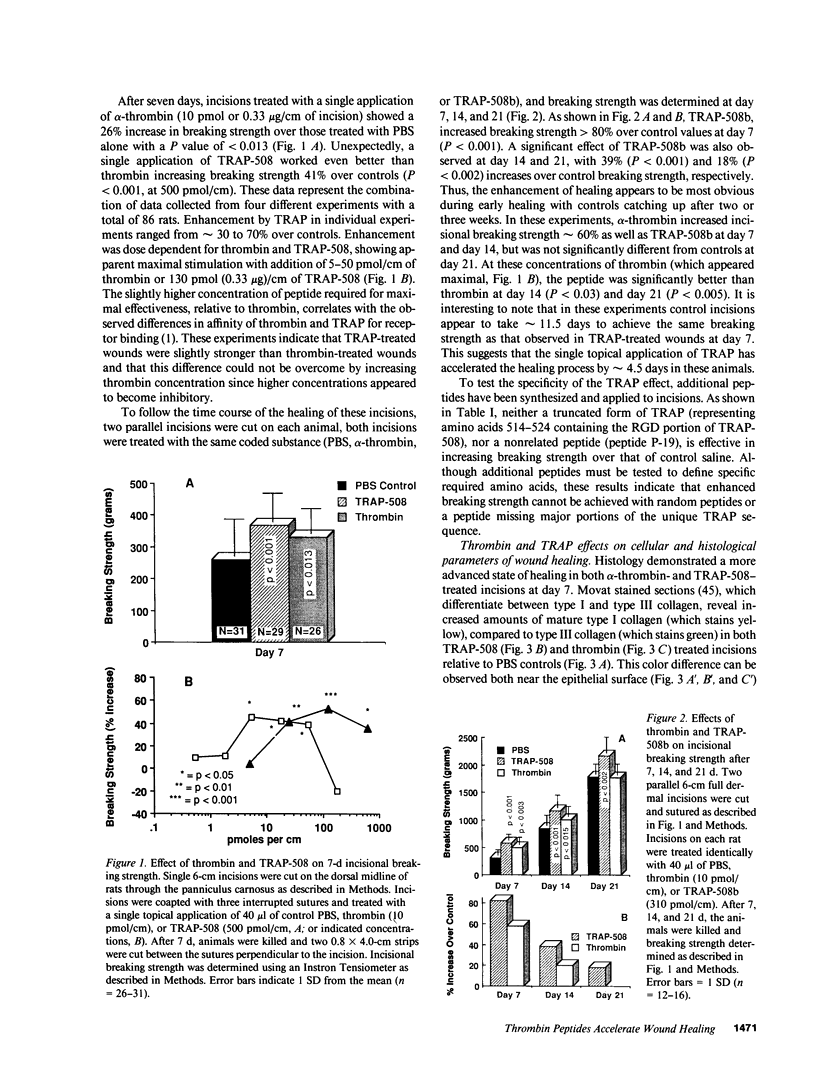
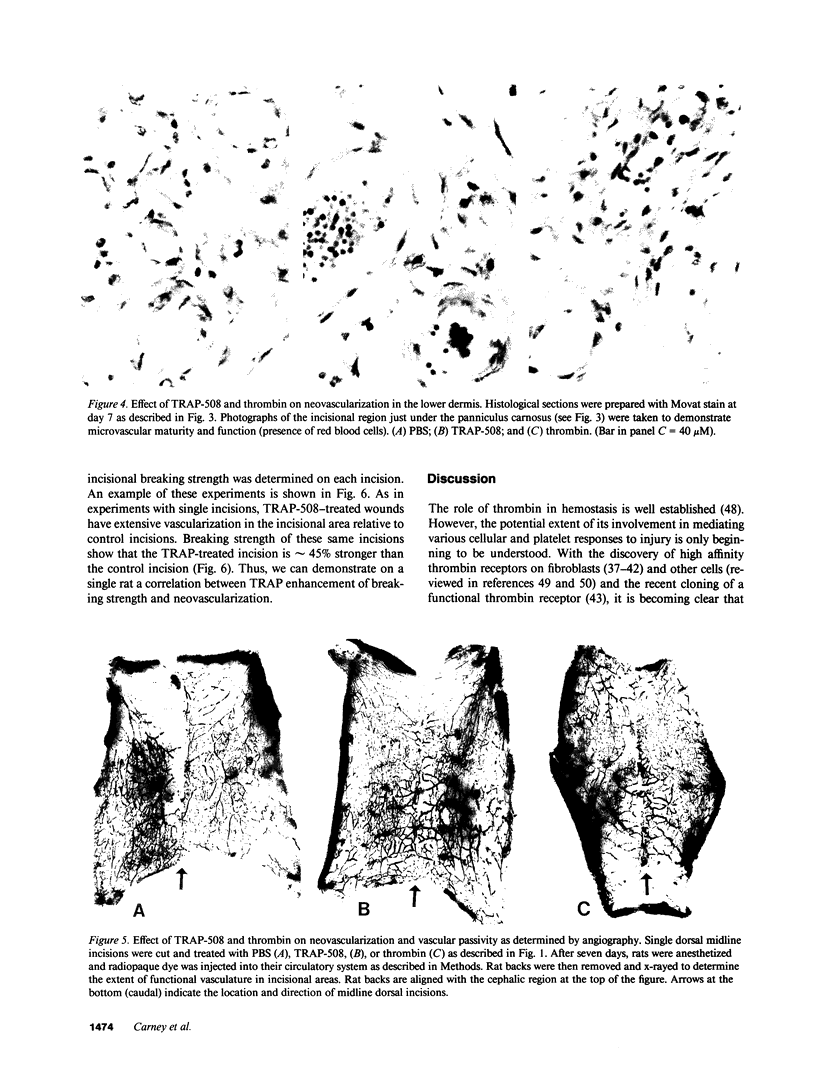
To better define thrombin-receptor interactions, we synthesized human thrombin peptides and identified binding-domain peptides that bind thrombin receptors and activate mitogenic signals (Glenn, K.C., G.H. Frost, J.S. Bergmann, and D.H. Carney. 1988. Pept. Res. 1:65-73). Treatment of full dermal dorsal incisions with a single topical application of thrombin receptor-activating peptide (TRAP-508) or human alpha-thrombin in saline enhances 7-d incisional breaking strength in normal rats up to 82% or 55% over saline-treated controls, respectively. Control wounds require approximately 11.5 d to achieve breaking strength equivalent to TRAP-treated wounds at day 7. Thus, a single application of TRAP accelerates healing, shifting the time course forward by up to 4.5 d. Histological comparisons at day 7 show more type I collagen, less evidence of prolonged inflammation, and an increase in number and maturity of capillaries in TRAP- and thrombin-treated incisions. Angiograms also show 50-65% more functional vascularization going across thrombin- and TRAP-treated surgical incisions. Thus, alpha-thrombin and thrombin peptides, such as those released following injury, appear to initiate or enhance signals required for neovascularization and wound healing. The ability to accelerate normal wound healing events with synthetic peptides representing receptor binding domains of human thrombin may offer new options for management of wound healing in man.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Eldor A., Vlodavsky I. Binding of thrombin to subendothelial extracellular matrix. Protection and expression of functional properties. J Clin Invest. 1989 Oct;84(4):1096–1104. doi: 10.1172/JCI114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A. J., Mann K. G., Wilner G. D. Growth-promoting effects of esterolytically inactive thrombin on macrophages. J Cell Biochem. 1986;32(4):261–272. doi: 10.1002/jcb.240320403. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A. J., Mann K. G., Wilner G. D. Identification of a thrombin sequence with growth factor activity on macrophages. Proc Natl Acad Sci U S A. 1986 Feb;83(4):976–980. doi: 10.1073/pnas.83.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Receptor-mediated chemotactic response of a macrophage cell line (J774) to thrombin. Lab Invest. 1983 Dec;49(6):702–707. [PubMed] [Google Scholar]
- Belloni P. N., Carney D. H., Nicolson G. L. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res. 1992 Jan;43(1):20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bizios R., Lai L. C., Cooper J. A., Del Vecchio P. J., Malik A. B. Thrombin-induced adherence of neutrophils to cultured endothelial monolayers: increased endothelial adhesiveness. J Cell Physiol. 1988 Feb;134(2):275–280. doi: 10.1002/jcp.1041340214. [DOI] [PubMed] [Google Scholar]
- Bizios R., Lai L., Fenton J. W., 2nd, Malik A. B. Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol. 1986 Sep;128(3):485–490. doi: 10.1002/jcp.1041280318. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L. J., White M., Mitchell R. O., Pietsch J., Nordquist R., von Fraunhofer A., Schultz G. S. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988 Dec;208(6):788–794. doi: 10.1097/00000658-198812000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Role of specific cell surface receptors in thrombin-stimulated cell division. Cell. 1978 Dec;15(4):1341–1349. doi: 10.1016/0092-8674(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Glenn K. C., Cunningham D. D. Conditions which affect initiation of animal cell division by trypsin and thrombin. J Cell Physiol. 1978 Apr;95(1):13–22. doi: 10.1002/jcp.1040950103. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Glenn K. C., Cunningham D. D., Das M., Fox C. F., Fenton J. W., 2nd Photoaffinity labeling of a single receptor for alpha-thrombin on mouse embryo cells. J Biol Chem. 1979 Jul 25;254(14):6244–6247. [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Williams L. T. Agents that increase cAMP accumulation block endothelial c-sis induction by thrombin and transforming growth factor-beta. J Biol Chem. 1987 Sep 5;262(25):11893–11896. [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fenton J. W., 2nd Regulation of thrombin generation and functions. Semin Thromb Hemost. 1988 Jul;14(3):234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- Frost G. H., Thompson W. C., Carney D. H. Monoclonal antibody to the thrombin receptor stimulates DNA synthesis in combination with gamma-thrombin or phorbol myristate acetate. J Cell Biol. 1987 Dec;105(6 Pt 1):2551–2558. doi: 10.1083/jcb.105.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. G., Siflinger-Birnboim A., Bizios R., Del Vecchio P. J., Fenton J. W., 2nd, Malik A. B. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986 Jul;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Frost G. H., Bergmann J. S., Carney D. H. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept Res. 1988 Nov-Dec;1(2):65–73. [PubMed] [Google Scholar]
- Gospodarowicz D., Brown K. D., Birdwell C. R., Zetter B. R. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978 Jun;77(3):774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Malik A. B. Thrombin-induced endothelial injury. Semin Thromb Hemost. 1986 Jul;12(3):184–196. doi: 10.1055/s-2007-1003549. [DOI] [PubMed] [Google Scholar]
- McGee G. S., Broadley K. N., Buckley A., Aquino A., Woodward S. C., Demetriou A. A., Davidson J. M. Recombinant transforming growth factor beta accelerates incisional wound healing. Curr Surg. 1989 Mar-Apr;46(2):103–106. [PubMed] [Google Scholar]
- McGee G. S., Davidson J. M., Buckley A., Sommer A., Woodward S. C., Aquino A. M., Barbour R., Demetriou A. A. Recombinant basic fibroblast growth factor accelerates wound healing. J Surg Res. 1988 Jul;45(1):145–153. doi: 10.1016/0022-4804(88)90034-0. [DOI] [PubMed] [Google Scholar]
- McGrath M. H. Peptide growth factors and wound healing. Clin Plast Surg. 1990 Jul;17(3):421–432. [PubMed] [Google Scholar]
- Meltzer T., Myers B. The effect of hyperbaric oxygen on the bursting strength and rate of vascularization of skin wounds in the rat. Am Surg. 1986 Dec;52(12):659–662. [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Lubenskyi W., Kivity E., Sonder S. A., Fenton J. W., 2nd Protease mitogenic response of chick embryo fibroblasts and receptor binding/processing of human alpha-thrombin. J Biol Chem. 1981 Mar 25;256(6):2767–2776. [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P. Proteases stimulate proliferation of human fibroblasts. J Cell Physiol. 1977 Jun;91(3):387–392. doi: 10.1002/jcp.1040910308. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rodriquez R., Franchi A., Pouysségur J. Growth factor requirements of Chinese hamster lung fibroblasts in serum free media: high mitogenic action of thrombin. Cell Biol Int Rep. 1981 Apr;5(4):347–357. doi: 10.1016/0309-1651(81)90004-7. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Reddan J. R., Dziedzic D. C., McGee S. J. Thrombin induces cell division in rabbit lenses cultured in a completely defined serum-free medium. Invest Ophthalmol Vis Sci. 1982 Apr;22(4):486–493. [PubMed] [Google Scholar]
- Russell H. K., Jr A modification of Movat's pentachrome stain. Arch Pathol. 1972 Aug;94(2):187–191. [PubMed] [Google Scholar]
- Rydel T. J., Ravichandran K. G., Tulinsky A., Bode W., Huber R., Roitsch C., Fenton J. W., 2nd The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990 Jul 20;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- Saegusa Y., Cavender D., Ziff M. Stimulation of mononuclear cell binding to human endothelial cell monolayers by thrombin. J Immunol. 1988 Dec 15;141(12):4140–4145. [PubMed] [Google Scholar]
- Sonne O. The specific binding of thrombin to human polymorphonuclear leucocytes. Scand J Clin Lab Invest. 1988 Dec;48(8):831–838. doi: 10.3109/00365518809088768. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]
- Zetter B. R., Antoniades H. N. Stimulation of human vascular endothelial cell growth by a platelet-derived growth factor and thrombin. J Supramol Struct. 1979;11(3):361–370. doi: 10.1002/jss.400110311. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates neutrophil adherence by an endothelial cell-dependent mechanism: characterization of the response and relationship to platelet-activating factor synthesis. Ann N Y Acad Sci. 1986;485:349–368. doi: 10.1111/j.1749-6632.1986.tb34596.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Prescott S. M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985 Dec;76(6):2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]