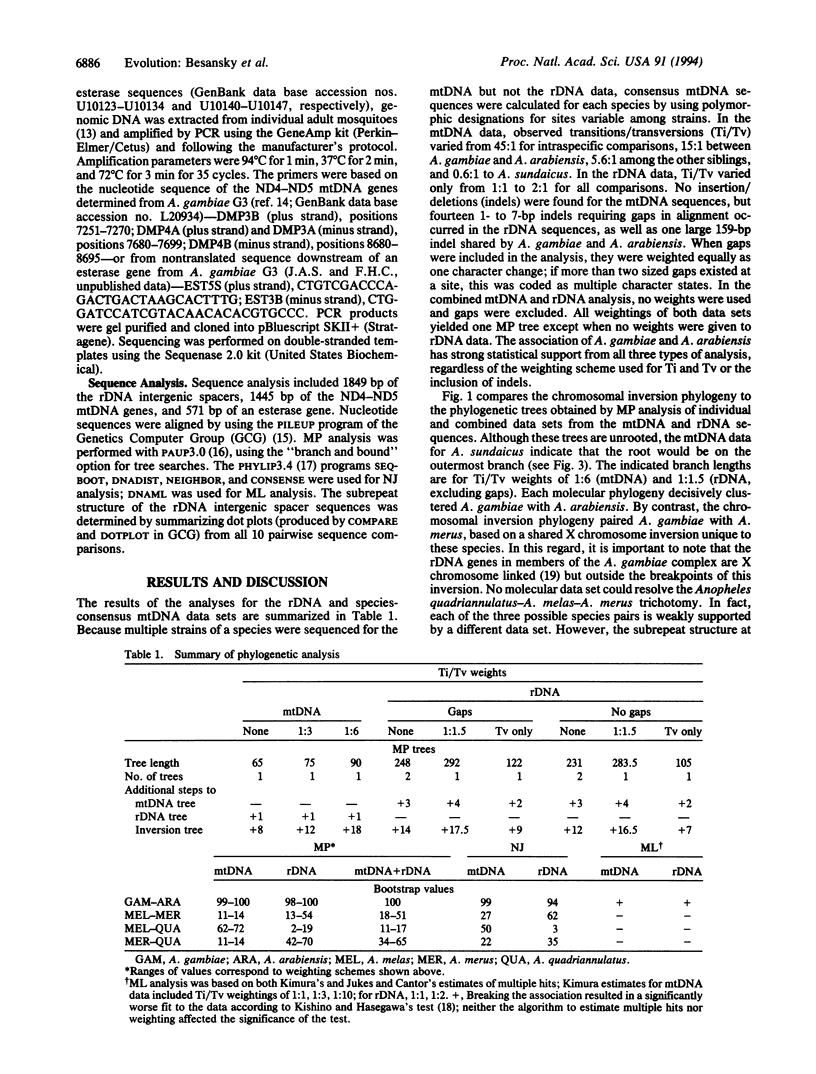
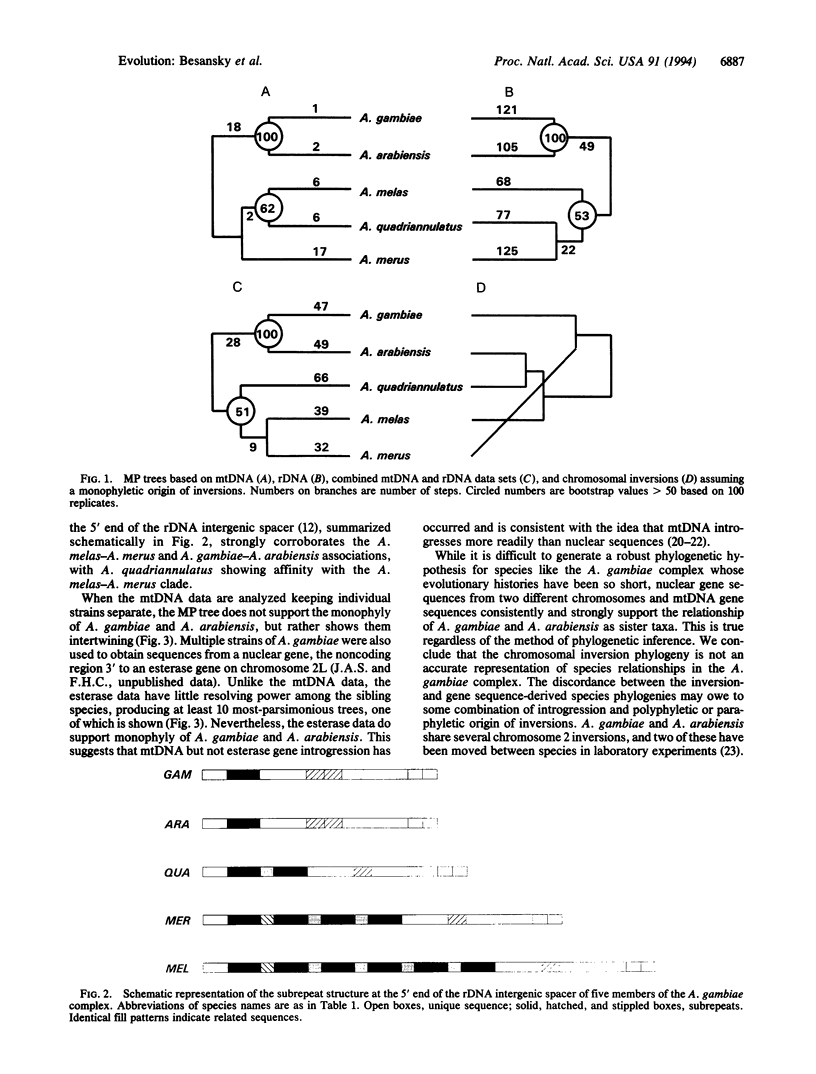
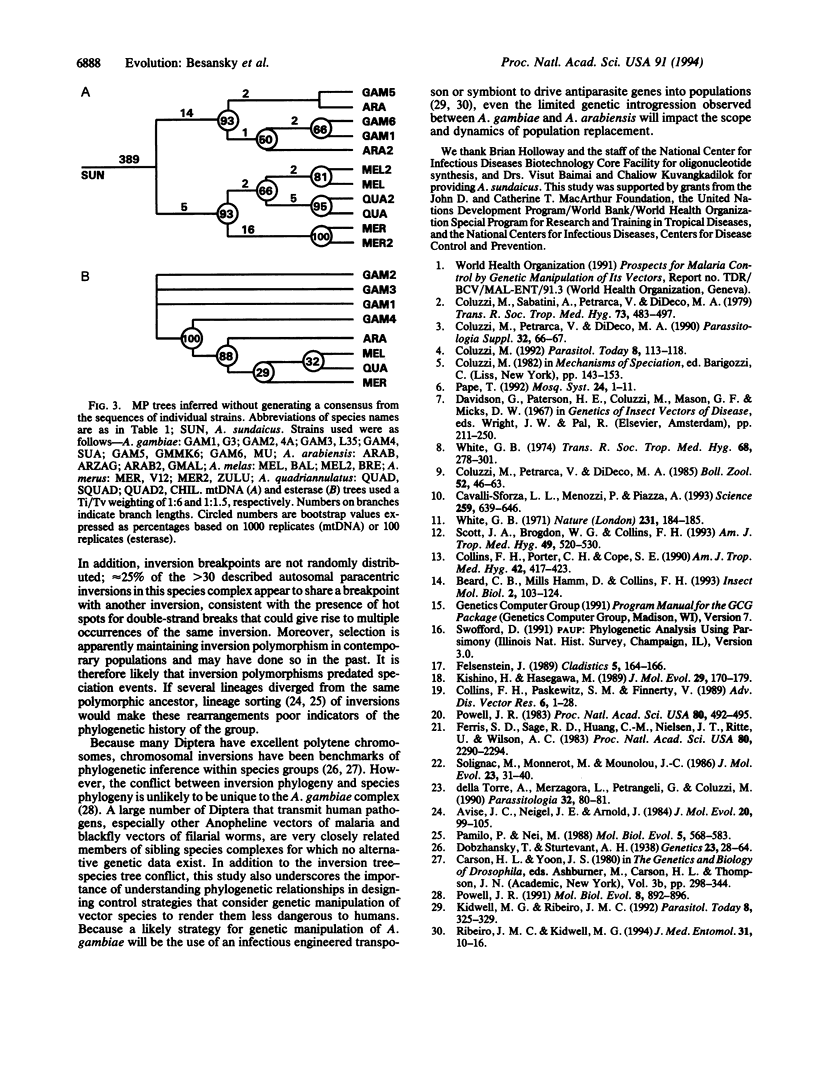
Abstract
The six Afrotropical species of mosquitoes comprising the Anopheles gambiae complex include the most efficient vectors of malaria in the world as well as a nonvector species. The accepted interpretation of evolutionary relationships among these species is based on chromosomal inversions and suggests that the two principal vectors, A. gambiae and Anopheles arabiensis, are on distant branches of the phylogenetic tree. However, DNA sequence data indicate that these two species are sister taxa and suggest gene flow between them. These results have important implications for malaria control strategies involving the replacement of vector with nonvector populations.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Neigel J. E., Arnold J. Demographic influences on mitochondrial DNA lineage survivorship in animal populations. J Mol Evol. 1984;20(2):99–105. doi: 10.1007/BF02257369. [DOI] [PubMed] [Google Scholar]
- Beard C. B., Hamm D. M., Collins F. H. The mitochondrial genome of the mosquito Anopheles gambiae: DNA sequence, genome organization, and comparisons with mitochondrial sequences of other insects. Insect Mol Biol. 1993;2(2):103–124. doi: 10.1111/j.1365-2583.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L., Menozzi P., Piazza A. Demic expansions and human evolution. Science. 1993 Jan 29;259(5095):639–646. doi: 10.1126/science.8430313. [DOI] [PubMed] [Google Scholar]
- Collins F. H., Porter C. H., Cope S. E. Comparison of rDNA and mtDNA in the sibling species Anopheles freeborni and A. hermsi. Am J Trop Med Hyg. 1990 May;42(5):417–423. doi: 10.4269/ajtmh.1990.42.417. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Malaria vector analysis and control. Parasitol Today. 1992 Apr;8(4):113–118. doi: 10.1016/0169-4758(92)90277-9. [DOI] [PubMed] [Google Scholar]
- Coluzzi M., Sabatini A., Petrarca V., Di Deco M. A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73(5):483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Spatial distribution of chromosomal inversions and speciation in Anopheline mosquitoes. Prog Clin Biol Res. 1982;96:143–153. [PubMed] [Google Scholar]
- Dobzhansky T, Sturtevant A H. Inversions in the Chromosomes of Drosophila Pseudoobscura. Genetics. 1938 Jan;23(1):28–64. doi: 10.1093/genetics/23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Huang C. M., Nielsen J. T., Ritte U., Wilson A. C. Flow of mitochondrial DNA across a species boundary. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2290–2294. doi: 10.1073/pnas.80.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Ribeiro J. M. Can transposable elements be used to drive disease refractoriness genes into vector populations? Parasitol Today. 1992 Oct;8(10):325–329. doi: 10.1016/0169-4758(92)90065-a. [DOI] [PubMed] [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989 Aug;29(2):170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Pamilo P., Nei M. Relationships between gene trees and species trees. Mol Biol Evol. 1988 Sep;5(5):568–583. doi: 10.1093/oxfordjournals.molbev.a040517. [DOI] [PubMed] [Google Scholar]
- Powell J. R. Interspecific cytoplasmic gene flow in the absence of nuclear gene flow: evidence from Drosophila. Proc Natl Acad Sci U S A. 1983 Jan;80(2):492–495. doi: 10.1073/pnas.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. R. Monophyly/paraphyly/polyphyly and gene/species trees: an example from Drosophila. Mol Biol Evol. 1991 Nov;8(6):892–896. doi: 10.1093/oxfordjournals.molbev.a040695. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Kidwell M. G. Transposable elements as population drive mechanisms: specification of critical parameter values. J Med Entomol. 1994 Jan;31(1):10–16. doi: 10.1093/jmedent/31.1.10. [DOI] [PubMed] [Google Scholar]
- Scott J. A., Brogdon W. G., Collins F. H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993 Oct;49(4):520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Solignac M., Monnerot M., Mounolou J. C. Mitochondrial DNA evolution in the melanogaster species subgroup of Drosophila. J Mol Evol. 1986;23(1):31–40. doi: 10.1007/BF02100996. [DOI] [PubMed] [Google Scholar]
- White G. B. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68(4):278–301. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- White G. B. Chromosomal evidence for natural interspecific hybridization by mosquitoes of the Anopheles gambiae complex. Nature. 1971 May 21;231(5299):184–185. doi: 10.1038/231184a0. [DOI] [PubMed] [Google Scholar]




