Abstract
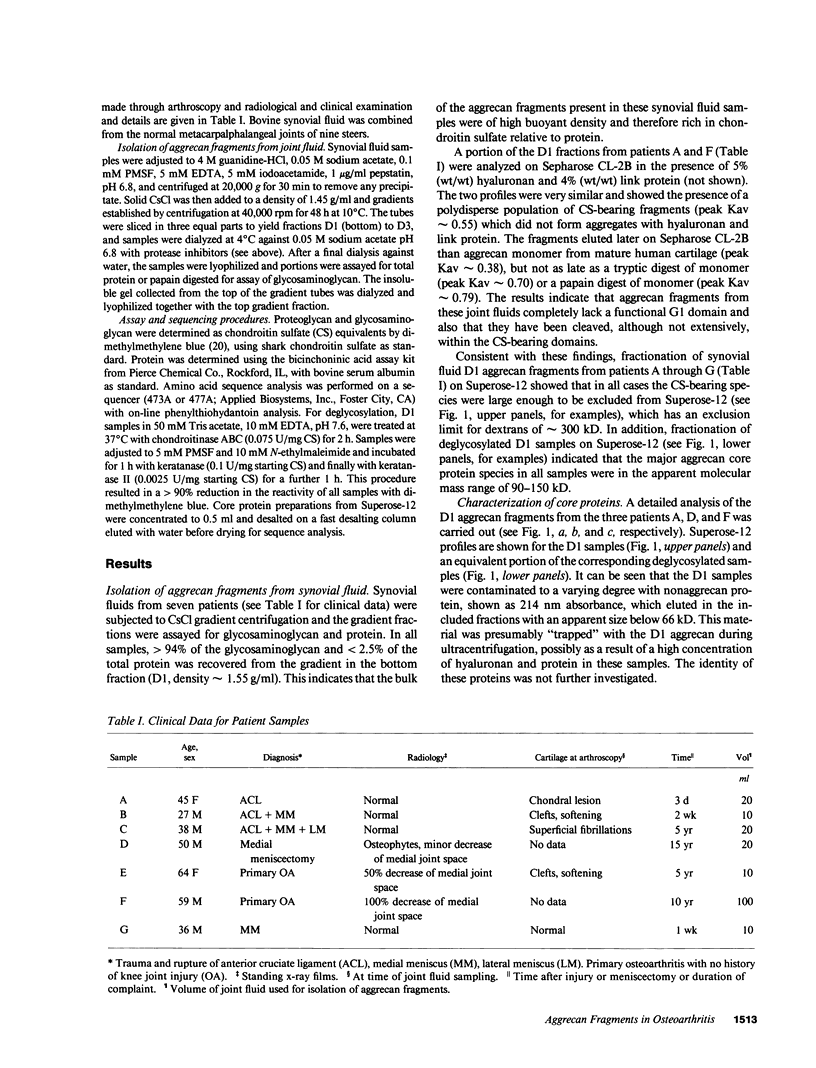
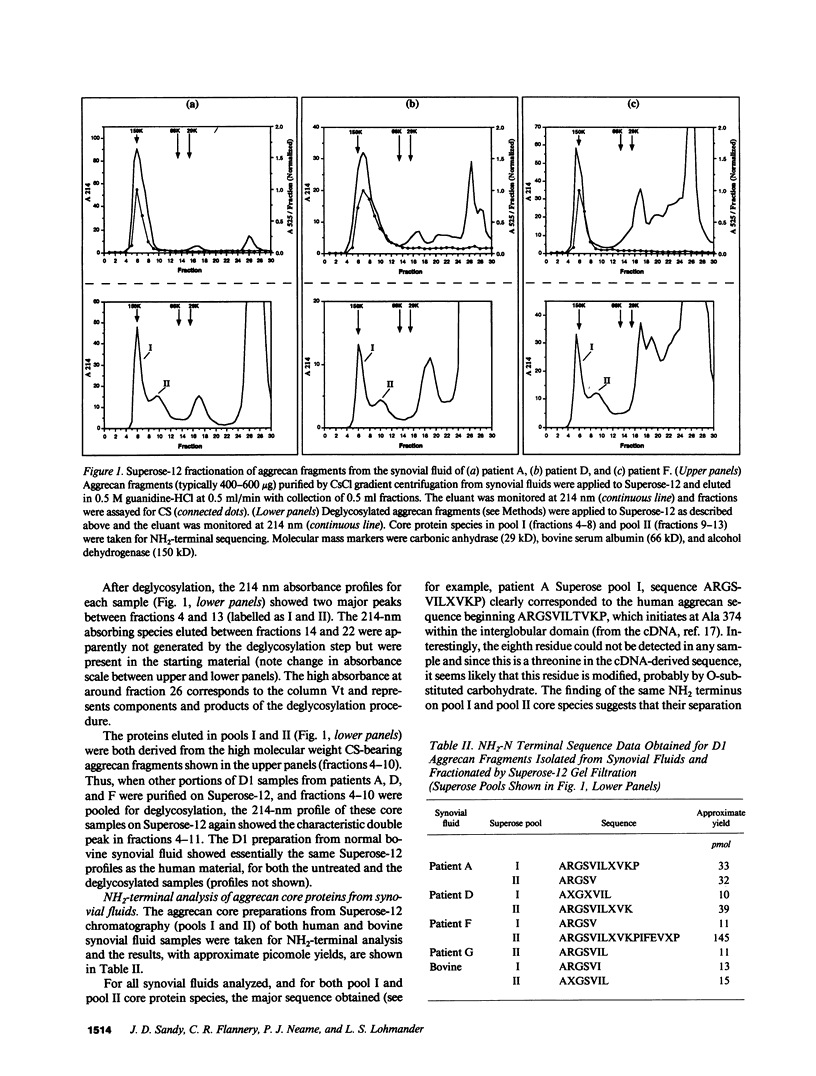
Synovial fluid was collected from patients with recent knee injury and from patients with early or late stage osteoarthritis. Chondroitin sulfate-substituted aggrecan fragments present in these fluids, and in normal bovine synovial fluid, were purified by cesium chloride gradient centrifugation, enzymically deglycosylated and fractionated by gel filtration on Superose-12. Each sample contained two major aggrecan core protein populations with apparent molecular masses of approximately 90 kD and 150 kD. For all samples, NH2-terminal analysis of both populations gave a single major sequence beginning ARGSV. This NH2 terminus results from cleavage of the human aggrecan core protein at the Glu 373-Ala 374 bond within the interglobular domain between the G1 and G2 domains. Cleavage at this site also occurs during control and interleukin-1 stimulated aggrecan catabolism in bovine cartilage explant cultures (Sandy, J., P. Neame, R. Boynton, and C. Flannery. 1991. J. Biol. Chem. 266:8683-8685). These results indicate that the major aggrecan fragments present in both osteoarthritic human synovial fluid and in normal bovine synovial fluid are large, being composed of a short NH2-terminal stretch of the interglobular domain, the G2 domain, the keratan sulfate domain, and variable lengths of the chondroitin sulfate domain(s). We conclude that the release of aggrecan fragments from articular cartilage into the synovial fluid seen at all stages of human osteoarthritis (Lohmander, L. S. 1991. Acta Orthop. Scand. 62:623-632) is promoted by the action of a normal cartilage proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Orthop Res. 1984;2(3):201–206. doi: 10.1002/jor.1100020301. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Structure of newly synthesised (35S)-proteoglycans and (35S)-proteoglycan turnover products of cartilage explant cultures from dogs with experimental osteoarthritis. J Orthop Res. 1985;3(2):140–147. doi: 10.1002/jor.1100030203. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Murphy G. The tissue metalloproteinase family and the inhibitor TIMP: a study using cDNAs and recombinant proteins. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):469–479. [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Grodzinsky A. J., Koob T. J., Albert M. L., Eyre D. R. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Dahlberg L., Ryd L., Heinegård D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989 Nov;32(11):1434–1442. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S. Markers of cartilage metabolism in arthrosis. A review. Acta Orthop Scand. 1991 Dec;62(6):623–632. doi: 10.3109/17453679108994513. [DOI] [PubMed] [Google Scholar]
- Lohmander S. Turnover of proteoglycans in guinea pig costal cartilage. Arch Biochem Biophys. 1977 Apr 15;180(1):93–101. doi: 10.1016/0003-9861(77)90012-1. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Hughes C. E., Fosang A. J., Hardingham T. E. Role and regulation of metalloproteinases in connective tissue turnover. Biochem Soc Trans. 1990 Oct;18(5):812–815. doi: 10.1042/bst0180812. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Liu J., Roughley P. J., Mort J. S. Link protein as a monitor in situ of endogenous proteolysis in adult human articular cartilage. Biochem J. 1991 Aug 15;278(Pt 1):143–147. doi: 10.1042/bj2780143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]



