Abstract
The objective of this case-control study was to investigate the risk of cardiovascular disease (CVD) following non-steroidal anti-inflammatory drug (NSAID) use in patients with ankylosing spondylitis (AS). A total of 10,763 new AS patients were identified from the National Taiwan Health Insurance claims database during the period from 1997 to 2008. In all, 421 AS patients with CVD were recruited as cases, and up to 2-fold as many sex- and age-matched controls were selected. Logistic regression models were used to estimate the odds ratio (OR) between NSAID use and CVD incidence. The medication possession rate (MPR) was used to evaluate NSAID exposure during the study period. AS patients had increased risk of CVD (OR, 1.68; 95% confidence interval (CI), 1.57 to 1.80). Among frequent (MPR≥80%) COX II users, the risks for all types of CVD were ten times lower than those among non-users at 24 months (OR, 0.08; 95% CI, 0.01 to 0.92). Among frequent NSAID users, the risks of major adverse cardiac event (MACE) were significantly lower at 12 months (OR, 0.23; 95% CI, 0.07 to 0.76)—a trend showing that longer exposure correlated with lower risk. Regarding non-frequent NSAID users (MPR<80%), short-term exposure did carry higher risk (for 6 months: OR, 1.41; 95% CI, 1.07 to 1.86), but after 12 months, the risk no longer existed. We conclude that long-term frequent use of NSAIDs might protect AS patients from CVD; however, NSAIDs still carried higher short-term risk in the non-frequent users.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed drugs in the world. For many musculoskeletal conditions and inflammatory diseases, toothache and even dysmenorrhea, NSAIDs are the drugs of choice [1,2]. Over the past 50 years, traditional NSAIDs have become notorious for their GI complications [3–5]. In the 1990s, a new class of NSAIDs that were designed for specific inhibition of cyclooxygenase II (COX II) was launched and proved to reduce the risk of GI complications [6,7]. Unfortunately, a new concern arose for COX II inhibitors: increased cardiovascular risk. In the Vioxx Gastrointestinal Outcomes Research (VIGOR) study, rofecoxib was shown to be associated with a much higher risk of myocardial infarction than the comparator drug (naproxen) [7]. Furthermore, data from clinical trials [8–10], epidemiological studies [11,12] and a meta-analysis [13] indicated that both traditional NSAIDs and COX II inhibitors increase the occurrence of cardiovascular events. In the latest meta-analysis, all NSAIDs increased hospitalization for heart failure, and coxibs and diclofenac increased the frequency of major vascular events [14]. Panic has spread to both patients and physicians, certain publications advocate that even a low event rate must be taken seriously, given that NSAIDs are prescribed primarily for symptom relief. It is well known that autoimmune/auto-inflammatory diseases are associated with an increased risk of cardiovascular disease (CVD) [15,16]. Rheumatoid arthritis (RA) is reported to be associated with a 2- to 3-fold increased risk [17]; systemic lupus erythematosus, with an at least 2-fold increased risk [18,19]; and ankylosing spondylitis (AS), with a 1.3- to 2.2-fold increased risk [20,21]. Autoimmune/auto-inflammatory diseases and atherosclerosis have been found to share similar inflammatory processes, suggesting that mechanisms of auto-inflammatory cascades contribute to the excessive cardiovascular risk of autoimmune/auto-inflammatory diseases [22]. Given that an inflammatory process mediates atherosclerosis, it has been suggested that the medications used to control inflammation may potentially reduce the cardiovascular risk of autoimmune/auto-inflammatory diseases. Tumor necrosis factor (TNF) inhibitors are widely used for the treatment of RA due to their strong anti-inflammatory reaction. Several studies have revealed that TNF inhibitors are effective in reducing cardiovascular risks [23–25]. In one study, Bili et al. found that the use of anti-TNF was associated with a 55% reduction in the risk of developing coronary artery disease in an incident cohort of RA patients and that this risk decreased further with prolonged use [26].
Theoretically, mediated by their anti-inflammatory effects, the use of NSAIDs can reduce the cardiovascular risk of autoimmune/auto-inflammatory diseases. However, NSAID use is known to increase cardiovascular risk in the general population. It would be very interesting to know whether the level of NSAID use is pro-CVD or anti-CVD. In this study, we chose patients with AS as the study population to investigate the impact of NSAID use on the risk of cardiovascular events.
Methods
Data Sources
The Taiwan National Health Insurance (NHI) database covers health data from 99% of the 23 million inhabitants of Taiwan. This database includes disease diagnosis, hospital admissions, outpatient visits, and prescriptions. The database has been released to researchers in an electronically encrypted form since 1999. The large sample size and the high quality of diagnosis recording, according to the coding system of the International Classification of Diseases—Ninth Revision, Clinical Modification (ICD-9-CM), have ensured that this dataset provides the opportunity to estimate the incidence of CVD in both the general populations and AS patients with different levels of NSAID exposure. The study protocol was approved by the Kaohsiung Medical University Hospital Ethics Committee. The study protocol was approved by the Kaohsiung Medical University Hospital Ethics Committee. Because patient records/information was anonymized and de-identified prior to analysis, informed consent is not required.
AS Cohort
The records of all patients who were diagnosed with AS using the ICD-9-CM (code 7200) and logged in the claims database between January 1, 1997, and December 31, 2008, were retrieved. In Taiwan, patients who fulfill the 1984 modified New York criteria for AS are defined in NHI database as AS. We recruited those AS patients who had at least two service claims or either ambulatory or inpatient care for further confirmation of their diagnosis. Clinical characteristics included information on overall comorbidity at the time of data mining, which was assessed by computing the Charlson comorbidity index (CCI) [27]. The index applies to 17 disease categories whose scores are totaled to obtain an overall score for each patient.
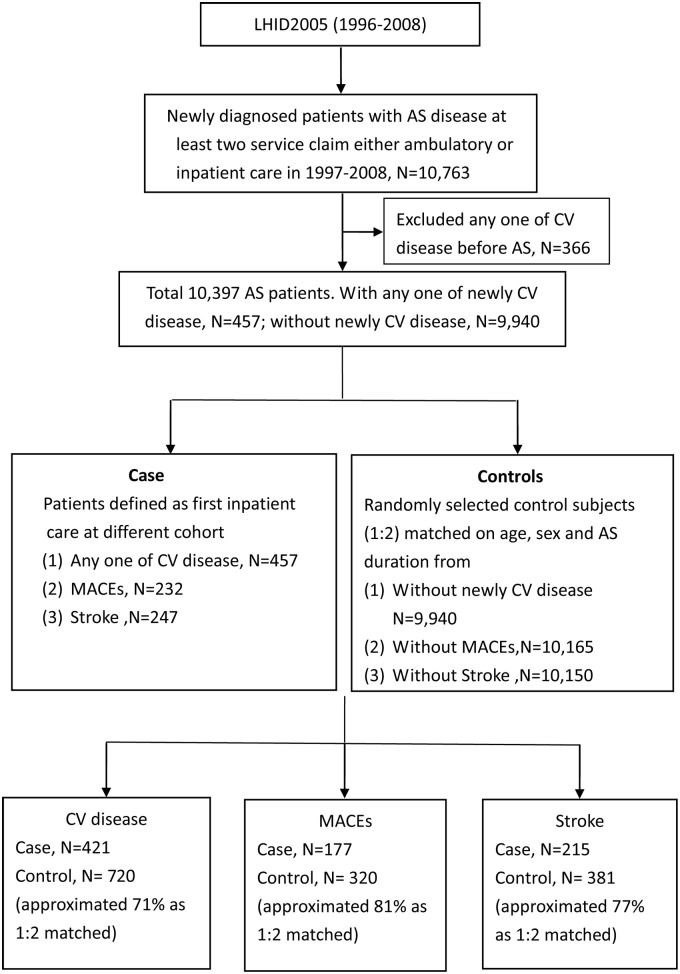
A total of 10,763 patients were selected by the above definition. We excluded 366 patients who had any CVD before the diagnosis of AS. As a result, the study cohort encompassed 10,397 patients. Among them, 457 patients who had newly onset CVD were included as cases. The other 9,940 patients served as the control group. For further analysis of the risk of stroke and major adverse cardiac events (MACEs), 247 and 232 patients who had stroke and MACEs, respectively, were included as cases. In total, 10,150 and 10,165 patients who did not have stroke or MACEs, respectively, were selected as controls. Risk-set sampling matched by sex, age (within 5 years), AS duration at the onset of CVD, and the frequency of NSAID use was used to select controls in the AS cohort. Up to 2 controls were selected for each case (Fig 1).
Fig 1. Consort diagram.
Abbreviations: AS, ankylosing spondylitis; CV disease, cardiovascular disease; MACEs, major cardiovascular events; LHID, longitudinal health insurance database.
NSAID Treatment
All of the NSAIDs prescribed in the claims database were further classified into non-selective NSAIDs and specific COX II inhibitors. We chose the medication possession rate (MPR) as a tool to assess the frequency of NSAID exposure. Three groups were classified: non-user, MPR<80% and MPR≥80%. The durations of drug exposure were defined as 3 months, 6 months, 12 months, 24 months and 36 months.
Outcomes
The combined endpoints of stroke (ICD-9 430–438), MACEs (acute coronary syndrome: ICD-9 410–410.9, heart failure: ICD-9 428.0–428.9, cerebrovascular accident, stroke: ICD-9 430–438) and any CVD (ICD-9 410–414, 425–426, 427.3, 428, 430–438, 443, 785.4, 444.2) were used as outcomes of NSAID exposure.
Statistical Analysis
In this study, conditional logistic regression was used to estimate the crude and adjusted odds ratios (ORs) and 95% confidence interval (CI) for the risk of CVD associated with NSAID use. Potential risk factors, including, sex, age, CCI, AS disease duration and other drugs used, were incorporated into the models. The frequency of NSAID exposure, which was assessed using the MPR, was included as a time-dependent variable. CIs were set to 95%, and a two-tailed p value less than 0.05 was considered significant.
Results
A total of 10,763 patients were recruited from the claims database. Compared with normal populations, the AS patients had an increased risk of CVD. The crude OR was 1.68 (95% CI, 1.57 to 1.80; p<0.0001). After exclusion of 366 patients with CVD onset before the diagnosis of AS, 10,397 patients were selected for further analysis. The baseline characteristics of the AS patients are reported in Table 1. More than 70% of patients were aged less than 55 years old. As shown in Table 1, approximately 53% of patients developed CVD more than 3 years after the diagnosis of AS.
Table 1. Baseline characteristics of AS patients and duration from diagnosis to the onset of CV disease.
| AS patients (N = 10,397) | AS patients with CV disease (N = 457) | |||
|---|---|---|---|---|
| Characteristics | N (%) | Duration (year) | N (%) | |
| Sex | Female | 5,705 (54.87) | <1 | 61 (13.35) |
| Male | 4,692 (45.13) | 1–2 | 86 (18.82) | |
| Age | <35 | 3,027 (29.11) | 2–3 | 66 (14.44) |
| 35–45 | 2,082 (20.03) | 3–4 | 50 (10.94) | |
| 45–55 | 2,336 (22.47) | >4 | 195 (42.45) | |
| 55–65 | 1,411 (13.57) | |||
| >65 | 1,541 (14.82) | |||
Abbreviation: AS, ankylosing spondylitis; CV, cardiovascular
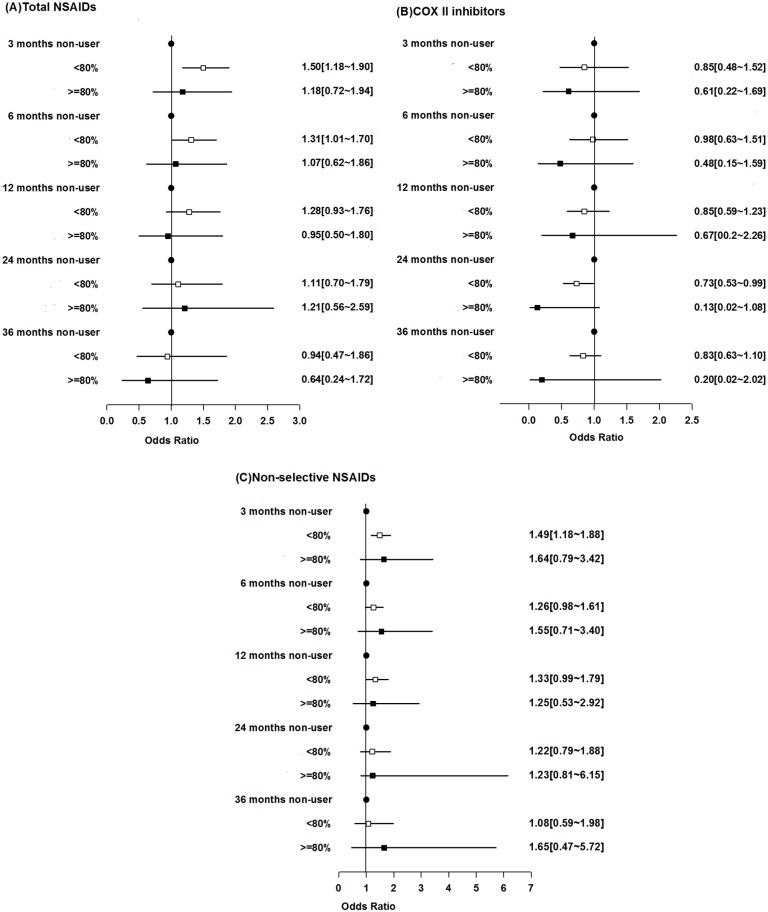
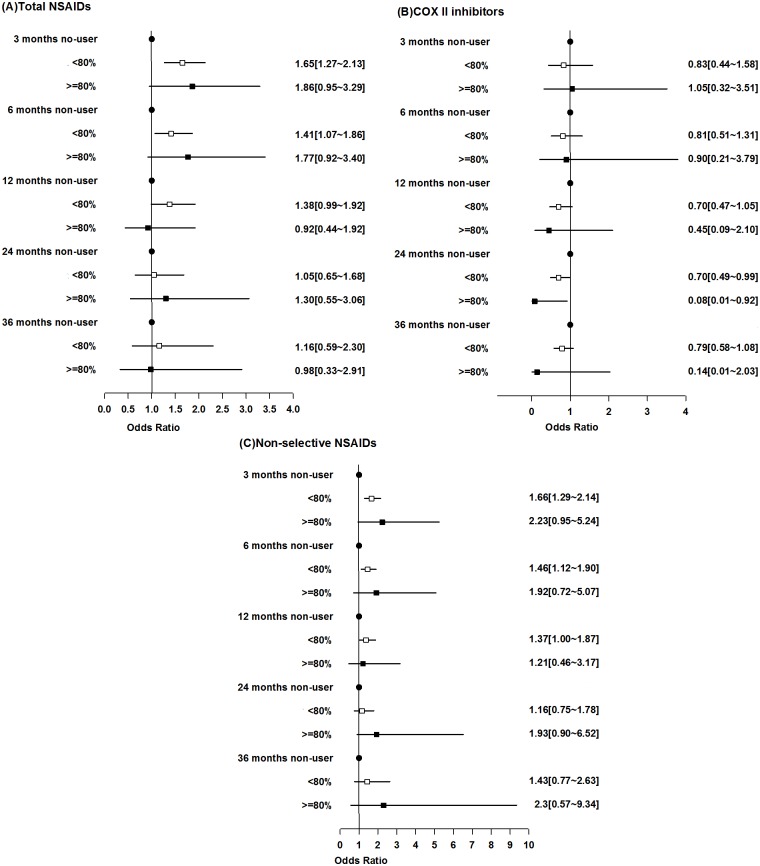
For all types of CVD as an endpoint, a total of 421 incident cases and 720 age-, sex- and disease duration-matched controls were identified. After adjustment for the CCI, among patients who were frequent NSAID users (MPR≥80%), there was no statistically significant increase in CVD risk compared with that among non-users at any time point after the initiation of NSAID. Moreover, there was a trend showing that the longer the exposure was, the lower the risk was. For non-frequent NSAID users (MPR<80), a statistically significant increase in CVD risk was found with short-term exposure (at 3 months: OR, 1.50; 95% CI, 1.18 to 1.90; p = 0.001 and at 6 months: OR, 1.31; 95% CI, 1.01 to 1.70; p = 0.0412), but after 12 months, a significant risk was no longer found (Fig 2A). After further adjustment for other drugs used, the results were similar (at 3 months: OR, 1.65; 95% CI, 1.27 to 2.13; p = 0.0002 and at 6 months: OR, 1.41; 95% CI, 1.07 to 1.86; p = 0.0137) (Fig 3A). We further stratified all NSAIDs into two groups: COX II inhibitors and non-selective NSAIDs. Compared with NSAID non-users, COX II users did not have an increased risk of any type of CVD. Instead, after adjustment for CCI and drug, among patients who were frequent COX II user for 24 months, the risks for all types of CVD were ten times lower than that among non-user (OR, 0.08; 95% CI, 0.01 to 0.92; p = 0.042). Even for those non-frequent users, the risk was significantly lower compared with that among non-user (OR, 0.70; 95% CI, 0.49 to 0.99; p = 0.043) (Fig 3B). There was also a trend showing that the longer the use was, the lower the risk was (Fig 2B and Fig 3B). For non-frequent non-selective NSAID users, a mild increase in CVD was found at 3 months (OR, 1.49; 95% CI, 1.18 to 1.88; p = 0.001) (Fig 2C). Similar results were found at 3 months (OR, 1.66; 95% CI, 1.29 to 2.14; p<0.001) after adjustment for other drugs (Fig 3C).
Fig 2. Risks of all types of cardiovascular diseases associated with non-steroidal anti-inflammatory drugs (NSAIDs).
(A) Total NSAIDs, (B) specific cyclooxygenase II (COX II) inhibitors, (C) non-selective NSAIDs. NSAID exposure was categorized into non-users, MPR<80% and MPR≥80%. The data were adjusted by the CCI.
Fig 3. Risks of all types of cardiovascular diseases associated with non-steroidal anti-inflammatory drugs (NSAIDs).
(A) Total NSAIDs, (B) specific cyclooxygenase II (COX II) inhibitors, (C) non-selective NSAIDs. NSAID exposure was categorized into non-users, MPR<80% and MPR≥80%. The data were adjusted by the CCI and other drugs used.
For MACEs as an endpoint, a total of 177 incident cases and 320 age-, sex- and disease duration-matched controls were identified. After adjustment for the CCI, no any NSAID user had a statistically significant increase in MACE risk compared with non-users, no matter for how long and how frequently they were exposed to NSAIDs (Table 2). After further adjustment for drugs, there was significant lower risk for those frequent users at 12 months (OR, 0.23; 95% CI, 0.07 to 0.76; p = 0.016) (S1 Table).
Table 2. Risk of MACEs associated with NSAIDs in patients with AS stratified by frequency of exposure and types of NSAIDs adjust for Charlson comorbility index.
| Total NSAIDs | COX-II | Non-selective NSAIDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | OR | 95%CI | P-value | ||||||
| 3 months | Non-user | 1 | - | - | 3 months | Non-user | 1 | - | - | 3 months | Non-user | 1 | - | - |
| <80% | 1.21 | 0.87–1.70 | 0.259 | <80% | 0.56 | 0.26–1.25 | 0.1576 | <80% | 1.36 | 0.98–1.90 | 0.0669 | |||
| ≥80% | 0.78 | 0.37–1.67 | 0.5227 | ≥80% | 0.38 | 0.07–2.18 | 0.275 | ≥80% | 0.86 | 0.29–2.52 | 0.7801 | |||
| 6 months | Non-user | 1 | - | - | 6 months | Non-user | 1 | - | - | 6 months | Non-user | 1 | - | . |
| <80% | 1.13 | 0.79–1.61 | 0.5125 | <80% | 0.74 | 0.40–1.35 | 0.3218 | <80% | 1.2 | 0.85–1.69 | 0.29 | |||
| ≥80% | 0.81 | 0.38–1.76 | 0.5995 | ≥80% | 0.15 | 0.02–1.45 | 0.1014 | ≥80% | 1.21 | 0.44–3.31 | 0.7155 | |||
| 12 months | Non-user | 1 | - | - | 12 months | Non-user | 1 | - | - | 12 months | Non-user | 1 | - | - |
| <80% | 0.85 | 0.55–1.31 | 0.4572 | <80% | 0.61 | 0.37–1.02 | 0.0582 | <80% | 1.03 | 0.68–1.54 | 0.9023 | |||
| ≥80% | 0.48 | 0.19–1.22 | 0.1252 | ≥80% | 0.43 | 0.06–3.15 | 0.4084 | ≥80% | 0.61 | 0.17–2.20 | 0.4536 | |||
| 24 months | Non-user | 1 | - | - | 24 months | Non-user | 1 | - | - | 24 months | Non-user | 1 | - | - |
| <80% | 0.92 | 0.48–1.76 | 0.8022 | <80% | 0.68 | 0.44–1.05 | 0.0801 | <80% | 0.89 | 0.50–1.58 | 0.6893 | |||
| ≥80% | 0.5 | 0.16–1.56 | 0.2317 | ≥80% | 1.41 | 0.08–24.67 | 0.8122 | ≥80% | 0.7 | 0.13–3.70 | 0.6704 | |||
| 36 months | Non-user | 1 | - | - | 36 months | Non-user | 1 | - | - | 36 months | Non-user | 1 | - | - |
| <80% | 0.95 | 0.34–2.69 | 0.9292 | <80% | 0.78 | 0.52–1.15 | 0.2013 | <80% | 1.1 | 0.47–2.53 | 0.8309 | |||
| ≥80% | 0.52 | 0.13–2.10 | 0.3613 | ≥80% | 1.43 | 0.08–25.09 | 0.8072 | ≥80% | 1.38 | 0.23–8.45 | 0.7263 | |||
Abbreviation: NSAIDs, non-steroidal anti-inflammatory drugs; Total NSAID, include COX-II inhibitors and non-selective NSAIDs; COX-II, cyclooxygenase II inhibitors; MACEs, major adverse cardiac events
For stroke as an endpoint, a total of 215 incident cases and 381 age-, sex- and disease duration-matched controls were identified. After adjustment for the CCI, only non-frequent non-selective NSAID users had a statistically significant increase in stroke risk compared with non-users at 3 months (OR, 1.44; 95% CI, 1.00 to 2.07; p = 0.049) (Table 3), but after further adjustment for the drugs used, a significant increase in stroke risk was no longer found (S2 Table).
Table 3. Risk of stroke associated with NSAIDs in patients with AS stratified by frequency of exposure and types of NSAIDs adjust for Charlson comorbility index.
| Total NSAIDs | COX-II | Non-selective NSAIDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | OR | 95%CI | P-value | ||||||
| 3 months | Non-user | 1 | - | - | 3 months | Non-user | 1 | - | - | 3 months | Non-user | 1 | - | - |
| <80% | 1.35 | 0.93–1.95 | 0.1128 | <80% | 0.87 | 0.36–2.12 | 0.7655 | <80% | 1.44 | 1.00–2.07 | 0.0494 | |||
| ≥80% | 0.9 | 0.39–2.05 | 0.796 | ≥80% | 0.38 | 0.07–2.15 | 0.2749 | ≥80% | 0.6 | 0.16–2.22 | 0.447 | |||
| 6 months | Non-user | 1 | - | - | 6 months | Non-user | 1 | - | - | 6 months | Non-user | 1 | - | - |
| <80% | 1.2 | 0.82–1.77 | 0.351 | <80% | 0.99 | 0.51–1.93 | 0.9747 | <80% | 1.26 | 0.87–1.83 | 0.226 | |||
| ≥80% | 0.97 | 0.43–2.20 | 0.939 | ≥80% | 0.18 | 0.02–1.85 | 0.1495 | ≥80% | 1.76 | 0.54–5.73 | 0.3459 | |||
| 12 months | Non-user | 1 | - | - | 12 months | Non-user | 1 | - | - | 12 months | Non-user | 1 | - | - |
| <80% | 1 | 0.63–1.59 | 0.9999 | <80% | 0.84 | 0.49–1.45 | 0.5321 | <80% | 1.21 | 0.78–1.87 | 0.3959 | |||
| ≥80% | 0.61 | 0.23–1.63 | 0.3214 | ≥80% | 0.29 | 0.04–1.84 | 0.1877 | ≥80% | 1.27 | 0.29–5.65 | 0.752 | |||
| 24 months | Non-user | 1 | - | - | 24 months | Non-user | 1 | - | - | 24 months | Non-user | 1 | - | - |
| <80% | 1.04 | 0.51–2.10 | 0.9231 | <80% | 0.79 | 0.49–1.26 | 0.3158 | <80% | 1 | 0.54–1.86 | 0.9933 | |||
| > = 80% | 0.72 | 0.22–2.36 | 0.5864 | ≥80% | 0.36 | 0.03–3.75 | 0.3894 | ≥80% | 1.07 | 0.19–6.16 | 0.9407 | |||
| 36 months | Non-user | 1 | - | - | 36 months | Non-user | 1 | - | - | 36 months | Non- user | 1 | - | - |
| <80% | 0.98 | 0.30–3.23 | 0.9755 | <80% | 0.97 | 0.63–1.49 | 0.8947 | <80% | 1.3 | 0.50–3.36 | 0.5905 | |||
| ≥80% | 0.55 | 0.12–2.60 | 0.4537 | ≥80% | 0.14 | 0.09–24.53 | 0.7907 | ≥80% | 1.36 | 0.21–8.97 | 0.7474 | |||
Abbreviation: NSAIDs, non-steroidal anti-inflammatory drugs; Total NSAID, include COX-II inhibitors and non-selective NSAIDs; COX-II, cyclooxygenase II inhibitors; MACEs, major adverse cardiac events
Discussion
Our results show that in the first 6 months, AS patients treated with NSAIDs did have a non-trivial risk of CVD among those who were non-frequent users. The risk tended to decline with long-term use. In frequent NSAID users, there was no significant risk of CVD, and interestingly, there was a trend showing that the longer the use was, the lower the risk was. Even more, long-term frequent use of COX II had a strong protective effect. The robustness of this finding was supported by the consistency of the results across several pre-specified analyses.
The conclusion that NSAIDs have the potential to increase the occurrence of CVD is based on three clinical trials and epidemiological studies. In the VIGOR study, the COX II inhibitor rofecoxib had a higher incidence of CVD compared with traditional NSAIDs, although in another trial, the CLASS study, celecoxib did not show the same trend. Unfortunately, the CVD risk of COX II inhibitors was further confirmed in two subsequent studies: the APC trial [8] and the APPROVE trial [9]. In these trials, compared with placebo, COX II inhibitors increased CVD risk in patients with a history of colon adenoma. Furthermore, the data showed that the higher the dose was, the higher the risk was. Later epidemiological studies [11] indicated that not only COX II inhibitors but also traditional NSAIDs have the potential to increase CVD risk. Compared with patients with autoimmune diseases, the patients recruited in the above trials were patients with colon adenoma, which is not an inflammatory disease. Additionally, those epidemiological studies examined cardiovascular outcomes in the general population. Few data on NSAIDs’ association with CVD risk within cohorts of patients with autoimmune diseases have been evaluated. Naproxen has been observed to have a cardio protective effect on patients with RA [28,29]. However, the cited reports were full of confounding factors (for example, the authors did not adjust for the drugs used, such as disease-modifying anti-rheumatic drugs (DMARDs), and there was considerable between-study heterogeneity. In a recent meta-analysis, no increased risk was associated with NSAIDs in patients with joint disease [30]. Again, between-study heterogeneity was high. Moreover, the event number was low, and most trials did not include cardiovascular events as the primary outcome, so it is not clear how ascertainment of events was performed in each trial. In an inflammatory polyarthritis cohort study, Goodson et al found that patients exposed to NSAIDs had a 2.5-fold reduction in cardiovascular death compared with non-users [31]. The limitations of their study were 1) the high heterogeneity of the patient population and 2) potential inaccuracy in assessing NSAID exposure. In a recent report from Denmark, Linhardsen et al. observed that the CVD risk associated with NSAID use is lower in RA patients than in patients without RA. Again, the limitation in this study is that the authors ignored the drugs used by the RA patients, which might have had an additional immunomodulatory effect [32].
The strength of the current study is that compared with previous studies, which derived their conclusions from patients with high heterogeneity, we specifically chose patients with AS as the study population to investigate the impact of NSAID use on the risk of cardiovascular events. One reason that we chose patients with AS is that in contrast to use in patients with RA, the use of NSAIDs in the AS patient population is very heterogeneous, so we could compare frequent users with non-frequent users. For RA patients, NSAIDs are usually indispensable. A second reason is that DMARDs (both synthetic and biologic) and steroids are seldom prescribed for the AS population, making the analysis more simple and straightforward.
Recently, interesting observations from clinical studies on patients with AS revealed that NSAIDs could potentially defer radiographic progression [33,34]. Compared with on-demand users, regular NSAID users had less radiographic progression. Meanwhile, patients with high inflammatory marker levels benefitted more from continuous NSAID use. Another observation from a German cohort of patients with ankylosing spondylitis was similar, associating high NSAID intake over 2 years with less mSASSS progression [35]. Based on these solid results, Nigil et al. suggested long-term regular use of NSAIDs among young AS patients without cardiovascular risk to retard radiographic progression, but for older patients, CVD risk should still be considered [36].
In this study we excluded 366 patients who had prior CVD before the diagnosis of AS. This exclusion criteria might cause a potential bias since these patients might have a delayed diagnosis of AS. We had done further analysis which included these 366 patients. The result was similar to the previous analysis. NSAIDs did increase CVD risk in non-frequent users in first 3 months. For frequent users, there was no significant risk of CVD (S3 Table). This study had certain limitations. First, using the data bank, we could not identify which patients had a higher inflammatory status. Inflammatory status might have been a confounding factor. However, theoretically, a higher inflammatory status may cause patients to be prescribed more NSAIDs to relieve symptoms. Second, we were unable to determine real compliance with the drugs prescribed. Third, several of the patients may have taken alternative medicine or may have even bought NSAIDs over the counter by themselves, and these data could not be traced in the database. However, patients only have to pay very low physician fee and one tenth of the drug price for each clinic visit. Total cost will be much lower than they buy the drugs in pharmacy stores. We considered that very low percentage of our patients (especially for those patients with chronic diseases such as AS) got their NSAIDs over the counter. Fourth, it is possible that patients with high CVD risk were channeled away from NSAIDs. This could cause the favorable CVD outcomes observed in NSAID users. Fifth, compared with randomized trials and cohort studies, the level of evidence derived from a case-control study is considered to be lower because the study design is subject to many biases, including in case and control selection, confounding adjustment, and outcome measures. Although we have adjusted for several confounding factors, a major limitation is that unknown confounders may remain, resulting from misclassification of variables or unmeasured variables. Additionally, it seemed that patients were quite old in our database. There are two possibilities: 1) due to delayed diagnosis. In Taiwan, in average, male AS had delayed diagnosis for 5 years, female AS had delayed diagnosis for 10 years. 2): an AS patient might have been ill for years when his data first appeared in the database.
Conclusion
The results of this study provided data from our cohort for clinicians and patients to judge the risks and benefits of medication when long-term NSAID use is necessary to relieve painful arthritis. Although our data showed that there were no increased risks of CVD in long-term NSAID users. Individual patient must balance the risk/benefit when long-term use is considered. Of course, we need more studies of other autoimmune/auto-inflammatory diseases to determine whether they have similar results as our study. Studies designed both prospectively and retrospectively may help to elucidate the impact of the long-term use of NSAIDs.
Supporting Information
(DOCX)
(DOCX)
(DOC)
Acknowledgments
The authors are thankful for help from the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Medical University Hospital. This study was based in part on data from the National Health Insurance Research Database, provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes (registration number 980178). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Wolfe F, Zhao S, Lane N (2000) Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum 43:378–85. [DOI] [PubMed] [Google Scholar]
- 2. Pincus T, Swearingen C, Cummins P, Callahan LF (2000) Preference for nonsteroidal antiinflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. J Rheumatol 27:1020–27. [PubMed] [Google Scholar]
- 3. Gabriel SE, Jaakkimainen L, Bombardier C (1991) Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med 115:787–96. [DOI] [PubMed] [Google Scholar]
- 4. Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA (1991) Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 114:257–63. [DOI] [PubMed] [Google Scholar]
- 5. Wolfe MM, Lichtenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 340:1888–99. [DOI] [PubMed] [Google Scholar]
- 6. Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A et al. (2000) Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284:1247–55. [DOI] [PubMed] [Google Scholar]
- 7. Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas, Davis B R et al. (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 343:1520–28. [DOI] [PubMed] [Google Scholar]
- 8. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K et al. (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352:1092–102. [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P et al. (2005) Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352:1071–80. [DOI] [PubMed] [Google Scholar]
- 10. Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A Parlow JL et al. (2005) Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352:1081–91. [DOI] [PubMed] [Google Scholar]
- 11. Hippisley-Cox J, Coupland C (2005) Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 330:1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Nørgård B, Sørensen HT (2005) Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med 165:978–84. [DOI] [PubMed] [Google Scholar]
- 13. Hennekens CH, Borzak S (2008) Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J Cardiovasc Pharmacol Ther 13:41–50. 10.1177/1074248407312990 [DOI] [PubMed] [Google Scholar]
- 14. Lader E (2013) Review: NSAIDs increase GI and CV events; coxibs increase mortality. Ann Intern Med 159:JC12 10.7326/0003-4819-159-12-201312170-02012 [DOI] [PubMed] [Google Scholar]
- 15. Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV (2006) Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33:2167–72. [PubMed] [Google Scholar]
- 16. Kitas GD, Erb N (2003) Tackling ischaemic heart disease in rheumatoid arthritis. Rheumatology 42:607–13. [DOI] [PubMed] [Google Scholar]
- 17. Kitas GD, Gabriel SE (2011) Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 70:8–14. 10.1136/ard.2010.142133 [DOI] [PubMed] [Google Scholar]
- 18. Schoenfeld SR, Kasturi S, Costenbader KH (2013) The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 43:77–95. 10.1016/j.semarthrit.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 19. Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, et al. (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44:2331–37. [DOI] [PubMed] [Google Scholar]
- 20. Bremander A, Petersson IF, Bergman S, Englund M (2011) Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res 63:550–56. 10.1002/acr.20408 [DOI] [PubMed] [Google Scholar]
- 21. Szabo SM, Levy AR, Rao SR, Kirbach SE, Lacaille D, Cifaldi M et al. (2011) Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum 63:3294–304. 10.1002/art.30581 [DOI] [PubMed] [Google Scholar]
- 22. Stevens RJ, Douglas KM, Saratzis AN, Kitas GD (2005) Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med 7:1–24. [DOI] [PubMed] [Google Scholar]
- 23. Jacobsson LT, Turesson C, Gulfe A, Kapetanovic MC, Petersson IF, Saxne T et al. (2005) Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol 32:1213–18. [PubMed] [Google Scholar]
- 24. Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P et al. (2011) Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis 70:576–82. 10.1136/ard.2010.129916 [DOI] [PubMed] [Google Scholar]
- 25. Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP et al. (2007) Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 56:2905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL et al. (2014) TNF-alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis patients. Arthritis Care Res 66:355–63. 10.1002/acr.22166 [DOI] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–83. [DOI] [PubMed] [Google Scholar]
- 28. Watson DJ, Rhodes T, Cai B, Guess HA (2002) Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med 162:1105–10. [DOI] [PubMed] [Google Scholar]
- 29. Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M (2004) Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 364:2021–29. [DOI] [PubMed] [Google Scholar]
- 30. Salpeter SR, Gregor P, Ormiston TM, Whitlock R, Raina P, Thabane L et al. (2006) Meta-analysis: cardiovascular events associated with nonsteroidal anti-inflammatory drugs. Am J Med 119:552–59. [DOI] [PubMed] [Google Scholar]
- 31. Goodson NJ, Brookhart AM, Symmons DP, Silman AJ, Solomon DH (2009) Non-steroidal anti-inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis 68:367–72. 10.1136/ard.2007.076760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindhardsen J, Gislason GH, Jacobsen S, Ahlehoff O, Olsen AM, Madsen OR et al. (2014) Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 73: 1515–1521 [DOI] [PubMed] [Google Scholar]
- 33. Wanders A, Heijde D, Landewe R, Béhier JM, Calin A, Olivieri I et al. (2005) Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 52:1756–65. [DOI] [PubMed] [Google Scholar]
- 34. Kroon F, Landewe R, Dougados M (2012) Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 71:1623–29. 10.1136/annrheumdis-2012-201370 [DOI] [PubMed] [Google Scholar]
- 35. Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, Zeidler H et al. (2012) Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 71:1616–22. 10.1136/annrheumdis-2011-201252 [DOI] [PubMed] [Google Scholar]
- 36. Haroon N, Kim TH, Inman RD (2012) NSAIDs and radiographic progression in ankylosing spondylitis Bagging big game with small arms? Ann Rheum Dis 71:1593–95. 10.1136/annrheumdis-2012-201844 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.