Abstract
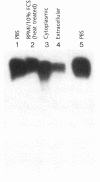
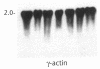
Colo 320 cells are colonic carcinoma cells known to express abundant c-myc mRNA. Based on the response of several hematopoietic cell lines to chemical inducers of differentiation, we reasoned that such agents might have similar inductive activity in Colo 320 cells. Accordingly, we exposed Colo 320 cells to 5 mM sodium butyrate (NaBT) for 7 d. C-myc expression decreased threefold and self-replicative potential decreased (defined as a greater than 60% decrease in colony-forming capacity in soft agar that did not contain inducer). In an effort to demonstrate a direct cause and effect between myc expression and the colony-forming capacity of Colo 320 cells, we exposed these cells to a 15-base antisense c-myc oligonucleotide (complementary to the translation initiation region of exon II). Cells were also exposed to equimolar (20 microM) amounts of sense and missense oligonucleotides. Subsequently, cells were incubated at 10, 20, 30, and 40 microM antisense DNA for 16 h, then washed and plated in oligonucleotide-free agar medium. We demonstrated that: (a) the oligomers were stable in the extracellular medium and in the cell cytoplasm; (b) the uptake of the oligonucleotides was 0.7%; (c) sense and missense oligonucleotides had no effect on colony-forming capacity; and (d) the antisense c-myc oligonucleotide resulted in a 40-75% concentration-dependent decrease in colony-forming capacity. The specific inhibition of colony-forming capacity by antisense DNA suggests that the role of myc expression in Colo 320 cells is similar to its role in hematopoiesis, and that the failure to inhibit myc expression maintains colony-forming capacity. This system provides a new strategy for inducing differentiation and may provide further insight into the genetic factors that govern the process of colonic carcinogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Buick R. N., Fry S. E., Salmon S. E. Application of in vitro soft agar techniques for growth of tumor cells to the study of colon cancer. Cancer. 1980 Mar 15;45(5 Suppl):1238–1242. doi: 10.1002/1097-0142(19800315)45:5+<1238::aid-cncr2820451333>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Cole M. D. Myc meets its Max. Cell. 1991 May 31;65(5):715–716. doi: 10.1016/0092-8674(91)90377-b. [DOI] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Dexter D. L., Hager J. C. Maturation-induction of tumor cells using a human colon carcinoma model. Cancer. 1980 Mar 15;45(5 Suppl):1178–1184. doi: 10.1002/1097-0142(19800315)45:5+<1178::aid-cncr2820451323>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Emilia G., Donelli A., Ferrari S., Torelli U., Selleri L., Zucchini P., Moretti L., Venturelli D., Ceccherelli G., Torelli G. Cellular levels of mRNA from c-myc, c-myb and c-fes onc-genes in normal myeloid and erythroid precursors of human bone marrow: an in situ hybridization study. Br J Haematol. 1986 Feb;62(2):287–292. doi: 10.1111/j.1365-2141.1986.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Filmus J., Buick R. N. Relationship of c-myc expression to differentiation and proliferation of HL-60 cells. Cancer Res. 1985 Feb;45(2):822–825. [PubMed] [Google Scholar]
- Gowda S. D., Koler R. D., Bagby G. C., Jr Regulation of C-myc expression during growth and differentiation of normal and leukemic human myeloid progenitor cells. J Clin Invest. 1986 Jan;77(1):271–278. doi: 10.1172/JCI112287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Kam W. K., Byrd J. C., Hicks J. W., Sleisenger M. H., Kim Y. S. Effects of sodium butyrate on human colonic adenocarcinoma cells. Induction of placental-like alkaline phosphatase. J Biol Chem. 1987 Jan 25;262(3):1092–1097. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZ F., NITOWSKY H. M. Alkaline phosphatase activity of human cell cultures: kinetic and physical-chemical properties. Arch Biochem Biophys. 1962 Mar;96:506–515. doi: 10.1016/0003-9861(62)90328-4. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Tsao D., Siddiqui B., Whitehead J. S., Arnstein P., Bennett J., Hicks J. Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer. 1980 Mar 15;45(5 Suppl):1185–1192. doi: 10.1002/1097-0142(19800315)45:5+<1185::aid-cncr2820451324>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kirkland S. C. Clonal origin of columnar, mucous, and endocrine cell lineages in human colorectal epithelium. Cancer. 1988 Apr 1;61(7):1359–1363. doi: 10.1002/1097-0142(19880401)61:7<1359::aid-cncr2820610714>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Cheng G. H., Skoultchi A. I. Transfection of mouse erythroleukemia cells with myc sequences changes the rate of induced commitment to differentiate. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6480–6484. doi: 10.1073/pnas.83.17.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G. D., Desai T. K., Conteas C. N., Moshier J. A., Silverman A. L. Biochemical markers in colorectal cancer: diagnostic and therapeutic implications. Gastroenterol Clin North Am. 1988 Dec;17(4):931–940. [PubMed] [Google Scholar]
- Mills S. E., Allen M. S., Jr, Cohen A. R. Small-cell undifferentiated carcinoma of the colon. A clinicopathological study of five cases and their association with colonic adenomas. Am J Surg Pathol. 1983 Oct;7(7):643–651. doi: 10.1097/00000478-198310000-00005. [DOI] [PubMed] [Google Scholar]
- Morita A., Tsao D., Kim Y. S. Effect of sodium butyrate on alkaline phosphatase in HRT-18, a human rectal cancer cell line. Cancer Res. 1982 Nov;42(11):4540–4545. [PubMed] [Google Scholar]
- Mulder K. M., Brattain M. G. Alterations in c-myc expression in relation to maturational status of human colon carcinoma cells. Int J Cancer. 1988 Jul 15;42(1):64–70. doi: 10.1002/ijc.2910420113. [DOI] [PubMed] [Google Scholar]
- Petrelli M., Tetangco E., Reid J. D. Carcinoma of the colon with undifferentiated, carcinoid, and squamous cell features. Am J Clin Pathol. 1981 Apr;75(4):581–584. doi: 10.1093/ajcp/75.4.581. [DOI] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Sarsfield P., Anthony P. P. Small cell undifferentiated ('neuroendocrine') carcinoma of the colon. Histopathology. 1990 Apr;16(4):357–363. doi: 10.1111/j.1365-2559.1990.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao D., Morita A., Bella A., Jr, Luu P., Kim Y. S. Differential effects of sodium butyrate, dimethyl sulfoxide, and retinoic acid on membrane-associated antigen, enzymes, and glycoproteins of human rectal adenocarcinoma cells. Cancer Res. 1982 Mar;42(3):1052–1058. [PubMed] [Google Scholar]
- Wick M. R., Weatherby R. P., Weiland L. H. Small cell neuroendocrine carcinoma of the colon and rectum: clinical, histologic, and ultrastructural study and immunohistochemical comparison with cloacogenic carcinoma. Hum Pathol. 1987 Jan;18(1):9–21. doi: 10.1016/s0046-8177(87)80187-9. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]