Abstract
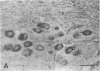
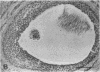
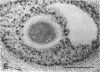
The actions, localization, and regulation of activin in the human ovary are unknown. Therefore, the aims of this study were (a) to define the effects of recombinant activin-A and its structural homologue, inhibin-A, on mitogenesis and steroidogenesis (progesterone secretion and aromatase activity) in human preovulatory follicular cells; (b) to localize the activin-A dimer in the human ovary by immunohistochemistry; and (c) to examine regulation of intracellular activin-A production in cultured human follicular cells. In addition to stimulating mitogenic activity, activin-A causes a dose- and time-dependent inhibition of basal and gonadotropin-stimulated progesterone secretion and aromatase activity in human luteinizing follicular cells on day 2 and day 4 of culture. Inhibin-A exerts no effects on mitogenesis, basal or gonadotropin-stimulated progesterone secretion and aromatase activity, and does not alter effects observed with activin-A alone. Immunostaining for dimeric activin-A occurs in granulosa and cumulus cells of human ovarian follicles and in granulosa-lutein cells of the human corpus luteum. cAMP, and to a lesser degree human chorionic gonadotropin and follicle-stimulating hormone, but not inhibin-A, activin-A, or phorbol 12-myristate 13-acetate, increased the immunostaining for activin-A in cultured granulosa cells. These results indicate that activin-A may function as an autocrine or paracrine regulator of follicular function in the human ovary.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis S. R., Dench F., Nikolaidis I., Clements J. A., Forage R. G., Krozowski Z., Burger H. G. Inhibin A-subunit gene expression in the ovaries of immature female rats is stimulated by pregnant mare serum gonadotrophin. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1191–1195. doi: 10.1016/s0006-291x(86)80408-9. [DOI] [PubMed] [Google Scholar]
- Demoulin A., Guichard A., Mignot T. M., Cedard L., Lambotte R., Franchimont P. Inhibin concentration in the culture media of human oocyte-cumulus-corona cell complexes is not related to subsequent embryo cleavage. Fertil Steril. 1986 Dec;46(6):1150–1152. doi: 10.1016/s0015-0282(16)49897-3. [DOI] [PubMed] [Google Scholar]
- Fluker M. R., Marshall L. A., Monroe S. E., Jaffe R. B. Variable ovarian response to gonadotropin-releasing hormone antagonist-induced gonadotropin deprivation during different phases of the menstrual cycle. J Clin Endocrinol Metab. 1991 Apr;72(4):912–919. doi: 10.1210/jcem-72-4-912. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Manchon C., Vale W. Activin-A, inhibin and transforming growth factor-beta modulate growth of two gonadal cell lines. Endocrinology. 1989 Sep;125(3):1666–1672. doi: 10.1210/endo-125-3-1666. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Miyamoto K., Abe Y., Nakamura T., Sugino H., Eto Y., Shibai H., Igarashi M. Induction of follicle stimulating hormone receptor by erythroid differentiation factor on rat granulosa cell. Biochem Biophys Res Commun. 1988 Oct 31;156(2):668–674. doi: 10.1016/s0006-291x(88)80894-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Miyamoto K., Iwamura S., Igarashi M. Changes in serum concentrations of inhibin in cyclic pigs. J Endocrinol. 1988 Aug;118(2):211–219. doi: 10.1677/joe.0.1180211. [DOI] [PubMed] [Google Scholar]
- Hutchinson L. A., Findlay J. K., de Vos F. L., Robertson D. M. Effects of bovine inhibin, transforming growth factor-beta and bovine Activin-A on granulosa cell differentiation. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1405–1412. doi: 10.1016/0006-291x(87)90806-0. [DOI] [PubMed] [Google Scholar]
- LaPolt P. S., Soto D., Su J. G., Campen C. A., Vaughan J., Vale W., Hsueh A. J. Activin stimulation of inhibin secretion and messenger RNA levels in cultured granulosa cells. Mol Endocrinol. 1989 Oct;3(10):1666–1673. doi: 10.1210/mend-3-10-1666. [DOI] [PubMed] [Google Scholar]
- Laherty R. F., Rotten D., Yamamoto M., Jaffe R. B. Effects of oestradiol and prolactin on progesterone production by rhesus monkey luteal cells in vitro. Acta Endocrinol (Copenh) 1985 Feb;108(2):266–272. doi: 10.1530/acta.0.1080266. [DOI] [PubMed] [Google Scholar]
- Lucas C., Bald L. N., Fendly B. M., Mora-Worms M., Figari I. S., Patzer E. J., Palladino M. A. The autocrine production of transforming growth factor-beta 1 during lymphocyte activation. A study with a monoclonal antibody-based ELISA. J Immunol. 1990 Sep 1;145(5):1415–1422. [PubMed] [Google Scholar]
- Marshall L. A., Fluker M. R., Jaffe R. B., Monroe S. E. Inhibition of follicular development by a potent antagonistic analog of gonadotropin-releasing hormone (detirelix). J Clin Endocrinol Metab. 1991 Apr;72(4):927–933. doi: 10.1210/jcem-72-4-927. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Spiess J., Rivier J., Rosenfeld M. G., Evans R. M., Vale W. Inhibin A-subunit cDNAs from porcine ovary and human placenta. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5849–5853. doi: 10.1073/pnas.83.16.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier H., Cajander S. B., Roberts V. J., Rivier C., Sawchenko P. E., Hsueh A. J., Vale W. Rapid changes in the expression of inhibin alpha-, beta A-, and beta B-subunits in ovarian cell types during the rat estrous cycle. Mol Endocrinol. 1988 Dec;2(12):1352–1363. doi: 10.1210/mend-2-12-1352. [DOI] [PubMed] [Google Scholar]
- O W. S., Robertson D. M., de Kretser D. M. Inhibin as an oocyte meiotic inhibitor. Mol Cell Endocrinol. 1989 Apr;62(2):307–311. doi: 10.1016/0303-7207(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Penschow J. D., Aldred G. P., Darling P. A., Haralambidis J., Hammond V. E., van Leeuwen B. H., Mason A. J., Niall H. D., Seeburg P., Coghlan J. P. Differential expression of inhibin alpha and beta A subunit genes in rat and mouse ovarian follicles during pregnancy. J Mol Endocrinol. 1990 Jun;4(3):247–255. doi: 10.1677/jme.0.0040247. [DOI] [PubMed] [Google Scholar]
- Rabinovici J., Jaffe R. B. Development and regulation of growth and differentiated function in human and subhuman primate fetal gonads. Endocr Rev. 1990 Nov;11(4):532–557. doi: 10.1210/edrv-11-4-532. [DOI] [PubMed] [Google Scholar]
- Rabinovici J., Spencer S. J., Jaffe R. B. Recombinant human activin-A promotes proliferation of human luteinized preovulatory granulosa cells in vitro. J Clin Endocrinol Metab. 1990 Nov;71(5):1396–1398. doi: 10.1210/jcem-71-5-1396. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Stuchbery S. J., Findlay J. K. Inhibin mRNAs in ovine and bovine ovarian follicles and corpora lutea throughout the estrous cycle and gestation. Mol Cell Endocrinol. 1989 Mar;62(1):95–101. doi: 10.1016/0303-7207(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Schwall R. H., Mason A. J., Wilcox J. N., Bassett S. G., Zeleznik A. J. Localization of inhibin/activin subunit mRNAs within the primate ovary. Mol Endocrinol. 1990 Jan;4(1):75–79. doi: 10.1210/mend-4-1-75. [DOI] [PubMed] [Google Scholar]
- Schwall R. H., Mason A. J., Wilcox J. N., Bassett S. G., Zeleznik A. J. Localization of inhibin/activin subunit mRNAs within the primate ovary. Mol Endocrinol. 1990 Jan;4(1):75–79. doi: 10.1210/mend-4-1-75. [DOI] [PubMed] [Google Scholar]
- Schwall R. H., Nikolics K., Szonyi E., Gorman C., Mason A. J. Recombinant expression and characterization of human activin A. Mol Endocrinol. 1988 Dec;2(12):1237–1242. doi: 10.1210/mend-2-12-1237. [DOI] [PubMed] [Google Scholar]
- Shukovski L., Findlay J. K. Activin-A inhibits oxytocin and progesterone production by preovulatory bovine granulosa cells in vitro. Endocrinology. 1990 Apr;126(4):2222–2224. doi: 10.1210/endo-126-4-2222. [DOI] [PubMed] [Google Scholar]
- Spencer S. J., Rabinovici J., Jaffe R. B. Human recombinant activin-A inhibits proliferation of human fetal adrenal cells in vitro. J Clin Endocrinol Metab. 1990 Dec;71(6):1678–1680. doi: 10.1210/jcem-71-6-1678. [DOI] [PubMed] [Google Scholar]
- Sugino H., Nakamura T., Hasegawa Y., Miyamoto K., Abe Y., Igarashi M., Eto Y., Shibai H., Titani K. Erythroid differentiation factor can modulate follicular granulosa cell functions. Biochem Biophys Res Commun. 1988 May 31;153(1):281–288. doi: 10.1016/s0006-291x(88)81219-1. [DOI] [PubMed] [Google Scholar]
- Sugino H., Nakamura T., Hasegawa Y., Miyamoto K., Igarashi M., Eto Y., Shibai H., Titani K. Identification of a specific receptor for erythroid differentiation factor on follicular granulosa cell. J Biol Chem. 1988 Oct 25;263(30):15249–15252. [PubMed] [Google Scholar]
- Tonetta S. A., diZerega G. S. Intragonadal regulation of follicular maturation. Endocr Rev. 1989 May;10(2):205–229. doi: 10.1210/edrv-10-2-205. [DOI] [PubMed] [Google Scholar]
- Torney A. H., Hodgson Y. M., Forage R., de Kretser D. M. Cellular localization of inhibin mRNA in the bovine ovary by in-situ hybridization. J Reprod Fertil. 1989 May;86(1):391–399. doi: 10.1530/jrf.0.0860391. [DOI] [PubMed] [Google Scholar]
- Tsonis C. G., McNeilly A. S., Baird D. T. Inhibin secretion by the sheep ovary during the luteal and follicular phases of the oestrous cycle and following stimulation with FSH. J Endocrinol. 1988 May;117(2):283–291. doi: 10.1677/joe.0.1170283. [DOI] [PubMed] [Google Scholar]
- Tsonis C. G., Messinis I. E., Templeton A. A., McNeilly A. S., Baird D. T. Gonadotropic stimulation of inhibin secretion by the human ovary during the follicular and early luteal phase of the cycle. J Clin Endocrinol Metab. 1988 May;66(5):915–921. doi: 10.1210/jcem-66-5-915. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Hsueh A., Campen C., Meunier H., Bicsak T., Vaughan J., Corrigan A., Bardin W., Sawchenko P. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res. 1988;44:1–34. doi: 10.1016/b978-0-12-571144-9.50005-3. [DOI] [PubMed] [Google Scholar]
- Woodruff T. K., D'Agostino J., Schwartz N. B., Mayo K. E. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science. 1988 Mar 11;239(4845):1296–1299. doi: 10.1126/science.3125611. [DOI] [PubMed] [Google Scholar]
- Woodruff T. K., Lyon R. J., Hansen S. E., Rice G. C., Mather J. P. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990 Dec;127(6):3196–3205. doi: 10.1210/endo-127-6-3196. [DOI] [PubMed] [Google Scholar]
- Xiao S., Findlay J. K., Robertson D. M. The effect of bovine activin and follicle-stimulating hormone (FSH) suppressing protein/follistatin on FSH-induced differentiation of rat granulosa cells in vitro. Mol Cell Endocrinol. 1990 Feb 12;69(1):1–8. doi: 10.1016/0303-7207(90)90082-j. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Ling N., Ueno N., Guillemin R. Inhibin and beta type transforming growth factor (TGF beta) have opposite modulating effects on the follicle stimulating hormone (FSH)-induced aromatase activity of cultured rat granulosa cells. Biochem Biophys Res Commun. 1986 May 14;136(3):969–975. doi: 10.1016/0006-291x(86)90427-4. [DOI] [PubMed] [Google Scholar]