Abstract
The impact of obesity on cardiovascular disease (CVD) outcomes in patients with type 2 diabetes mellitus (T2DM) and established coronary artery disease (CAD) is controversial; whether BMI and/or waist circumference correlate with atherothrombotic risk factors in such patients is uncertain. We sought to evaluate whether higher BMI or waist circumference are associated with specific risk factors among 2,273 Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study participants with T2DM and documented CAD (baseline data, mean age 62 years, 66% non-Hispanic white, 71% men). Multiple linear regression models were constructed after adjusting for sex, age, race/ ethnicity, US vs. non-US site, diabetes duration, exercise, smoking, alcohol, and relevant medication use. First-order partial correlations of BMI with risk factors after controlling for waist circumference and of waist circumference with risk factors after controlling for BMI were also evaluated. Ninety percent of the patients were overweight (BMI ≥25 kg/m2); 68% of men and 89% of women had high-risk waist circumference measures (≥102 and ≥88 cm, respectively). BMI and waist circumference, in separate models, explained significant variation in metabolic (insulin, lipids, blood pressure (BP)) and inflammatory/procoagulation (C-reactive protein, PAI-1 activity and antigen, and fibrinogen) risk factors. In partial correlation analyses BMI was independently associated with BP and inflammatory/procoagulation factors, waist circumference with lipids, and both BMI and waist circumference with insulin. We conclude that, in cross-sectional analyses, both BMI and waist circumference, independently, are associated with increased atherothrombotic risk in centrally obese cohorts such as the BARI 2D patients with T2DM and CAD.
INTRODUCTION
Generalized obesity measured by the BMI, and abdominal obesity measured by the waist circumference, are associated with a variety of cardiovascular disease (CVD) risk factors and increase the risk of type 2 diabetes mellitus (T2DM) and CVD development in patients without T2DM (1–11). Guidelines for the clinical use of BMI and waist circumference cutoffs values reflecting increased disease risk have been published (10). However these guidelines do not specify whether BMI and/or waist circumference measurements have the same utility in predicting CVD risk in patients with established T2DM compared to those without T2DM (10,12). Moreover the usefulness of BMI in predicting increased CVD mortality in patients with established T2DM or documented CVD has been recently challenged (13–20). The influence of obesity on both traditional atherogenic and also novel inflammatory and procoagulation/thrombotic CVD risk factors is of particular interest in patients with established T2DM as these risk factors are thought to contribute to increased CVD mortality in this population (21–25).
The putative mechanisms underlying the risk for increased CVD events in patients with established T2DM and coronary artery disease (CAD) include not only insulin resistance and dyslipidemia but also endothelial dysfunction, chronic release of mediators of inflammation, procoagulation, and impaired fibrinolysis (21,22). In cross-sectional and longitudinal studies, both traditional atherogenic risk factors (high triglycerides (TG), low high-density lipoprotein (HDL-cholesterol) levels, high diastolic and systolic BP, hyperglycemia) (11,12,23) and novel atherogenic and proinflammatory/thrombotic risk factors (high insulin, albumin/creatinine (AC) ratio, plasminogen-activator inhibitor (PAI)-1, C-reactive protein (CRP), fibrinogen and D-dimer) were hypothesized to confer increased CVD risk in patients with or without T2DM (11,21–25). However the associations between obesity and CVD risk factors, in patients with established T2DM and documented CAD, have been less well studied (26). In these populations the impact of obesity on CVD risk could be confounded by variables such as duration of diabetes, glycemic control and type or number of antidiabetic or other medications used to control glucose, lipids and BP levels and be modulated by risk taking behaviors such as smoking and alcohol use.
We therefore used the cross-sectional baseline measurements in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) study to determine associations between indexes of obesity and atherothrombotic risk factors in patients with established T2DM and documented CAD. The (BARI 2D) study is a randomized clinical trial designed to determine simultaneously, in patients with T2DM and stable, documented CAD and in the presence of uniform control of glycemia, dyslipidemia, hypertension, and lifestyle factors, whether 5-year mortality is lower if: (i) myocardial ischemia is managed by immediate revascularization plus aggressive medical therapy compared with aggressive medical therapy alone with deferred revascularization as needed; and (ii) diabetes is managed by an insulin providing strategy compared with an insulin sensitizing strategy (27,28). We analyzed the baseline data from the BARI 2D study and sought to evaluate among BARI 2D patients whether a higher BMI or a higher waist circumference is associated with specific CVD risk factors and whether a higher waist circumference is associated with CVD risk factors independent of BMI among BARI 2D patients.
METHODS AND PROCEDURES
Study design and patient population
BARI 2D was designed to compare treatment strategies for patients with established T2DM and angiographically documented CAD suitable for elective revascularization (27). Briefly, patients with age ≥25, treated with insulin, oral hypoglycemic agents, diet, and exercise or a combination of these were eligible provided that T2DM was documented by review of medical record or made on the basis of plasma glucose measurements. The CAD inclusion criteria were coronary arteriogram showing ≥1 vessel amenable to revascularization (≥50% stenosis) and objective documentation of ischemia or subjectively documented typical angina with ≥70% stenosis in ≥1 artery. The exclusion criteria included: type 1 diabetes, definite need for revascularization such as prompt control of severe or unstable angina, left main coronary artery stenosis ≥50%, prior coronary artery bypass graft or percutaneous coronary intervention within the past 12 months, class III or IV congestive heart failure (CHF), creatinine greater than 2mg/dl, or hemoglobin A1c(HbA1c) ≥ 13%, noncardiac illness expected to limit survival, current alcohol abuse, chronic steroid use judged to interfere with the control of hyperglycemia, hepatic disease with alanine aminotransferase ≥2 times upper limit of normal, fasting plasma triglyceride >1,000 mg/dl in the presence of HbA1c <9%, known, suspected or planned pregnancy, plus logistic study exclusions as previously described in detail (27). The main referral sources at each study sites were: the cardiac catheterization laboratories (54%), the cardiology clinics (27%), the diabetes clinics (4%) and the cardiac stress test laboratories (3%). Determination of suitability for BARI 2D was carefully made by physician-investigators at each participating site. A detailed description of the eligibility criteria for participation and the process of establishing the eligibility were published in ref. 27.
A total of 2,368 patients were recruited at 49 international clinical sites, from January 2001 to March 2005. There were 2,273 patients whose BMI was ≥18.5 kg/m2 and in whom waist circumference measurements and at least 85% of baseline information were available at baseline. These patients were included in the current analysis. All participants signed informed consents before enrollment in the study. The study and the consent forms were approved by institutional review boards at all participating clinical sites.
Measurements of indexes of obesity: BMI and waist circumference
BMI was the ratio of weight (kg) divided by squared height (m2) based on measurements obtained during the baseline study visit (randomization visit). For eight patients who had undergone lower-limb amputations before randomization, weight was estimated by adding back an appropriate proportion to the observed body weight (29). Waist circumference was measured at the same baseline visit according to a protocol developed by the BARI 2D nonpharmacologic working group and used after standard training at each site (11).
Risk factors measurements
At the baseline visit, before randomization, fasting blood, and urine samples were obtained and sent to the Core Laboratories for specific assays. HbA1c, standardized lipid values (total cholesterol, HDL-cholesterol, and TG levels) and urinary AC ratios were measured in the Biochemistry Core Laboratory. Low-density lipoprotein cholesterol was estimated using the Friedewald formula. Insulin and PAI-1 antigen/activity were measured in the fibrinolysis and coagulation core laboratory. Additional plasma markers of inflammation, and of pro-coagulation, including CRP, fibrinogen, and D-dimer, were measured as part of one of the BARI 2D ancillary studies titled “Inflammation, Procoagulation, and Plaque Vulnerability” (R01HL71306, National Institutes of Health, 2000–2007; B.E.S., principal investigator). At the same baseline visit, sitting systolic and diastolic BP were obtained following a standardized protocol and the mean of three BP readings was used (11).
Clinical history and lifestyle characteristics
The clinical history data were obtained by various means, such as review of medical records, exercise or radionuclide test results, primary care physicians’ medical records and reports, or patients’ self-report. The lifestyle data, such as physical activity and alcohol consumption, were self-reported by the patients. The levels of physical activity were defined as follows: (i) sedentary included very little to no physical activity, walking less than one block or only one flight of stairs at a time; (ii) mild included walking 1–2 blocks on a level and climbing >1 flight of stairs at a time, or photography, fishing, and light gardening; (iii) moderate included walking >2 blocks at a time, ping pong, golf, bowling, and light house work; (iv) strenuous meant active sports such as tennis, jogging, basketball, and swimming (30,31).
Statistical methods
For the purpose of descriptive data presentation and to demonstrate the association between obesity and baseline clinical and lifestyle characteristics (Table 1) or baseline atherothrombotic risk factors (Table 2), BMI was categorized into five classes in an ascending order: normal weight (BMI <25), overweight (BMI 25–29.9), obesity class I (BMI 30–34.9), obesity class II (BMI 35–39.9), and severe obesity (BMI ≥40), according to the National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel–determined obesity classes by BMI categories in similar populations without established T2DM (10).
Table 1.
Baseline clinical history and lifestyle characteristics categorized by BMI
| Characteristicsa | Total N = 2,273 |
BMI <25 N = 216 |
25–29.9 N = 776 |
30–34.9 N = 733 |
35–39.9 N = 347 |
≥40 N = 202 |
P Valueb |
|---|---|---|---|---|---|---|---|
| Sex: female (row %) | 100% | 10% | 29% | 31% | 17% | 13% | <0.001 |
| Sex: male (row %) | 100% | 9% | 36% | 33% | 14% | 7% | |
| Age at entry | 62.4 ± 8.9 | 62.6 ± 8.8 | 63.4 ± 8.9 | 62.8 ± 8.7 | 61.0 ±9.2 | 58.5 ± 8.3 | <0.001 |
| Race: white (row %) | 100% | 7% | 34% | 34% | 16% | 9% | <0.001 |
| Race: black (row %) | 100% | 8% | 30% | 35% | 16% | 12% | |
| Race: Hispanic (row %) | 100% | 18% | 35% | 28% | 13% | 5% | |
| Race: others (row %) | 100% | 21% | 52% | 14% | 8% | 5% | |
| Years since DM diagnosisc | 10.4 ± 8.6 | 11.1 ± 8.7 | 10.5 ± 8.6 | 10.3 ± 8.8 | 10.1 ± 8.8 | 10.1 ± 8.0 | 0.7 |
| Positive medical history | |||||||
| Myocardial infarction | 32% | 36% | 34% | 31% | 30% | 26% | 0.1 |
| Congestive heart failure | 6.6% | 5.7% | 4.8% | 6.0% | 5.8% | 17.4% | <0.001 |
| Hypercholesterolemia | 82% | 77% | 80% | 85% | 83% | 83% | 0.06 |
| Hypertension | 83% | 73% | 79% | 85% | 86% | 92% | <0.001 |
| Chronic renal dysfunction | 3% | 1% | 2% | 3% | 5% | 3% | 0.03 |
| Number of relevant drug classes (For the indicated diagnoses) | |||||||
| Dyslipidemia | 0.89 ± 0.57 | 0.79 ± 0.50 | 0.86 ± 0.56 | 0.94 ± 0.57 | 0.93 ± 0.55 | 0.87 ± 0.62 | 0.002 |
| Hypertension | 2.23 ± 1.03 | 1.88 ± 1.0 | 2.06 ± 0.96 | 2.29 ± 1.02 | 2.44 ± 1.07 | 2.65 ± 1.0 | <0.001 |
| Diabetes | 1.57 ± 0.86 | 1.44 ± 0.83 | 1.52 ± 0.82 | 1.56 ± 0.87 | 1.67 ± 0.91 | 1.78 ± 0.92 | <0.001 |
| Current use of insulin | 28% | 19% | 23% | 28% | 33% | 42% | <0.001 |
| Smoking status | |||||||
| Current smoker | 12% | 16% | 13% | 11% | 10% | 11% | 0.21 |
| Former smoker | 55% | 52% | 56% | 55% | 54% | 54% | 0.86 |
| Number of years smoked | 18.2 ± 17.2 | 19.0 ± 17.5 | 19.3 ± 17.5 | 18.3 ± 17.1 | 17.0 ± 17.2 | 15.1 ± 15.5 | 0.02 |
| Physical activity level | |||||||
| Sedentary | 22% | 27% | 19% | 20% | 22% | 32% | 0.004 |
| Mild | 42% | 36% | 42% | 43% | 44% | 38% | |
| Moderate | 34% | 33% | 36% | 35% | 30% | 28% | |
| Strenuous | 3% | 3% | 3% | 2% | 4% | 1% | |
| Exercise regularly | 26% | 33% | 29% | 25% | 19% | 19% | <0.001 |
| Alcohol use status | |||||||
| Regular alcohol drink | 24% | 21% | 27% | 24% | 20% | 20% | 0.05 |
| History of binge drink | 11% | 10% | 10% | 11% | 13% | 9% | 0.54 |
Data are shown as row % for sex and race, as column % of those categorized as “yes” for all other binary variables where “yes” or “no” answers were used and as mean ± s.d. for continuous variables.
For categorical variables, Pearson χ2-tests were performed to test the general associations with the BMI categories. For continuous variables, F-test was performed to test the differences of means among the BMI categories.
Years since diabetes diagnosis are estimated.
Table 2.
Baseline atherothrombotic risk factors categorized by BMI
| Risk factors | BMI <25 N = 216 |
25–29.9 N = 776 |
30–34.9 N = 733 |
35–39.9 N = 347 |
≥40 N = 202 |
P value |
|---|---|---|---|---|---|---|
| HDL-cholesterola (mg/dl) | 40 ± 11 | 39 ± 10 | 38 ± 10 | 37 ± 9 | 39 ± 11 | 0.014 |
| Triglyceridesb (mg/dl) | 137 (95, 197) | 144 (103, 210) | 156 (105, 224) | 159 (118, 235) | 152 (101, 228) | <0.001 |
| LDL-cholesterola (mg/dl) | 98 ± 37 | 97 ± 34 | 95 ± 31 | 95 ± 33 | 102 ± 36 | 0.11 |
| Systolic BPa (mm Hg) | 129 ± 21 | 131 ± 20 | 133 ± 20 | 134 ± 20 | 131 ± 19 | 0.05 |
| Diastolic BPa (mm Hg) | 74 ± 11 | 74 ± 11 | 75 ± 12 | 75 ± 12 | 74 ± 12 | 0.35 |
| Urine AC ratiob (mg/g) | 12 (5, 63) | 11 (5, 34) | 13 (5, 49) | 14 (5, 70) | 17 (6, 63) | 0.02 |
| HbA1ca (%) | 7.9 ± 1.8 | 7.6 ± 1.6 | 7.6 ± 1.6 | 7.7 ± 1.6 | 7.6 ± 1.6 | 0.24 |
| Insulinb (muU/ml) | 6 (3, 12) | 8 (5, 13) | 11 (7, 19) | 13 (8, 21) | 14 (8, 23) | <0.001 |
| PAI-1 activityb (au/ml) | 12 (8, 19) | 14 (9, 23) | 16 (10, 27) | 22 (13, 31) | 20 (12, 29) | <0.001 |
| PAI-1 antigenb (ng/ml) | 19 (12, 27) | 21 (14, 32) | 23 (15, 34) | 27 (17, 40) | 28 (18, 40) | <0.001 |
| CRPb (ug/ml) | 1 (1, 4) | 2 (1, 4) | 2 (1, 6) | 4 (2, 8) | 6 (3, 10) | <0.001 |
| Fibrinogena (mg/dl) | 358 ± 88 | 354 ± 95 | 362 ± 98 | 388 ± 95 | 399 ± 103 | <0.001 |
| D-dimerb (ug/ml) | 0.35 (0.19, 0.69) | 0.30 (0.18, 0.59) | 0.30 (0.18, 0.55) | 0.32 (0.20, 0.59) | 0.35 (0.20, 0.69) | 0.11 |
BP, blood pressure; AC ratio, urinary albumin/creatinine ratio; PAI-1, plasminogen-activator inhibitor 1; CRP: C-reactive protein.
Data are shown as mean ± s.d. and a F-test was performed to test the differences of means among the BMI categories.
Due to the skewness of distribution, data are shown as median (first quartile, third quartile) and the Kruskal–Wallis test was performed to test the difference of Wilcoxon rank score among the BMI categories.
Data are shown in Table 1 as row percentages for sex and race and as column percentages of those categorized as “yes” for all other binary variables where “yes” or “no” answers were used. The binary variables were: clinical history of myocardial infarction, CHF, hypercholesterolemia, hypertension, chronic renal dysfunction, current use of insulin, regular exercise, current smoking status, regular alcohol drinking, and binge drinking. Data are shown as mean ± s.d. for continuous variables i.e., age at study entry, years since diabetes mellitus diagnosis and number of relevant drug classes for the indicated diagnoses. Hyperlipidemia drug classes used were statins, fibrates, niacin, bile acid sequestrants, ω-3 fatty acids (administered as supplements), and cholesterol absorption inhibitor, hypertension drug classes used were angiotensin–converting enzyme inhibitor, angiotensin-receptor blocker, β-blocker, calcium channel blocker, and diuretics and diabetes drug classes used were metformin, thiazolidinediones, sulfonylurea, meglitinide, phenylalanine derivative, α-glucosidase inhibitor. For categorical baseline characteristic variables, Pearson χ2-tests were performed to test the general association with the categorical BMI. For continuous baseline characteristic variables, an F-test was performed to test any mean differences among the BMI categories (Table 1).
Data are shown in Table 2 as mean ± s.d. for HDL-cholesterol and low-density lipoprotein cholesterol, systolic and diastolic BP, HbA1c and fibrinogen; an F-test was performed for P value to test the significant differences of means among the BMI categories. Due to the skewness of distribution, data are presented as median (first quartile, third quartile) for TG, urine AC ratio, insulin, PAI-1 activity and antigen, CRP and D-dimer; the Kruskal–Wallis test was performed for P value to test the difference of Wilcoxon rank score among the BMI categories (Table 2).
Multiple linear regression models were built to evaluate the association of BMI and waist circumference with risk factors (Table 3). BMI, waist circumference and risk factors were treated as continuous variables; TG, AC ratio, insulin, PAI-1 activity and antigen, CRP, and D-dimer were transformed logarithmically to satisfy the normality assumptions for linear regression analyses. Each model involved one risk factor, explained by one obesity index (BMI or waist circumference) and relevant covariates. The covariates were: age at study entry, sex, race/ethnicity, US or non-US sites, regular exercise, current smoking status, number of years of smoking, regular alcohol drinking, binge drinking, duration of diabetes, and relevant medications. Risk factors were grouped to adjust for relevant medications using the number of relevant drugs as a covariate. There were four groups of risk factors. Models involving HDL-cholesterol and TG were adjusted for the number of lipid drug classes and models involving BP and AC ratio were controlled for number of hypertension drug classes. Models involving HbA1c, insulin, and PAI-1 were adjusted for the number of diabetes drug classes and insulin and models involving CRP, fibrinogen, and D-dimer were adjusted for the number of cardiac medications, such as antiangina, antihypertension, antiplatelet, and anticoagulant drug classes. In Table 3, each risk factor was first explained by all covariates (the initial R2 represented the variability explained by these covariates) and then either BMI or waist circumference was added to the existing model. The increment of R2 was calculated for each obesity index, standing for the marginal contribution to the explanation of the variability. P values were generated by partial F-tests for the significance of the marginal contribution (Table 3).
Table 3.
BMI and waist circumference (Wc) explain significant variation in atherothrombotic risk factors, respectively
| Linear regression model (no BMI or WC)a
|
Adding BMI only (for each 5 kg/m2 increase)
|
Adding WC only (for each 10 cm increase)
|
|||||
|---|---|---|---|---|---|---|---|
| Risk factor | R2 | Increment of R2 | Regression coefficient | Partial F-test P value | Increment of R2 | Regression coefficient | Partial F-test P value |
| Controlled for number of lipid drug classes | |||||||
| HDL-cholesterol | 0.16 | 0.002 | −0.45 | 0.012 | 0.007 | −0.66 | <0.001 |
| Log of triglycerides | 0.08 | 0.006 | 0.04 | <0.001 | 0.009 | 0.04 | <0.001 |
| Controlled for number of hypertension drug classes | |||||||
| Systolic BP | 0.07 | 0.003 | 1.05 | 0.006 | 0.001 | 0.60 | 0.062 |
| Diastolic BP | 0.16 | 0.003 | 0.61 | 0.002 | 0.003 | 0.48 | 0.005 |
| Log of AC ratio | 0.12 | 0.003 | 0.08 | 0.014 | 0.002 | 0.07 | 0.017 |
| Controlled for number of diabetes drug classes | |||||||
| HbA1c | 0.16 | 0.003 | −0.08 | 0.009 | 0.001 | –0.03 | 0.207 |
| Log of insulin | 0.03 | 0.056 | 0.20 | <0.001 | 0.064 | 0.18 | <0.001 |
| Log of PAI-1 activity | 0.11 | 0.044 | 0.13 | <0.001 | 0.049 | 0.12 | <0.001 |
| Log of PAI-1 antigen | 0.08 | 0.029 | 0.09 | <0.001 | 0.029 | 0.08 | <0.001 |
| Controlled for number of cardiac medications | |||||||
| Log of CRP | 0.11 | 0.053 | 0.27 | <0.001 | 0.048 | 0.21 | <0.001 |
| Fibrinogen | 0.08 | 0.012 | 9.90 | <0.001 | 0.012 | 8.27 | <0.001 |
| Log of D-dimer | 0.14 | 0.003 | 0.05 | 0.004 | 0.003 | 0.04 | 0.007 |
BP, blood pressure; AC ratio, urinary albumin/creatinine ratio; PAI-1, plasminogen-activator inhibitor 1; CRP, C-reactive protein.
Overall adjusted for age, sex, race ethnicity, US or non-US site, years of diabetes duration, regular exercise, current smoking, years of smoking, history of alcohol use, and number of relevant drug classes for: hyperlipidemia (statins, fibrate, niacin, bile-sequestrants, ω-3 fatty acid, cholesterol absorption inhibitor), hypertension (angiotensin–converting enzyme inhibitor, angiotensin-receptor blocker, β-blocker, calcium channel blocker, diuretics), diabetes (metformin, thiazolidinediones, sulfonylurea, meglitinide, phenylalanine derivative, α-glucosidase inhibitor, insulin), cardiac disease (antiangina, antihypertension, antiplatelet, anticoagulant) drug classes.
To compare the association of BMI, independent of waist circumference, and of waist circumference, independent of BMI, with risk factors, first-order partial Pearson correlation coefficients were computed (Table 4). The first-order partial correlation coefficient is a statistical estimator representing the unique association of one index with risk factors, apart from another index that correlates highly with the preceding index (32). Each risk factor (each row) in Table 4 was evaluated in two separate models. One model tested BMI after the linear effect of waist circumference has been removed from both the risk factor and BMI. The other model tested waist circumference after the linear effect of BMI was removed from both the risk factor and the waist circumference. BMI and waist circumference were not entered into a single model simultaneously because of the co linearity problem, which may affect the validity of the correlation estimate. We considered that one obesity index (for example, BMI), independent of another obesity index (like waist circumference), had a stronger association with a risk factor if the partial correlation coefficient had a significant absolute value, and also persisted in the direction that was consistent with common sense (Table 4).
Table 4.
First-order partial correlation for BMI or waist circumference (Wc) with atherothrombotic risk factors
| CVD risk factors | Partial correlation for BMI with a risk factor (independent of WC)
|
Partial correlation for WC with a risk factor (independent of BMI)
|
||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| HDL-cholesterol | 0.135 | <0.001 | −0.191 | <0.001 |
| Log of triglycerides | −0.020 | 0.346 | 0.072 | <0.001 |
| Systolic BP | 0.066 | 0.002 | −0.050 | 0.018 |
| Diastolic BP | 0.042 | 0.044 | −0.035 | 0.096 |
| Log of AC ratio | 0.035 | 0.106 | 0.006 | 0.776 |
| HbA1c | 0.02 | 0.342 | −0.038 | 0.067 |
| Log of insulin | 0.071 | <0.001 | 0.104 | <0.001 |
| Log of PAI-1 activity | 0.08 | <0.001 | 0.061 | 0.005 |
| Log of PAI-1 antigen | 0.080 | <0.001 | 0.034 | 0.242 |
| Log of CRP | 0.179 | <0.001 | −0.010 | 0.621 |
| Fibrinogen | 0.093 | <0.001 | −0.021 | 0.315 |
| Log of D-dimer | 0.033 | 0.121 | −0.014 | 0.509 |
To see if waist circumference, independent of BMI, or BMI independent of waist circumference, has the stronger association with a risk factor, the partial correlation coefficient should have significant absolute value and persist in the original direction consistent with common sense.
BP, blood pressure; AC ratio, urinary albumin/creatinine ratio; PAI-1, plasminogen-activator inhibitor 1; CRP. C-reactive protein.
As multiple comparisons were made for 12 risk factors simultaneously throughout the analysis, the present article used a Bonferroni-type procedure to control the false positive rate. Hence, a test with P value <0.004 was deemed a statistically significant result. SAS v9.1.3 (Cary, NC) was used for all analyses and R 2.3.1 (R Core Development Team, http://www.r-project.org/) was used to generate the figure in the present article.
Results
Demographics
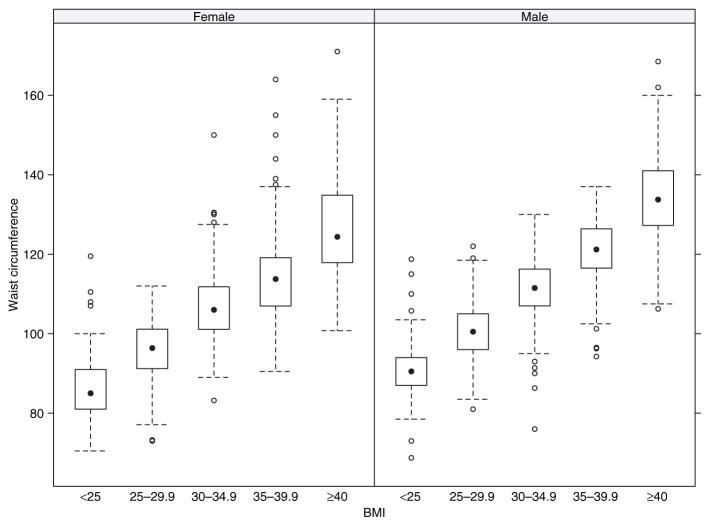
Demographics, clinical, and lifestyle characteristics (Table 1) or atherothrombotic risk factors (Table 2) are shown by BMI categories: BMI was categorized into five classes in an ascending order, according to the National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel–determined obesity classes (10). As seen in Figure 1, BMI and waist circumference were highly correlated (Pearson correlation = 0.82, P value < 0.0001). Use of quintiles of waist circumference values to correspond to the BMI categories gave practically the same results as those presented in Tables 1 and 2.
Figure 1.
Box- and-whisker plot representing the distribution of waist circumference across BMI categories stratified by sex in the Bypass Angioplasty Revascularization Investigation 2 Diabetes population. The solid circle is the median of each group. The box corresponds to the interquartile range (the first to third quartile). The whiskers extend both sides to data points within the 1.5 times the interquartile range. The circles outside of the whiskers are outliers.
The mean BMI was 31.7 ± 5.9 kg/m2 and only 10% of patients had normal weight (Table 1). Women were heavier than men (BMI 33 ± 6.8 vs. 31 ± 5.4 kg/m2, respectively), non-Hispanic whites and blacks were heavier than Asians and others (BMI 32 ± 5.7 and 33 ± 6.8 vs. 29 ± 5.5 kg/m2, respectively), and patients in US were heavier than those in other countries (BMI 33 ± 6.2 vs. 30 ± 5.0 kg/m2, respectively) (P < 0.0001 for all). The mean waist circumference was 108.7 cm for men and 105.7 cm for women (Figure 1). In all, 68% of men and 89% of women had waist circumference above the National Heart, Lung, and Blood Institute Obesity Education Initiative Expert Panel’s determined cutoffs for high-risk values in populations without established T2DM, i.e., ≥102 cm and ≥88 cm, respectively (10). Although there was a strong correlation between BMI and waist circumference, there was significant variability in waist circumference, below and above the previously mentioned cutoffs. The rate of increase in waist circumference with the increase of BMI was lower in women than in men (P < 0.0001) and lower in Hispanic (P = 0.02) and in non-Hispanic blacks (P < 0.0001), compared with non-Hispanic whites. Asian and others did not significantly differ from the non-Hispanic white group (P = 0.5).
Clinical history and lifestyle characteristics
The mean number of years following diagnosis of T2DM was 10.4 years and, in each BMI category, the majority of patients had hypercholesterolemia and/or hypertension (73–92%) (Table 1). These diagnoses were slightly more frequent in the heavier patients (BMI ≥35 vs. BMI ≤29.9), as was chronic renal dysfunction (3–5% vs. 1–2%). The heavier patients were taking a greater number of agents in lipid, hypertension, and diabetes drug classes and were more likely to be on insulin. Patients with severe obesity (BMI ≥40) had significantly more heart failure compared to the other groups. However, a history of myocardial infarction was less frequent in patients with BMI ≥35 (26–30%) than in those with BMI ≤29.9 (34–36%).
In all, 55% of patients were former smokers and 12% were current smokers. Smoking was inversely related to weight. Patients with BMI ≥35 reported fewer years of smoking (15.1–17.0 years) than those with BMI ≤29.9 (19–19.3 years). Seventy-four percent of patients reported no regular exercise. Weight was inversely related to physical activity. Fewer patients with BMI ≥35 exercised regularly (19%) compared with those with BMI ≤29.9 (29–33%).
Atherothrombotic risk factors: relationships with BMI and/or waist circumference
Raw values for the atherothrombotic risk factors are shown by BMI category in Table 2. Unadjusted, observed mean (median) values for TG, insulin, PAI antigen and activity, CRP, and fibrinogen were significantly higher (P < 0.001) at higher compared to lower BMI categories. Unadjusted, observed mean (median) values for HDL-cholesterol, low-density lipoprotein cholesterol, BP, urine AC ratio, HbA1c, and D-dimer did not differ significantly by BMI category.
The significant linear relationships of BMI, and of waist circumference, with atherothrombotic risk factors are shown in Table 3. After adjusted for the relevant covariates, in separate models, either a higher BMI or a higher waist circumference had significant associations with a higher TG, insulin, PAI-1 activity and antigen, CRP, and fibrinogen (P < 0.001). A higher BMI was also associated with a higher diastolic BP (P = 0.002) and a higher waist circumference was associated with a lower HDL-cholesterol (P < 0.001). In Table 3, the covariates alone explained between 3–16% of the variation in the risk factors. Addition of either BMI or waist circumference explained <1% additional variation in HDL-cholesterol, TG and BP, but between 1–6% additional variation in insulin, PAI-1 activity or antigen, CRP, and fibrinogen.
The first-order partial correlation coefficients between BMI and waist circumference with the atherothrombotic risk factors are shown in Table 4. Considering both the magnitude and the direction of these coefficients, BMI, independent of waist circumference, had stronger unique associations with systolic BP, PAI-1 antigen and activity, CRP, and fibrinogen, whereas waist circumference, independent of BMI, had stronger unique associations with HDL-cholesterol and TG. Both BMI and waist circumference had similar partial correlations with insulin.
DISCUSSION
The baseline BARI 2D data provided us with the opportunity to examine the associations between indexes of generalized and central obesity and a multitude of atherothrombotic risk factors in a unique population, patients with T2DM and documented CAD. Most BARI 2D patients were overweight (≥90%), more than half were obese, as in other T2DM cohorts (33,34). Most BARI 2D patients had waist circumference measures above the cutoffs established as high-risk for patients without T2DM (10,12). Similar to patients without T2DM, the BMI and waist circumference of BARI 2D patients were highly correlated and this relationship differed by sex and by ethnicity (35,36). Our main finding is that even in this population with an extremely high prevalence of central obesity according to established criteria (10,12) we found significant linear relationships between BMI and waist circumference with multiple atherothrombotic risk factors. Although most of the BARI 2D patients had higher-risk waist circumference values, the relationships between obesity indexes and risk factors were linear and were evident after adjusting for multiple potential confounders which could influence the atherothrombotic risk but were also related to obesity.
As expected, patients with a higher BMIs reported lower levels of physical activity, negative current smoking status, and lower number of cigarettes smoked (7,9,37). They were also more likely to have a diagnosis of hypertension, hypercholesterolemia, chronic renal dysfunction, and heart failure and to be on a higher number of drug classes used to treat hypertension, dyslipidemia, and diabetes and to be on insulin. These findings obtained by clinical history, as the results of our analyses, are consistent with the idea that even in patients with advanced disease (established T2DM and CAD), obesity is significantly associated with CVD risk. However, BARI 2D patients with a higher BMI had a lower prevalence of myocardial infarction by history. This finding is similar to those of longitudinal studies where, in cohorts with established T2DM and/or CAD, a higher BMI did not predict or predicted less CVD morbidity and mortality (13,14,19,20). Aggressive CVD risk treatment for the more obese patients was proposed as an explanation (20); alternatively, the more obese patients in our cohort could have had less severe CAD despite the associations of obesity indexes with the atherothrombotic risk factors.
A higher BMI or waist circumference, in separate models, explained quite a small, albeit significant, percent of variability (<1%) in metabolic risk factors such as high BP, high TG, or low HDL-cholesterol (12). They were not significantly related to low-density lipoprotein cholesterol, a higher AC ratio (a marker for endothelial dysfunction) (38) or HbA1c. Risk factors such as dyslipidemia, high BP, and glucose are known to have stronger associations with obesity in patients without T2DM or CAD. For example an increase of 10 kg body weight was associated with a 3 mm Hg increase in systolic BP in patients without T2DM (4) whereas a 5 kg/m2 increase in BMI was associated with only about a 1 mm Hg increase in systolic BP in BARI 2D. It is possible that we could not fully account for the more aggressive treatment of these risk factors in the BARI 2D patients with higher BMI despite our statistical adjustments. Although we adjusted for the number of medications in one class of drugs, we did not account for medication dosages. Although obesity indexes correlate with traditional CVD risk factors in patients without T2DM or CAD (8), once T2DM and CAD are established and such risk factors are aggressively treated, these associations may diminish.
BMI and waist circumference (as well as the waist to height ratio, data not shown), in separate models, explained a larger percent of variability (1–6%) in novel atherothrombotic CVD risk factors such as fasting insulin, markers of inflammation (CRP) and of procoagulation and impaired fibrinolysis (fibrinogen and PAI-1 activity and antigen). From these cross-sectional associations we cannot determine cause and effect however central obesity is known to be associated with elevated fasting insulin and fasting insulin level was specifically found to be a prospective risk factor for myocardial infarction in patients without T2DM (39). Still, the elevated fasting insulin level’s role as a CVD risk factor vis-à-vis insulin treatment in T2DM is uncertain (21,40,41). Both PAI-1 and insulin levels in the BARI 2D patients could have been heavily influenced by the T2DM treatment thus it is remarkable that we found these significant associations with obesity in our cohort. The associations of obesity with CRP, PAI-1, and fibrinogen are similar to those reported in cohorts without T2DM (42). Insulin and proinsulin are known to stimulate PAI-1 production in vitro, in endothelial, hepatic and fat cells (21,40) and elevated fasting insulin levels were associated with specific procoagulation and impaired fibrinolysis markers in other studies (41). Overall our findings support the hypothesis that the novel atherothrombotic risk factors could be important mediators of the relationship between obesity and CVD outcomes in patients with established T2DM and CAD (13,14,18,20).
Although the BMI and waist circumference of BARI 2D patients were highly correlated, for each BMI category there were waist circumference values both above and below the established criteria for high-risk values (10). BMI, after controlling for waist circumference, and waist circumference, after controlling for BMI, had unique associations with atherothrombotic risk factors in our cohort. We are not aware of similar previous reports in populations with established T2DM and documented CAD. We found unique relationships for BMI with BP and inflammatory/procoagulation risk factors and for waist circumference with lipids. Both indexes had independent associations with insulin. Longitudinally, it has been proposed that obesity affects CVD outcome mostly through its relationship with risk factors (13,15,43). In some studies, in cohorts with established T2DM and/or CAD, higher BMI did not predict or predicted less CVD morbidity and mortality (13,14,19,20). Lack of longitudinal data specifically utilizing measures of central obesity (waist, waist-to-hip ratio) in patients with T2DM and/or CAD (8,17–19) could be one explanation. In our analyses, either BMI and waist circumference, independently, were associated with atherothrombotic risk factors, therefore both obesity indexes should be included as potential risk factors for CVD outcomes in longitudinal analyses.
The strengths of our current study are the unique population and the large number of patients studied. Our study has several limitations. BMI and waist circumference were highly correlated and we used them as continuous variables, tested in separate models; thus we did not establish cutoff values for high risk or interactions between their associations with risk factors. We did not collect data on nutritional habits of the patients at baseline which may have influenced both obesity and atherothrombotic risk factors. Finally, the interpretation of the analysis is limited by its cross-sectional nature that precludes determination of cause and effect. Confounding variables such as age or duration of diabetes may have different effects in longitudinal studies than in cross-sectional associations. Analyses of longitudinal data will determine whether baseline BMI and waist circumference, or changes in them, will independently predict CVD events and death in the BARI 2D patients before or after adjusting for confounding variables or any of the related atherothrombotic risk factors.
Acknowledgments
The Bypass angioplasty Revascularization Investigation 2 Diabetes Trial is funded by the National Heart, Lung, and Blood Institute, grant nos U01 HL061746, U01 HL061748, U01 HL063804 and receives substantial funding from the National Institute of Diabetes and Digestive and Kidney Diseases, grant no HL061744. BaRI 2D receives significant supplemental funding from GlaxoSmithKline, Bristol-Myers Squibb Medical Imaging, Inc., astellas Pharma US, Inc., Merck & Co., Inc., abbott Laboratories, Inc., and Pfizer, Inc, and generous financial support from abbott Laboratories Ltd., MediSense Products, Bayer Diagnostics, Becton, Dickinson and Company, J.R. Carlson Laboratories, Inc., Centocor, Inc., Eli Lilly and Company, LipoScience, Inc., Merck Sante, Novartis Pharmaceuticals Corporation, and Novo Nordisk, Inc. The BaRI 2D Trial is coordinated by the Epidemiology Data Center at the University of Pittsburgh, Graduate School of Public Health. Other NIH grant support includes R01 HL71306, B.E.S., principal investigator.
APPENDIX
BARI 2d sites
**University of São Paulo Heart Institute, **Toronto General Hospital/University Health Network, **Texas Health Science @ San Antonio/South Texas, *Mayo Clinic-Rochester, *Mexican Institute of Social Security, *University Hospitals of Cleveland/ CASE Medical School, *Memphis VA Medical Center/University of Tennessee, *Montréal Heart Institute/Hôtel-Dieu-CHUM, *Albert Einstein College of Medicine/Montefiore, *Fuqua Heart Center/Piedmont Hospital, *University of Alabama @ Birmingham, *Northwestern University Medical Center, *Na Homolce Hospital, *Ottawa Heart Institute/Ottawa Hospital-Riverside Campus, *New York Medical College/Westchester Medical Center, *Emory University, *Washington Hospital Center /Georgetown University, *Quebec Heart Institute/Laval Hospital, *University of British Columbia/Vancouver Hospital, NYU School of Medicine, Lahey Clinic Medical Center, University of Virginia, University of Minnesota, St Luke’s/ Roosevelt Hospital Center, University of Florida, St Louis University, University of Texas @ Houston, Kaiser-Permanente Medical Center, Henry Ford Heart & Vascular Institute, Boston Medical Center, Fletcher Allen Health Care, Greater Fort Lauderdale Heart Group Research, Baylor College of Medicine, Duke University, University of Maryland Hospital, University of Chicago Medical Center, University of Pittsburgh Medical Center, Washington University/Barnes Jewish Hospital, Mount Sinai Medical Center, Mid America Heart Institute, University of Michigan, Johns Hopkins Bayview Medical Center, Brown University/Rhode Island Hospital, Houston VA Medical Center, New York Hospital Queens, Wilhelminen Hospital, St Joseph Mercy Hospital/Michigan Heart PC, Ohio State University Medical Center, Mayo Clinic-Scottsdale.
**≥100 participants; *≥50 and <100 participants; North America: 1937 participants (USA: 1499 participants, Canada: 353 participants, Mexico: 85 participants); South America: 356 participants; Europe (75 participants).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Wang Z, Heshka S, et al. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 3.Schneider HJ, Glaesmer H, Klotsche J, et al. DETECT Study Group. Accuracy of anthropometric indicators of obesity to predict cardiovascular risk. J Clin Endocrinol Metab. 2007;92:589–594. doi: 10.1210/jc.2006-0254. [DOI] [PubMed] [Google Scholar]
- 4.Poirier P, Giles TD, Bray GA, et al. American Heart Association. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113:2943–2946. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- 6.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 9.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung and Blood Institute (NHLBI) Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 11.Albu J, Gottlieb SH, August P, Nesto RW, Orchard TJ Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Modifications of coronary risk factors. Am J Cardiol. 2006;97:41G–52G. doi: 10.1016/j.amjcard.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Zoppini G, Verlato G, Leuzinger C, et al. Body mass index and the risk of mortality in type II diabetic patients from Verona. Int J Obes Relat Metab Disord. 2003;27:281–285. doi: 10.1038/sj.ijo.802199. [DOI] [PubMed] [Google Scholar]
- 14.Bruno G, Merletti F, Biggeri A, et al. Casale Monferrato Study. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 15.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 16.Tseng CH. Body mass index and waist circumference as determinants of coronary artery disease in Taiwanese adults with type 2 diabetes mellitus. Int J Obes (Lond) 2006;30:816–821. doi: 10.1038/sj.ijo.0803218. [DOI] [PubMed] [Google Scholar]
- 17.Hoefle G, Saely CH, Aczel S, et al. Impact of total and central obesity on vascular mortality in patients undergoing coronary angiography. Int J Obes (Lond) 2005;29:785–791. doi: 10.1038/sj.ijo.0802985. [DOI] [PubMed] [Google Scholar]
- 18.Dagenais GR, Yi Q, Mann JF, et al. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C, Køber L TRACE study group. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98:123–131. doi: 10.1016/j.ijcard.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Sobel BE. Optimizing cardiovascular outcomes in diabetes mellitus. Am J Med. 2007;120:S3–11. doi: 10.1016/j.amjmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Sobel BE BARI 2D Trial Investigators. Ancillary studies in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial: Synergies and opportunities. Am J Cardiol. 2006;97:53G–58G. doi: 10.1016/j.amjcard.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Eckel RH. Mechanisms of the components of the metabolic syndrome that predispose to diabetes and atherosclerotic CVD. Proc Nutr Soc. 2007;66:82–95. doi: 10.1017/S0029665107005320. [DOI] [PubMed] [Google Scholar]
- 24.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 25.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 26.Kahn HS, Dunbar VG, Ziemer DC, Phillips LS. Diabetes in urban African Americans. XII. Anthropometry for assessing municipal hospital outpatients recently diagnosed with type 2 diabetes. Obes Res. 1998;6:238–245. doi: 10.1002/j.1550-8528.1998.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 27.Brooks MM, Frye RL, Genuth S, et al. Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Frye RL, August P, Brooks MM, et al. BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozumdar A, Roy SK. Method for estimating body weight in persons with lower-limb amputation and its implication for their nutritional assessment. Am J Clin Nutr. 2004;80:868–875. doi: 10.1093/ajcn/80.4.868. [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- 31.Myers J, Bader D, Madhavan R, Froelicher V. Validation of a specific activity questionnaire to estimate exercise tolerance in patients referred for exercise testing. Am Heart J. 2001;142:1041–1046. doi: 10.1067/mhj.2001.118740. [DOI] [PubMed] [Google Scholar]
- 32.Kelinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariable Methods. Duxbury Press, Brooks/Cole Publishing; Pacific Grove, CA: 1998. [Google Scholar]
- 33.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–1527. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 34.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes and obesity-related health risk factors, 2001. J Am Med Assoc. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 35.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 36.Stanforth PR, Jackson AS, Green JS, et al. Generalized abdominal visceral fat prediction models for black and white adults aged 17–65 y: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 2004;28:925–932. doi: 10.1038/sj.ijo.0802563. [DOI] [PubMed] [Google Scholar]
- 37.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 38.Liese AD, Hense HW, Döring A, Stieber J, Keil U. Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens. 2001;15:799–804. doi: 10.1038/sj.jhh.1001266. [DOI] [PubMed] [Google Scholar]
- 39.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 40.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 41.Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia and impaired hemostasis. The Framingham offspring study. J Am Med Assoc. 2000;283:221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 42.Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- 43.Thomas F, Bean K, Pannier B, et al. Cardiovascular mortality in overweight subjects: the key role of associated risk factors. Hypertension. 2005;46:654–659. doi: 10.1161/01.HYP.0000184282.51550.00. [DOI] [PubMed] [Google Scholar]