Abstract
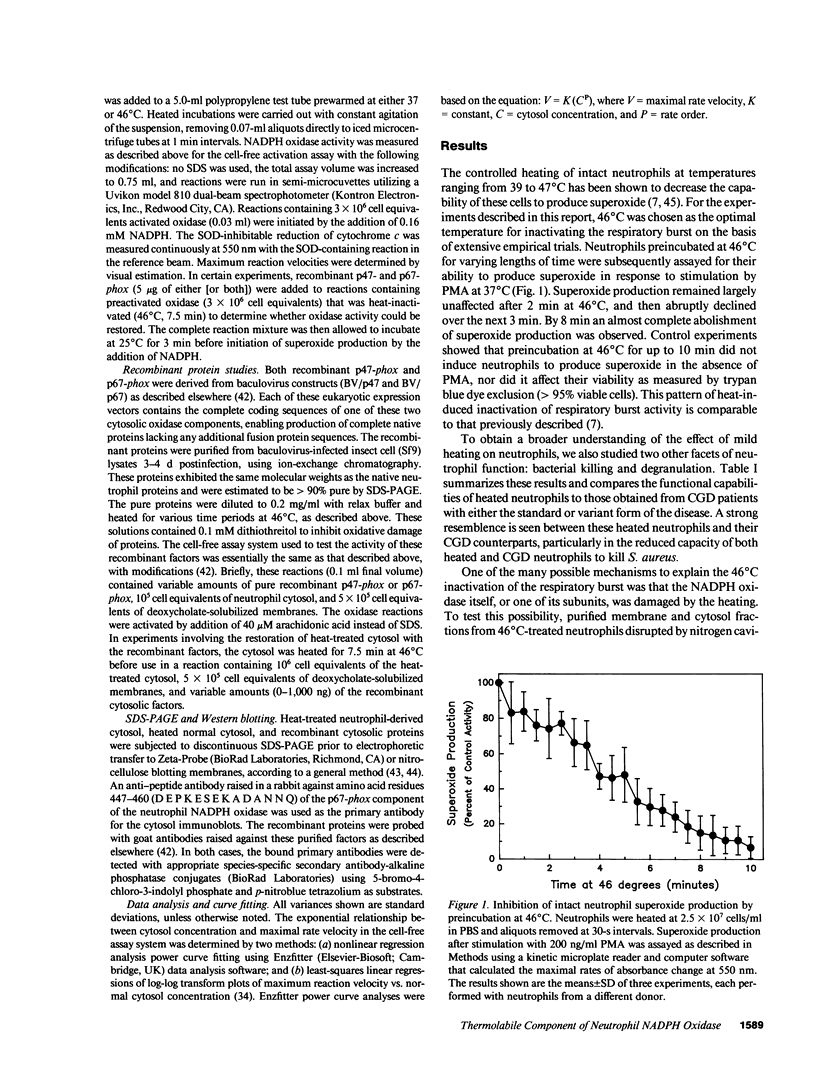
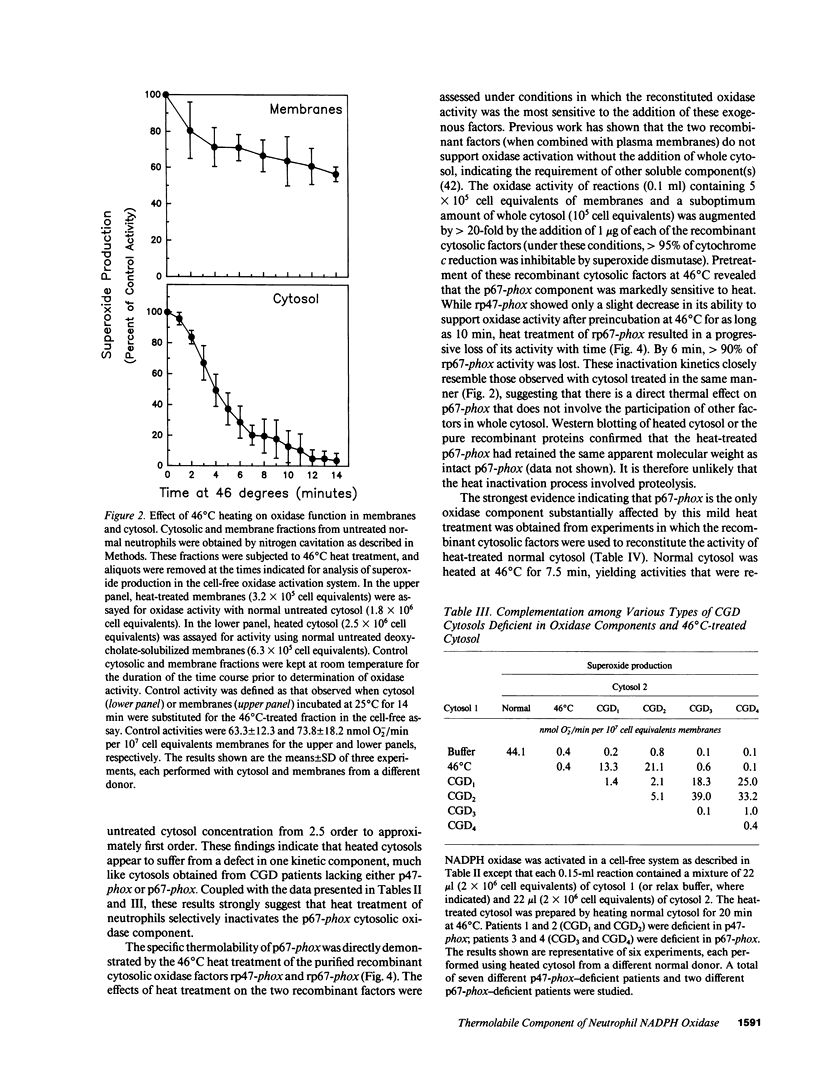
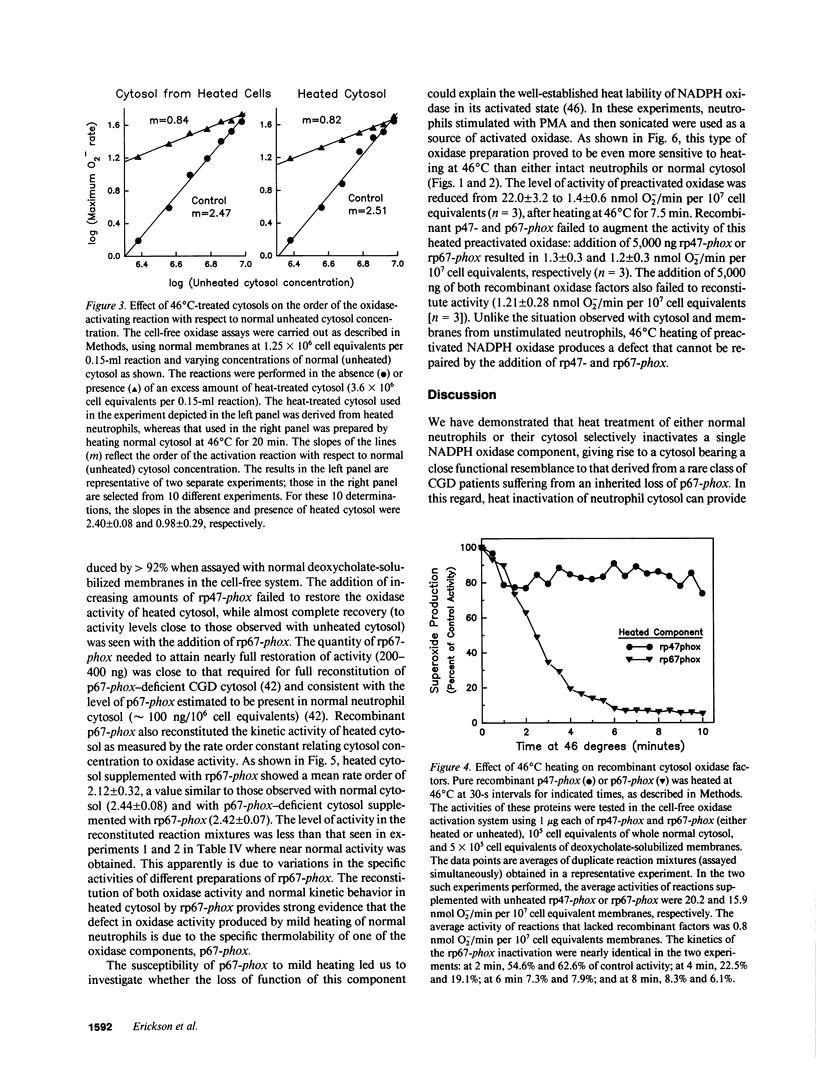
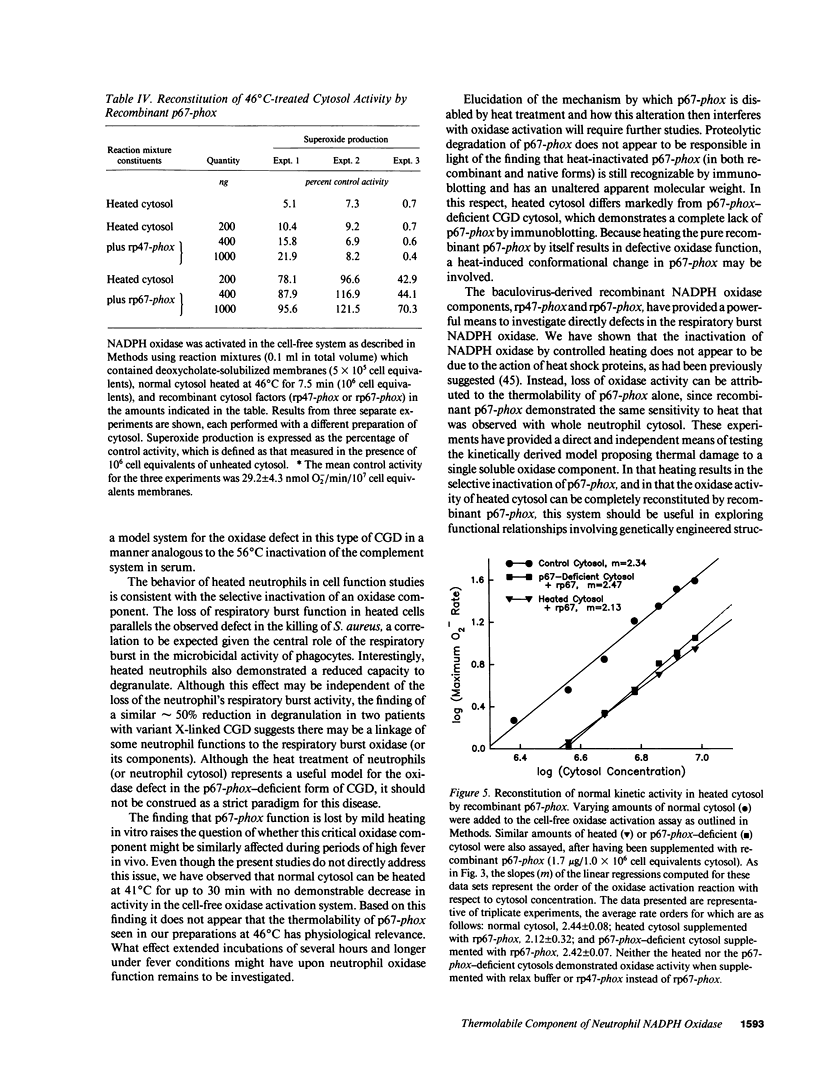
Mild heating of human neutrophils inactivates the respiratory burst oxidase, producing a defect in superoxide production and bacterial killing comparable to that seen in patients afflicted with chronic granulomatous disease (CGD). We have now investigated the mechanism and specificity of this inactivation by examining the effect of mild heating on the known oxidase components: the membrane-bound subunits of the cytochrome b558 (gp91-phox and p22-phox) and the two cytosolic oxidase factors (p47-phox and p67-phox). Heating (46 degrees C for 7.5 min) caused intact neutrophils to lose greater than 85% of their capacity to produce superoxide, a defect which was localized to the cytosolic, but not the membrane, fraction. Complementation studies with CGD cytosols deficient in either p47-phox or p67-phox suggested that the defective component of heat-inactivated cytosol was p67-phox. This was confirmed by experiments showing that recombinant p67-phox, but not p47-phox, exhibited lability at 46 degrees C and completely reconstituted oxidase activity of heat-treated cytosol. These studies indicate that mild heating of either intact neutrophils or normal neutrophil cytosol results in a selective inactivation of p67-phox, providing a model oxidase system for the extremely rare p67-phox-deficient form of CGD.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kuver R., Curnutte J. T. Kinetics of activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1988 Feb 5;263(4):1713–1718. [PubMed] [Google Scholar]
- Babior B. M., Woodman R. C. Chronic granulomatous disease. Semin Hematol. 1990 Jul;27(3):247–259. [PubMed] [Google Scholar]
- Casimir C. M., Bu-Ghanim H. N., Rodaway A. R., Bentley D. L., Rowe P., Segal A. W. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2753–2757. doi: 10.1073/pnas.88.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Malech H. L., Gallin J. I., Nunoi H., Volpp B. D., Pearson D. W., Nauseef W. M., Curnutte J. T. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med. 1989 Sep 7;321(10):647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Berkow R. L., Roberts R. L., Shurin S. B., Scott P. J. Chronic granulomatous disease due to a defect in the cytosolic factor required for nicotinamide adenine dinucleotide phosphate oxidase activation. J Clin Invest. 1988 Feb;81(2):606–610. doi: 10.1172/JCI113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Babior B. M. Activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1987 May 15;262(14):6450–6452. [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Babior B. M. Functional defect in neutrophil cytosols from two patients with autosomal recessive cytochrome-positive chronic granulomatous disease. J Clin Invest. 1989 Apr;83(4):1236–1240. doi: 10.1172/JCI114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Mayo L. A. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989 Feb;86(3):825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Curnutte J. T., Rosen H., Orkin S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989 Dec;84(6):2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990 Nov;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Erickson R. W., Muhlebach T. J., Messner H., Orkin S. H., Seger R. A., Curnutte J. T. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Naccache P. H., Sha'afi R. I. Stimulated cytokineplasts from human polymorphonuclear leukocytes mobilize calcium and polymerize actin. Cytoplasts made in cytochalasin B retain a defect in actin polymerization. J Clin Invest. 1986 Jan;77(1):34–37. doi: 10.1172/JCI112297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Van Blaricom G., Melnick D. A., Malech H. L. Functional integrity of cytokineplasts: specific chemotactic and capping responses. J Immunol. 1985 Sep;135(3):2090–2094. [PubMed] [Google Scholar]
- Francke U., Hsieh C. L., Foellmer B. E., Lomax K. J., Malech H. L., Leto T. L. Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2) and 7q11.23 (NCF1). Am J Hum Genet. 1990 Sep;47(3):483–492. [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Hurst J. K., Loehr T. M., Curnutte J. T., Rosen H. Resonance Raman and electron paramagnetic resonance structural investigations of neutrophil cytochrome b558. J Biol Chem. 1991 Jan 25;266(3):1627–1634. [PubMed] [Google Scholar]
- Keller H. U., Bessis M. Chemotaxis and phagocytosis in anucleated cytoplasmic fragments of human peripheral blood leucocytes. Nouv Rev Fr Hematol. 1975 Jul-Aug;15(4):439–446. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Garrett M. C., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991 Oct 15;266(29):19812–19818. [PubMed] [Google Scholar]
- Leto T. L., Lomax K. J., Volpp B. D., Nunoi H., Sechler J. M., Nauseef W. M., Clark R. A., Gallin J. I., Malech H. L. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990 May 11;248(4956):727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Lomax K. J., Leto T. L., Nunoi H., Gallin J. I., Malech H. L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989 Jul 28;245(4916):409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982 Dec;95(3):960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G., Cretella S. B. Cytokineplasts from human blood polymorphonuclear leukocytes. Lack of oxidase activity and extended functional longevity. Inflammation. 1985 Mar;9(1):99–106. doi: 10.1007/BF00915416. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G. Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: preservation of both motile function and respiratory burst oxidase activity. Proc Natl Acad Sci U S A. 1987 Jan;84(2):454–458. doi: 10.1073/pnas.84.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G. Phagocytic capacity of cytokineplasts from human blood polymorphonuclear leukocytes. Blood Cells. 1986;12(1):167–177. [PubMed] [Google Scholar]
- Maridonneau-Parini I., Clerc J., Polla B. S. Heat shock inhibits NADPH oxidase in human neutrophils. Biochem Biophys Res Commun. 1988 Jul 15;154(1):179–186. doi: 10.1016/0006-291x(88)90667-5. [DOI] [PubMed] [Google Scholar]
- Mayo L. A., Curnutte J. T. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Okamura N., Babior B. M., Mayo L. A., Peveri P., Smith R. M., Curnutte J. T. The p67-phox cytosolic peptide of the respiratory burst oxidase from human neutrophils. Functional aspects. J Clin Invest. 1990 May;85(5):1583–1587. doi: 10.1172/JCI114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Jesaitis A. J., Orkin S. H., Curnutte J. T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989 May 1;73(6):1416–1420. [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J Clin Invest. 1989 Jun;83(6):1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Curnutte J. T. Molecular basis of chronic granulomatous disease. Blood. 1991 Feb 15;77(4):673–686. [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Donelson J. E., Moser D. R., Clark R. A. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7195–7199. doi: 10.1073/pnas.86.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]



