Abstract
Intravital two-photon microscopy allows for the analysis of single-cell dynamics within intact tissues. As it is well recognized that molecular cues that regulate leukocyte trafficking into inflammatory sites differ between various tissues, it is important to study organ-specific responses. Recently, intravital two-photon microscopy has been expanded to moving organs in the mouse such as beating hearts. Unlike previous experimental approaches to image cardiac tissue explants or isolated perfused heart preparations by two-photon microscopy, intravital imaging accounts for the mechanical force transmitted to vessels by the heartbeat and accurately assesses dynamic leukocyte behavior in the coronary vessels and myocardial tissue. Intravital two-photon imaging of beating hearts is a promising experimental tool that will help elucidate cellular and molecular immune processes that contribute to a variety of cardiovascular diseases.
Introduction
Although the development of new therapeutic approaches has resulted in a decline in the mortality from cardiovascular diseases, ischemic heart disease due to coronary artery atherosclerosis remains the most common cause of death in the United States (Roger et al., 2011). While timely reconstitution of blood flow in coronary arteries is an important strategy to protect the ischemic heart, the process of reperfusing ischemic myocardium can have deleterious effects. Myocardial ischemia reperfusion injury can cause microvascular dysfunction, neutrophilic infiltration, activation of mitochondrial apoptosis as well as tissue necrosis, which can ultimately result in cardiac failure (Prasad et al., 2009; Verma et al., 2002) Similarly, ischemia reperfusion injury is an obligatory component of cardiac transplantation, as a donor cadaveric heart graft must be harvested and placed in cold preservation solution before implantation into a recipient.
Myocardial ischemia reperfusion injury results in a massive influx of neutrophils into the inflamed heart. Once neutrophils enter the myocardial tissue, they attach to cardiomyocytes and mediate cardiac injury. Consistent with observations in other models of sterile inflammation, murine studies have shown that myocardial ischemia reperfusion injury is ameliorated when neutrophils are depleted or when adhesion molecules that mediate neutrophil infiltration into heart tissue are blocked (Kono and Rock, 2008; Romson et al., 1983; Vinten-Johansen, 2004). Importantly, in the setting of organ transplantation, ischemia reperfusion injury-associated neutrophil influx into grafts can also shape alloimmune responses. Our group has recently shown that neutrophils augment alloimmunity by enhancing IL-12 production by donor antigen presenting cells, which skews T cell differentiation towards a Th1 phenotype (Kreisel et al., 2011). In a mouse model of heart transplantation, perioperative neutrophil depletion synergizes with costimulatory blockade to prolong graft survival (El-Sawy et al., 2005).
Neutrophil recruitment is a complex, multistep process orchestrated by integrins and chemotactic signals (Ley et al., 2007). Molecular cues that regulate neutrophil recruitment to sites of inflammation differ between various tissues and organs (Petri et al., 2008). To devise new therapeutic strategies for myocardial ischemia reperfusion injury, it is imperative to study these inflammatory processes in heart models. However, how leukocyte behavior is regulated during myocardial ischemia reperfusion injury is not well understood. Two-photon microscopy offers a powerful tool to visualize dynamic leukocyte trafficking and cellular interactions within inflamed tissues (Cahalan et al., 2002; Miller et al., 2002). Moving tissues were long thought not to be amenable to intravital imaging by two-photon microscopy. Our group has recently developed new techniques to stabilize moving tissues that allowed us to image leukocyte trafficking in the murine lung in vivo (Kreisel et al., 2010). We subsequently extended these techniques to image dynamic neutrophil behavior intravitally in transplanted cardiac grafts as well as native hearts in the mouse (Li et al., 2012). Undoubtedly, direct visualization of the highly dynamic nature of leukocyte behavior in a beating heart holds great promise to provide us fundamental insight into the molecular requirements for leukocyte trafficking under inflammatory conditions and may lead to the development of new therapeutic strategies for myocardial ischemia reperfusion injury and other cardiovascular diseases. While immunostaining of hearts can provide valuable information about tissue infiltration of various cells and assess their spatial associations, this technology renders only a static rather than a dynamic picture of how hosts mount immune responses. In addition to its capacity to monitor the dynamic behavior of immune cells during various phases of inflammation, further advantages of intravital two-photon microscopy over traditional immunostaining include the ability to image a time course of cell functions in living tissues such as apoptosis, division and synapse formation between T lymphocytes and antigen presenting cells.
Two-photon microscopy
Two-photon excitation, a principle first proposed in the 1930’s, defines a process where a fluorophore is excited after simultaneously absorbing energy from two photons. Once the excited electron in the fluorophore returns to its ground state, most of the energy is emitted as fluorescence. The emitted fluorescent photon has a shorter wavelength than the two photons used to excite the fluorophore. Compared to conventional single-photon confocal microscopy, two-photon microscopy offers the advantages of deeper tissue penetration and less bleaching. These properties are largely due to the low energy, long wavelength photons used for excitation. Improvements in laser technology in the 1990s have led to more wide-spread use of two-photon microscopy in biomedical research (Denk et al., 1990). The superior spatio-temporal resolution, deep tissue penetration and excellent image quality make two-photon microscopy a valuable modality to study individual behaviors of different cell types in various tissues and organs (Tabuchi et al., 2008). In order to visualize specific cells by two-photon microscopy, fluorescent dyes can be used to label purified cell populations of interest ex vivo prior to their adoptive transfer into a host. Commonly used dyes include 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) and 5-(and-6) -(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (CMTMR). Labeling cells with fluorescent dyes in vitro has some disadvantages. These include a limited number of certain cell populations that can be recovered after ex vivo manipulation and potential toxicity of the dye that can result in loss of cells during the labeling process. An alternative experimental strategy that avoids the cell labeling is the isolation of cells from transgenic mice that express various fluorescent proteins under an actin promoter. However, a potential concern with these approaches relates to altered cell behavior secondary to the ex vivo processing. Numerous transgenic mice exist that express fluorescent proteins under cell-specific promoters. Examples that our group and others have used include LysM-GFP mice that express green fluorescent protein under the lysozyme M promoter and can be used to visualize neutrophils, and CD11c-EFP mice that express enhanced yellow fluorescent protein under a CD11c promoter and allow for the detection of dendritic cells in various tissues(Gelman et al., 2009; Kreisel et al., 2010; Kreisel et al., 2011; Looney et al., 2011).
We have used intravenous injection of quantum dots to label blood vessels in both lungs and hearts (Kreisel et al., 2010; Li et al., 2012). Quantum dots are nanoparticles that have narrow emission spectra and whose fluorescence is stable over prolonged periods of time. We have reported that phagocytic cells within the circulation such as monocytes can take up quantum dots and can therefore be visualized (Kreisel et al., 2010). Moreover, collagen is birefringent and can be detected by two photon microscopy due to a second harmonic signal, which is defined as the generation of a single photon of half the wavelength of the exciting photons. For cell morphological and functional studies, two-photon microscopy has been used to image tissue both ex vivo as well as intravitally. While the use of explants may allow for more facile positioning of the tissue of interest than intravital imaging, this approach has several drawbacks as it may not adequately represent events in live tissue in its anatomical position. Two-photon imaging of explants relies on placing the tissue in chambers that are perfused with oxygenated warm media. Therefore, when examining dynamic cell behavior specifically in the heart, interpretation of ex vivo imaging may be limited by lack of blood flow and absent contractions. Nevertheless, intravital imaging is technically more demanding as it requires stabilization of the tissue. This is particularly the case for moving organs such as ventilated lungs or beating hearts, where the respiratory and cardiac motions have long been thought to be insurmountable obstacles to intravital imaging. These technical challenges have been recently addressed by our group and others, which allowed for the visualization of dynamic neutrophil behavior in the lung at steady state and under inflammatory conditions (Kreisel et al., 2010; Looney et al., 2011). Our group used a small amount of glue to attach the lung tissue to the bottom of a cover glass, while Looney and colleagues used a suction device for stabilization.
Two-photon microscopy in cardiovascular research
Several investigators have taken advantage of visualizing autofluorescence by two-photon microscopy in combination with the generation of second harmonic signals, which relies on endogenous properties of tissue components, to gain insight into the structure of hearts and blood vessels. Zoumi and co-workers combined two-photon excitation autofluorescence and second harmonic generation to image and differentiate structural components of walls of rabbit aortas and porcine coronary arteries such as collagen, elastin and smooth muscle (Zoumi et al., 2004). Analogous techniques have been used to image cardiomyocytes that were isolated from sheep fetuses and fixed in paraformaldehyde, where second harmonic generation allowed for clear visualization of myosin filaments within the cells and cell morphology and volume were determined based on autofluorescence signals (Wallace et al., 2008). Wallenburg and colleagues used two-photon excitation autofluorescence and second harmonic generation to image formalin-fixed sections of rat hearts, that were derived from healthy controls and animals that had undergone permanent ligation of the left anterior descending artery and either received an intramyocardial injection of mesenchymal stem cells transfected with human elastin or left untreated (Wallenburg et al., 2011). Specifically, these investigators evaluated changes in myocyte and collagen components that were visualized by two-photon autofluorescence and second harmonic signal, respectively. More recently, Caorsi provided a quantitative analysis of collagen deposition in chronically infracted rat hearts (Caorsi et al., 2013). Another application of second harmonic generation has been the evaluation of the arrangement of collagen fibers in human atrial tissue from patients suffering from atrial fibrillation and compared to controls that had normal sinus rhythm (Tsai et al., 2010).
Two-photon microscopy has also been utilized to perform functional studies of hearts. For example, Rubart and colleagues applied this technology to examine the fate of cardiomyocytes after injection into recipient hearts (Rubart et al., 2003). To accomplish this goal, these investigators transplanted fetal cardiomyocytes isolated from transgenic mice that express green fluorescent protein under the α-cardiac myosin heavy chain promoter into recipient mice and measured intracellular calcium fluxes after perfusing recipient hearts with calcium fluorophores ex vivo. They found that calcium transients occurred in essentially simultaneous fashion in donor and host cardiomyocytes indicating that transplantation of cardiomyocytes results in their functional integration with host myocardium. In follow-up studies the same group used analogous methods to evaluate whether transplantation of skeletal myoblasts could be used for cellular therapy. Here they injected skeletal myoblasts isolated from transgenic mice that express green fluorescent protein under a β-actin promoter and measured calcium transients in Langendorff preparations as in their previous study. While the majority of transplanted cells were functionally dissociated from the host myocardium, a small number of donor myoblasts had undergone fusion with host cardiomyocytes with some evidence of functional coupling (Rubart et al., 2004). Matsumoto-Ida and colleagues used two-photon laser scanning microscopy to visualize the loss of mitochondrial membrane potential in ex vivo perfused rat hearts, which were subjected to ischemia reperfusion injury (Matsumoto-Ida et al., 2006). The ability to monitor mitochondrial function in the context of inflammatory insults could provide a platform to develop new therapeutic strategies for the clinics. However, the authors acknowledged that their study was limited by the lack of cardiac contractions in their ex vivo preparation, which may not mirror events during reperfusion of ischemic myocardium under physiological conditions in vivo. Further limitations of isolated heart preparations relate to the fact that perfusion is not maintained by a beating heart, potential activation of leukocytes due to interactions with the circuit and trauma associated with surgical extirpation. The major obstacles to image hearts by two-photon microscopy in vivo have been the constant motion associated with the heartbeat as well as motion artifacts related to respiration. The recent development of new techniques to gate image acquisition to respiratory and cardiac cycles and to stabilize beating hearts has allowed for real time imaging of beating hearts over extended periods of time (Lee et al., 2012; Li et al., 2012)
Intravital two-photon imaging of leukocyte trafficking in beating hearts
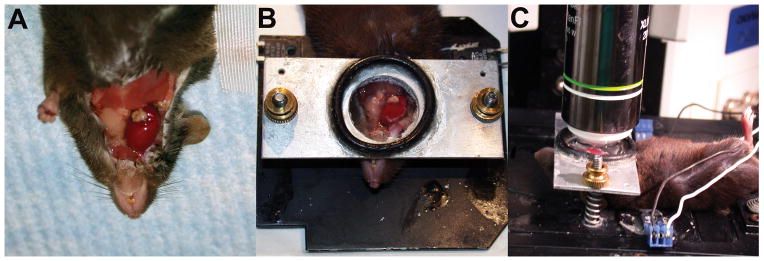
Leukocytes including neutrophils, monocytes and lymphocytes are rapidly recruited to inflammatory sites. Intravital two-photon microscopy has advanced our understanding how trafficking of leukocytes is regulated and how interactions between various cell populations shape immune responses. For example, we have observed that monocytes mediate the transendothelial migration of neutrophils into lungs that are subjected to ischemia reperfusion injury (Kreisel et al., 2010). Similar to our previous observations during lung ischemia reperfusion injury neutrophils rapidly infiltrate freshly reperfused ischemic heart tissue. Due to technical considerations we established our intravital imaging techniques of beating hearts in heterotopic heart transplants that were transplanted into the neck of recipient animals (Figure 1). Once we mastered this approach we extended our stabilization techniques to the native heart, where we evaluated neutrophil trafficking after transient ligation of the coronary artery. Interestingly, unlike the case in other tissues, where neutrophils exit the circulation in post-capillary venules, neutrophils extravasate from larger caliber coronary veins in the heart. Differences in leukocyte emigration requirements between various organs have been previously described and could at least in part be explained by tissue-specific anatomical or structural characteristics and distinct molecular recruitment mechanisms (Petri et al., 2008). Neutrophil recruitment into inflamed tissue is a multi-step process characterized by rolling, slow rolling, adhesion, crawling, and transendothelial migration (Nourshargh et al., 2010). A recent study described uropod elongation as the final step in the transmigration of leukocytes through the vascular endothelium prior to entering the interstitial space (Hyun et al., 2012). We were able to visualize how neutrophils are recruited into myocardial tissue during ischemia reperfusion injury. Neutrophils roll with velocities of approximately 146 μm/second and then slow down to adhere to the vessel wall. Subsequently, they crawl either in the direction of the blood flow or against it with velocities of approximately 8.8 μm/minute, presumably to their sites of optimum extravasation. There they form large intravascular clusters prior to exiting the circulation and entering the myocardial tissue. While we could not make definitive statements whether neutrophils left the circulation trans- or paracellularly, most neutrophils exited the vessels at “hot spots” in single file fashion suggesting a paracellular route. Similar to previous observations in infectious models and in transplant-mediated pulmonary ischemia reperfusion injury neutrophils formed large dynamic clusters in the myocardial tissue (Chtanova et al., 2008; Kreisel et al., 2010). Extravasated neutrophils that were approaching clusters displayed chemotactic behavior as evidenced by a meandering index (ratio of the distance a cell travels from its starting point to the track length) greater than 0.8. Future studies will need to determine the source and nature of the chemotactic signals that direct the migration of neutrophils within inflamed heart tissue. Neutrophil chemotaxins could be potentially secreted by tissue-resident macrophages, tissue-infiltrating blood borne cells such as monocytes or cardiomyocytes undergoing necrosis. Neutrophil trafficking in freshly reperfused cardiac grafts is impaired when the β2 integrins lymphocyte function-associated antigen-1 (LFA-1) or macrophage 1 antigen (Mac-1) are blocked pharmacologically. Specifically, neutrophils fail to adhere to the vessel walls when LFA-1 is blocked and, consequently, virtually no neutrophils infiltrate the myocardial tissue. In contrast, when Mac-1 is inhibited, neutrophils adhere to the vascular endothelium, but their crawling velocity is reduced and fewer neutrophils enter the transplant, consistent with prior work in other tissues (Phillipson et al., 2006). Furthermore, the few neutrophils that left the circulation after treatment with Mac1-blocking antibodies moved slower and in random fashion through the myocardial tissue.
Figure 1.
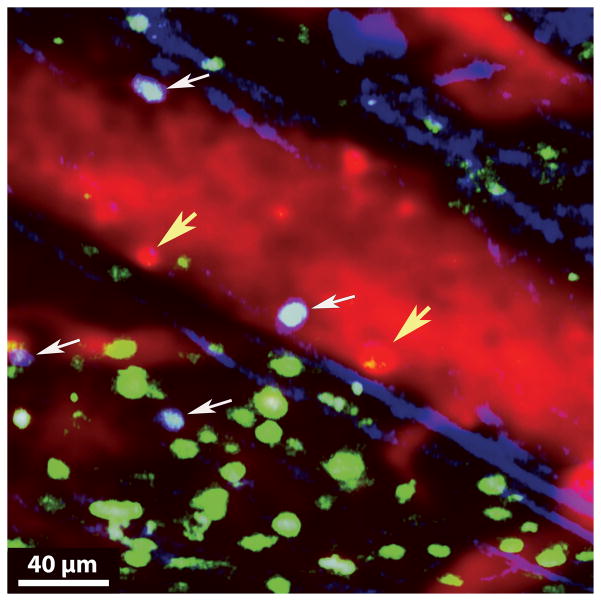
Using CX3CR1/GFP+ mice as heart transplant recipients, we observed that CX3CR1-expressing cells, which are most likely monocytes, are rapidly recruited during myocardial ischemia reperfusion injury. Others have reported that CX3CR1+ monocytes rapidly invade inflamed tissues where they play a critical role in initiating innate immune responses (Auffray et al., 2007). Whether monocytes regulate neutrophil behavior in inflamed hearts similar to our observations in lungs will be the topic of future investigations (Kreisel et al., 2010). T lymphocytes are critical mediators of cardiac allograft rejection and also play an important role in regulating ischemia reperfusion injury. To assess T cell infiltration into cardiac allografts at early time points we transplanted Balb/c hearts into B6 CD11c-EYFP hosts that express enhanced yellow fluorescent protein under a CD11c promoter (Figure 2). At the time of transplantation we injected 107 T cells that were isolated from spleen and pooled lymph nodes of B6 actin-CFP mice that express cyan fluorescent protein under an actin promoter. 24 hours after transplantation we observed numerous recipient-derived CD11c+ cells, some of which display dendritic cell morphology, within the tissue of the transplanted heart. We injected quantum dots intravenously immediately prior to imaging to label the vessels red and, interestingly, similar to our previous observations in transplanted lungs, we detected cells within the circulation that were labeled by the quantum dots and appeared red (Kreisel et al., 2010). We have previously shown that these cells are phenotypically consistent with monocytes. As graft-infiltrating monocytes can differentiate into either dendritic cells or macrophages it is possible that the recipient CD11c+ cells within the grafts arose from these quantum dot labeled cells (Gelman et al., 2010). We have observed that graft-infiltrating T cells interact with vessel walls of coronary veins. Previous studies have suggested that neutrophils facilitate the entry of T cells into inflammatory sites (Engeman et al., 2004). Whether and at what specific steps neutrophils regulate the recruitment of T cells to cardiac allografts can be accurately evaluated by intravital two-photon microscopy. Similar to our previous observations in lung grafts, recipient T cells form stable interactions with recipient-derived antigen presenting cells within cardiac allografts (Gelman et al., 2010). In the case of lung transplantation we have previously shown that graft-infiltrating recipient CD11c+ antigen presenting cells can express both donor and recipient MHC class II molecules and can therefore activate both direct and indirect allorecognition pathways (Gelman et al., 2010). Furthermore, unlike the case for lungs which we and others have shown provide a suitable environment for the activation of T cells, heart allografts can not be rejected if the recipient lacks secondary lymphoid organs (Gelman et al., 2009; Lakkis et al., 2000). Therefore, future studies will need to address the functional consequence of interactions between T cells and antigen presenting cells in cardiac allografts.
Figure 2.
Other imaging modalities for beating murine hearts
A variety of imaging techniques are available that can be used to gain insight into coronary blood flow and the regulation of cellular and molecular processes in hearts of living animals during steady state and in the context of inflammation. For example, Kiyooka and colleagues visualized the epicardial capillary network in beating canine hearts in vivo up to depths of 50 μm with a pencil-lens probe video microscope (Kiyooka et al., 2005). These investigators documented that capillary flow velocities increased after administration of adenosine or after reperfusion of transiently occluded coronary arteries. Schramm and coworkers imaged the subepicardial microcirculation of heterotopically transplanted mouse hearts with intravital fluorescence microscopy (Schramm et al., 2007). They observed that prolonged cold ischemic storage of the cardiac grafts resulted in marked decreases in capillary flow velocities. Furthermore, these investigators labeled circulating leukocytes through intravenous injection of rhodamine and found an increase in interactions between leukocytes and vessel walls when graft storage time was extended.
Monocytes are recruited to ischemic myocardium, where they can differentiate into macrophages and play an important role in the remodeling process after myocardial infarctions. Magnetic resonance imaging has been used to image infiltration of monocytes into murine heart tissue. Monocytes can be labeled with iron oxide particles and then detected by using T2 weighted magnetic resonance imaging (Yang et al., 2010) or, alternatively, with T1-shortening gadolinium-labeled liposomes (Naresh et al., 2012). This approach allows for serial non-invasive imaging over several days and can therefore provide a spatiotemporal assessment of monocyte infiltration into the myocardium in clinically relevant models.
Single Photon Emission Computed Tomography (SPECT) is a nuclear medicine imaging modality that detects gamma emission by a radionuclide that has been previously introduced into the subject. Brown and colleagues used this technology to examine sites of lymphatic drainage after heterotopic heart transplantation in mice (Brown et al., 2011). For this purpose they injected radiolabeled human albumin nanoparticles into the left ventricle of cardiac grafts that had been transplanted heterotopically into the abdomen of recipient mice, which led to the identification of mediastinal lymph nodes as the main draining stations and likely sites where alloreactive T cells encounter donor antigen.
Conclusions
Advances in biomedical research will continue to be facilitated by the development of new technologies and the wider application of existing technology. Recent advances in intravital two-photon microscopy and the generation of new cell-specific reporter mice offers investigators the opportunity to visualize dynamic cell trafficking and cellular interactions within living animals in real time. While intravital two-photon imaging of beating hearts is demanding it holds great promise to advance our understanding of mechanisms that contribute to cardiovascular disease. However, although our imaging technique yields stable images of the beating mouse heart for prolonged periods of time, there exist some important shortcomings. Most importantly, the use of glue to stabilize the heart does not allow for serial imaging of the heart in the same animal. In addition, motion artifacts secondary to cardiac contractions are evident when we image deeper areas such as coronary arteries (Li et al., 2012). These issues can be addressed through the development of more refined stabilization techniques and gated acquisition (Lee et al., 2012). In conclusion, intravital two-photon microscopy of the beating heart represents an important addition to other available experimental tools that are in use to study cardiovascular disease. Intravital two-photon imaging has the potential to elucidate organ-specific molecular requirements for the recruitment of leukocytes in the setting of sterile inflammation and infectious processes. Through the use of gene-deficient donor grafts the heart transplant model offers a platform to dissect the role of organ-resident cells such as vascular endothelial cells in the regulation of innate and adaptive immune responses.
References
- Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Brown K, Badar A, Sunassee K, et al. SPECT/CT lymphoscintigraphy of heterotopic cardiac grafts reveals novel sites of lymphatic drainage and T cell priming. American Journal of Transplantation. 2011;11:225–234. doi: 10.1111/j.1600-6143.2010.03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nature Reviews Immunology. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caorsi V, Toepfer C, Sikkel MB, Lyon AR, Macleod K, Ferenczi MA. Non-linear optical microscopy sheds light on cardiovascular disease. PLOS ONE. 2013;8:e56136. doi: 10.1371/journal.pone.0056136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Schaeffer M, Han SJ, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- Engeman T, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. Journal of Leukocyte Biology. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. Journal of Immunology. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman AE, Okazaki M, Sugimoto S, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. American Journal of Transplantation. 2010;10:1189–1199. doi: 10.1111/j.1600-6143.2010.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun YM, Sumagin R, Sarangi PP, et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. The Journal of Experimental Medicine. 2012;209:1349–1362. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyooka T, Hiramatsu O, Shigeto F, et al. Direct observation of epicardial coronary capillary hemodynamics during reactive hyperemia and during adenosine administration by intravital video microscopy. American Journal of Physiology. 2005;288:H1437–1443. doi: 10.1152/ajpheart.00088.2004. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Reviews Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel D, Nava RG, Li W, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature Medicine. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- Lee S, Vinegoni C, Feruglio PF, et al. Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nature Communications. 2012;3:1054. doi: 10.1038/ncomms2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li W, Nava RG, Bribriesco AC, et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. The Journal of Clinical Investigation. 2012;122:2499–2508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nature Methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006;114:1497–1503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Naresh NK, Xu Y, Klibanov AL, et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264:428–435. doi: 10.1148/radiol.12111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nature Reviews Molecular Cell Biology. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. Journal of Immunology. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. The Journal of Experimental Medicine. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circulation Research. 2003;92:1217–1224. doi: 10.1161/01.RES.0000075089.39335.8C. [DOI] [PubMed] [Google Scholar]
- Rubart M, Soonpaa MH, Nakajima H, Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. The Journal of Clinical Investigation. 2004;114:775–783. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm R, Menger MD, Kirsch S, et al. The subepicardial microcirculation in heterotopically transplanted mouse hearts: an intravital multifluorescence microscopy study. The Journal of Thoracic and Cardiovascular Surgery. 2007;134:210–217. doi: 10.1016/j.jtcvs.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. Journal of Applied Physiology. 2008;104:338–346. doi: 10.1152/japplphysiol.00348.2007. [DOI] [PubMed] [Google Scholar]
- Tsai MR, Chiu YW, Lo MT, Sun CK. Second-harmonic generation imaging of collagen fibers in myocardium for atrial fibrillation diagnosis. Journal of Biomedical Optics. 2010;15:026002. doi: 10.1117/1.3365943. [DOI] [PubMed] [Google Scholar]
- Verma S, Fedak PW, Weisel RD, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–2336. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovascular Research. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Wallace SJ, Morrison JL, Botting KJ, Kee TW. Second-harmonic generation and two-photon-excited autofluorescence microscopy of cardiomyocytes: quantification of cell volume and myosin filaments. Journal of Biomedical Optics. 2008;13:064018. doi: 10.1117/1.3027970. [DOI] [PubMed] [Google Scholar]
- Wallenburg MA, Wu J, Li RK, Vitkin IA. Two-photon microscopy of healthy, infarcted and stem-cell treated regenerating heart. Journal of Biophotonics. 2011;4:297–304. doi: 10.1002/jbio.201000059. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang Y, Yanasak N, Schumacher A, Hu TC. Temporal and noninvasive monitoring of inflammatory-cell infiltration to myocardial infarction sites using micrometer-sized iron oxide particles. Magnetic Resonance in Medicine. 2010;63:33–40. doi: 10.1002/mrm.22175. [DOI] [PubMed] [Google Scholar]
- Zoumi A, Lu X, Kassab GS, Tromberg BJ. Imaging coronary artery microstructure using second-harmonic and two-photon fluorescence microscopy. Biophysical Journal. 2004;87:2778–2786. doi: 10.1529/biophysj.104.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]