Abstract
Exposure to organic dusts is associated with increased respiratory morbidity and mortality in agricultural workers. Organic dusts in dairy farm environments are complex, polydisperse mixtures of toxic and immunogenic compounds. Previous toxicological studies focused primarily on exposures to the respirable size fraction, however, organic dusts in dairy farm environments are known to contain larger particles. Given the size distribution of dusts from dairy farm environments, the nasal and bronchial epithelia represent targets of agricultural dust exposures. In this study, well-differentiated normal human bronchial epithelial cells and human nasal epithelial cells were exposed to two different size fractions (PM10 and PM>10) of dairy parlor dust using a novel aerosol-to-cell exposure system. Levels of pro-inflammatory transcripts (IL-8, IL-6, and TNF-α) were measured two hr after exposure. Lactate dehydrogenase (LDH) release was also measured as an indicator of cytotoxicity. Cell exposure to dust was measured in each size fraction as a function of mass, endotoxin, and muramic acid levels. To our knowledge, this is the first study to evaluate the effects of distinct size fractions of agricultural dust on human airway epithelial cells. Our results suggest that both PM10 and PM>10 size fractions elicit a pro-inflammatory response in airway epithelial cells and that the entire inhalable size fraction needs to be considered when assessing potential risks from exposure to agricultural dusts. Further, data suggest that human bronchial cells respond differently to these dusts than human nasal cells and, therefore, the two cell types need to be considered separately in airway cell models of agricultural dust toxicity.
Keywords: in vitro, NHBE, HNE, pro-inflammatory, agricultural, endotoxin, aerosol
Introduction
Dust exposure is a major source of respiratory morbidity and mortality among agricultural workers (Schenker, 2000, Reynolds et al., 2013, Cleave et al., 2009, ATS, 1998, Linaker and Smedley, 2002). Previous studies indicated that dairy workers, in particular, have increased risks for asthma, rhinitis, sinusitis, mucus membrane inflammation syndrome, bronchitis, chronic obstructive pulmonary disease (COPD), hypersensitivity pneumonitis, and organic dust toxic syndrome (Gainet et al., 2007, Kullman et al., 1998, May et al. 2012, Reynolds et al., 2013). Agricultural aerosols span particle sizes from the respirable (aerodynamic particle diameter (dp) < 4 μm) to the inhalable range (dp < 100 μm). The respirable size fraction (PM<4) is often the focus of epidemiological and toxicological studies because respirable particulate matter (PM) penetrate deep into the lungs. Larger dust sizes present risks of adverse health effects (Rask-Andersen et al., 1989, Kateman et al., 1990), however PM larger than 10 μm have not been studied extensively in epidemiologic or toxicological contexts, even though such exposures are known to exist. Kullman et al. (1998) measured dairy farm dust with a mass median aerodynamic diameter of 13.5 μm (with a geometric standard deviation of 2.1). Following these findings, more recent epidemiologic studies included the entire inhalable dust fraction during exposure assessment (Garcia et al., 2013, Reynolds et al., 2013, Burch et al., 2010).
Dairy farm dusts are complex mixtures that contain both toxic and immunogenic compounds (Kullman et al., 1998). The organic fraction of these dusts may contain yeasts, molds, mesophilic and thermophilic bacteria (G-positive and G-negative), histamine, cow urine antigen, mite antigen, endotoxins, pharmaceutical compounds, and pesticides (Kullman et al., 1998, Donham, 1986, Kemper, 2008). The inorganic dust fraction may contain silicates, clays, pesticides, and metals (Schenker, 2000). Of the potential microbial etiologic constituents found in dairy farm dusts, endotoxin is the most often studied.
A universal dose-response for endotoxin has yet to be established because previous studies reported conflicting results. Rask-Andersen et al. (1989) observed high endotoxin exposure (5000 EU/m3) without symptoms, while Kateman et al. (1990) noted a dose-response in workers exposed to lower concentrations (0.29, 0.3, and 1.02 EU/m3). Both research groups analyzed only a fraction of the total inhalable dust (dp < 5 μm for Rask-Andersen et al. (1989) and dp < 8.5 μm for Kateman et al. (1990)), and Rask-Andersen et al (1989) found that 75% of the endotoxin activity was observed in the non-respirable (dp > 5 μm) fraction. Previous studies of swine workers suggested an exposure limit of 100 EU/m3 (Donham et al., 2000). Alternatively, the Netherlands proposed an occupational exposure limit of 50 EU/m3 in 1998, however, the 50 EU/m3 limit was shortly thereafter increased to 200 EU/m3 by the Dutch Social Affairs Ministry, before being eventually abandoned (Duquenne et al., 2012, Heederik and Douwes, 1997). The Dutch Expert Committee on Occupational Safety proposed a new exposure limit of 90 EU/m3 in 2010. To date, no occupational exposure limit for endotoxin has been established in the U.S. or internationally (Duquenne et al., 2012). Differences in sampling techniques and analysis, as well as the failure to consider the total inhalable dust size fraction, may contribute to the lack of a clear dose-response for endotoxin exposure (Reynolds et al. 1996, 2002, 2005, Duquenne et al. 2012). Further, recent studies suggested that the inflammatory potential of such dusts does not depend solely on endotoxin (Poole et al. 2010, Harting et al. 2012).
Previous studies suggested that human nasal epithelial (HNE) cells might serve as a reliable (and more accessible) surrogate for bronchial cell toxicology (McDougall et al., 2008). Here, a novel aerosol-to-cell exposure system was used to examine acute responses of normal human bronchial epithelial (NHBE) and HNE cells following exposure to two different size fractions of dairy parlor dust: particulate matter less than 10 μm in diameter (PM10) and particulate matter (PM) greater than 10 μm in diameter (PM>10). Exposure levels were designed to achieve similar PM mass loadings between treatments (cell type and size range). Cytotoxicity and transcripts associated with pro-inflammatory cytokines and chemokines (IL-8, IL-6, and TNF-α) were measured two hr after exposure.
Until recently, in vitro models of aerosol exposure have been limited by biological, physiological, and environmental relevance. Traditionally, the most common technique for in vitro lung cell exposures relied upon growing cells submerged in growth media and exposing the cells to particulate extracts or PM resuspended in a liquid. Recent advancements with air-liquid interfaced (ALI) cell culture allow for greater physiological relevance than previous, submerged cell cultures (Adler and Li, 2001; Whitcutt et al., 1988; Gruenert et al., 1995). To complement ALI cultures, direct air-to-cell exposure systems were developed to preserve the chemical and physical characteristics of aerosols during exposure and provide better control over deposited PM levels (Teeguarden et al., 2007, Volckens et al., 2009). To our knowledge, this is the first study to use a direct air-to-cell exposure system with ALI cultures to evaluate the human airway cell response to dairy dust exposure. Further, it is postulated that this is the first study to examine (1) pro-inflammatory responses of airway epithelial cells after exposure to dairy parlor dusts across the inhalable size fraction and (2) differences in pro-inflammatory responses between two different airway cell populations (upper and lower airway cell populations), after exposure to two different particle size fractions.
Materials and Methods
Cell Culture
Normal human bronchial epithelial (NHBE) cells were obtained by brush biopsy from two healthy, non-smoking human volunteers (EPA, Research Triangle Park, NC, USA) in accordance with a human studies protocol approved by the Institutional Review Board at the University of North Carolina. Cell populations were expanded through two passages with Bronchial Epithelial Growth Media (BEGM kit; Lonza, Walkersville, MD, USA) before being plated onto collagen-coated, porous, polycarbonate membranes (0.4 μm Snapwell membrane; Corning, Inc., Corning, NY, USA) at a seeding density approximately 150,000 cells/cm2. All ALI cultures were carried for a minimum of 21 days (prior to exposure) to allow progressive differentiation into basal, ciliated, and mucin-producing cell types within a pseudo-stratified columnar epithelium (Ross et al., 2007). Mucus production was visually apparent by day 10 of ALI and excess mucus was removed with a saline rinse, every three days thereafter.
Human nasal epithelial (HNE) cells from two different human donors were obtained from Celprogen Inc. (Celprogen Inc., San Pedro, CA). The HNE cells were cultured following the same protocol described above for the NHBE cells. The only protocol difference to note was a saline rinse every two days (as opposed to three) due to increased mucus production by HNE cells.
Dairy Dust Collection and Extraction
Airborne dust from a local dairy parlor was sampled and segregated by size using a high-volume cascade impactor (IESL v2, (Collett et al., 1995)) over the course of single 72 hr period. The cascade impactor operated at an airflow of 1500 L/min and collected dust onto Teflon™ substrates at three aerodynamic size ranges: 3–10 μm, 10–30 μm, and 30–100 μm. PM less than 3 μm in aerodynamic diameter (PM3) was collected downstream of the impactor on an 8″×11″ Teflon™ filter (Zeflour, Pall Inc, Ann Arbor, MI). The PM3 filter was replaced every 12 hr to prevent overloading. An annotated image of the cascade impactor sampler is shown in Supplementary Figure 1 (Schaeffer et al. 2013). Relative humidity and temperature inside of the parlor varied from 45–80% and 8–14°C, respectively. Immediately after sampling, the impactor was transported to the lab and collected PM was scraped from each substrate. Each size fraction was then placed in cryovials and stored at −20°C until use. Each PM3 filter was placed into 100 ml of acetone in non-pyrogenic glass vials and allowed to soak for 10 min. The filters were then vortexed for two min and finally shaken for two hr at 100 rpm and 22°C. Acetone was used as the solvent for particle extractions to ensure that both polar and nonpolar constituents were extracted from the polytetrafluoroethylene (PTFE) filter. The filters were carefully removed from the acetone solution, and acetone was allowed to evaporate in pure, dry nitrogen in a fume hood overnight. To ensure the extraction process exerted no effect upon endotoxin levels, rFC analysis was performed with a standardized assay kit and protocol and 1 mg/ml solutions of untreated PM3-10 dairy dust in acetone or Tween (Supplementary Figure 2) (Pyrogene Recombinant Factor C Assay; Lonza Group Ltd., Walkersville, MD) (Thorne et al., 2010). The PM3 fraction was combined with PM3-10 at approximately a 1:2 mass ratio in sterile, pyrogen free H2O, to achieve a 1.25% stock solution PM10 for cell exposures. Standard curves for endotoxin units (EU) as a function of PM mass were prepared for each particle size fraction (Supplementary Figure 3).
Cell Exposures to Dairy Parlor Dust
Well-differentiated NHBE or HNE cells (cultured at ALI for a minimum of 21 days) were exposed to re-suspended dust samples in a gravity settling chamber (n = 10 per treatment group). A schematic of the settling chamber can be seen in Supplementary Figure 4. A heated water bath served to maintain temperature (37°C) and humidity (85–90%) inside the chamber at near-physiologic conditions. Supplemental CO2 (5% by volume) was provided to the chamber to maintain cellular pH. An experimental matrix for cellular exposures is provided in Supplementary Table 1. Dairy dust was sonicated in water for 30 min prior to cell exposures. Dairy dust was then nebulized for 5 min using a three-jet nebulizer (BGI Incorporated, Waltham, MA) until a stable, steady-state mass concentration of either 1.4 or 2.8 mg/m3 was achieved inside the settling chamber. Particle mass concentrations inside the settling chamber were monitored with a DustTrak Aerosol Monitor (DustTrak Aerosol Monitor 8520; TSI, Shoreview, MN, USA) that was calibrated by gravimetric analysis. An Aerodynamic Particle Sizer (APS; TSI, Shoreview, MN, USA) was used to measure the particle size distributions immediately after the steady-state concentration was reached. A diffusion dryer was placed upstream of both instruments to minimize measurement bias from condensation effects. Cells were exposed for two hr to allow each dust sample to settle completely onto the cultured cells. Control cells were exposed to the same conditions stated above except that sterile, pyrogen free H2O diluent, with no dairy dust, was nebulized. The response of PM10 exposed cells was compared to the response of control cells, two hr after exposure. Each exposure was repeated on two separate test days.
A similar approach was used for cell exposures to PM>10. However, a larger settling chamber (Supplementary Figure 5) was used to disperse and settle these dusts. Because the PM>10 size fraction contained less endotoxin per mg of dust than the PM10 size fraction (Supplementary Figure 3) an additional PM>10 exposure level of higher mass was included. Preliminary studies with this chamber indicated that 8 mg of PM>10 loaded into the aspirator corresponded to a deposition level of approximately 1 μg/cm2 of cellular growth area. Therefore, mass loadings of 8 mg were used to establish the highest PM>10 exposure group and 0.8 and 4 mg mass loadings were used to establish low and medium exposure groups, respectively. On each test day, NHBE or HNE cells were placed in the chamber and then PM>10 dust was re-suspended into the chamber using a venturi-style aspirator (Supplementary Figure 5); re-suspended dust was then allowed to settle for 10 min. Given the high terminal settling velocities of PM>10, 10 min was sufficient to allow complete settling of all aspirated dust. The cell plate was then transferred to the same gravitational settling chamber used for the PM10 exposures and cells were exposed to the same conditions (physiological temperature, humidity, CO2) and same total duration of exposure (two hr) as used for PM10 exposed cells. Control cells were treated similarly, except that no PM>10 was aspirated. The response of PM>10 exposed cells was compared to the response of control cells, two hr after exposure.
Levels of PM mass deposited to cells were calculated by direct measurement of endotoxin present on a 12-well plate that was co-located with cell cultures in the exposure chamber. Each culture plate also contained two empty cell wells that were analyzed for quality control. Unseeded wells were filled with 1.5 ml non-pyrogenic Tween solution (0.05% by volume). Dust PM was collected into these 14 wells during cell exposures, and total endotoxin units (EU) per ml were measured in triplicate with a commercial rFC assay and standardized protocol (Pyrogene Recombinant Factor C Assay; Lonza Group Ltd., Walkersville, MD). Sample coefficient of variations were less than 25%. Total mass deposited was estimated with the standard curve equations for EU per mg PM for either the PM10 or PM>10 size fractions (Supplementary Figure 3). Equations 1 (PM10) and 2 (PM>10) below were used to estimate total mass deposited,
| Equation (1) |
| Equation (2) |
where PMmass represents mass of PM deposited per cellular growth area (μg per cm2), EU represents measured endotoxin concentration in each well (EU per ml), V represents total volume Tween solution per cell well (1.5 ml) and A represents the total area of a single well from a standard 12-well plate (3.83 cm2). The constants on the right-hand side of equations 1 and 2 account for units conversion between endotoxin mass content and total dust mass for each size fraction (taken from serial calibration curves of known mass content).
Muramic acid content of the two dust size fractions were measured with gas chromatography mass-spectrometry using an Agilent 6890 gas chromatograph (Agilent Technologies, Loveland, CO) with a Micromass Quattro Micro mass spectrometer (Waters Corporation, Milford, MA) and a standardized protocol (Poole et al., 2010). A 150 μl (120 μg total mass) aliquot of a 1.25% (by mass) solution of each particle dust size fraction was frozen at −80°C until GC-MS analysis could be performed. Samples were lyophilized prior to GC-MS analysis for muramic acid. Measured levels of muramic acid were reported as ng per μg dust.
Transcript Production in ALI NHBE Cells
Transcripts coding for proteins that are often used to characterize the cellular pro-inflammatory response observed in humans exposed to agricultural dusts were quantified (Interleukin 8, IL-8; Interleukin 6, IL-6, and Tumor Necrosis Factor alpha, TNF-α) (Burch et al., 2010, Reynolds et al., 2013). All mRNA transcript analyses were quantified by RT-PCR (CFX96, Bio-Rad Laboratories, Hercules, CA) in accordance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (Bustin et al., 2009). Expression profiles for each transcript were normalized to GAPDH (Barber et al., 2005). Transcript levels of IL-8, IL-6, and TNF-α were measured two hr after exposure. All transcript expression profiles were normalized to control expression levels of each transcript.
Cytotoxicity in ALI NHBE Cells
Lactate dehydrogenase (LDH) is expressed constituently in NHBE and HNE cells. The loss of membrane integrity during cell injury and death produces extracellular release of LDH, which may be used as an indicator of cytotoxicity (Allan and Rushton, 1994). Extracellular LDH was assayed at two hr post-exposure to dairy dust using a standard kit and protocol (Promega Cytotox96 Non-radioactive Cytotoxicity Assay, Promega Corporation, Madison, WI, USA). Percent cytotoxicity was calculated by following the standard protocol established by Promega for an assay with a single cell type (Promega, 2012).
Statistical Analysis
Transcript data were log-transformed to satisfy model assumptions of normality and homoscedasticity. The effects of exposure type, exposure level, donor phenotype, and experimental repeat (and their interactions) were evaluated relative to the expression of IL-8, IL-6, and TNF-α transcripts and extracellular LDH (cytotoxicity) using a PROC MIXED procedure in SAS. Cell donor and experimental replicate were treated as random effects. Statistical analyses were conducted with SAS software (v9.3 SAS Institute Inc., Cary, NC, USA) with a type I error rate of 0.05.
Results
Dairy Dust Characteristics
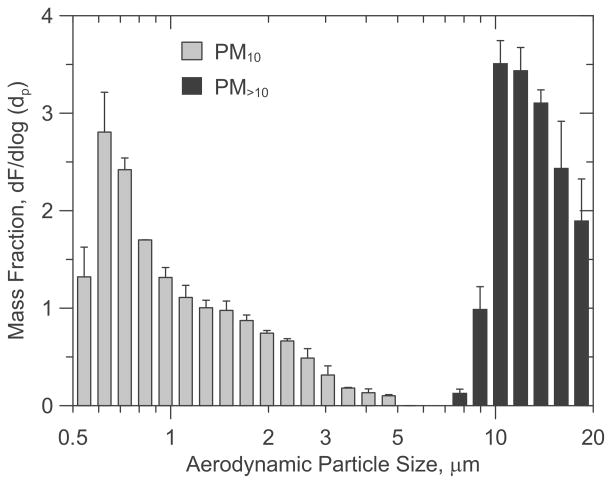
The average size distribution (by mass) for PM10 and PM>10 cell exposures are shown in Figure 1. Mass median diameters (MMD) during PM10 and PM>10 cell exposures were 0.87 μm (GSD = 1.31) and 12.4 μm (GSD = 1.26), respectively. PM10 mass concentrations inside the gravity settling chamber at the start of each experiment ranged from 1.3–1.5 mg/m3 and 2.6 to 3 mg/m3 for the low and high exposure groups, respectively. After two hr, these starting concentrations resulted in 0.1 – 0.2 and 0.3 – 0.4 μg of settled PM10 per cm2 of cellular growth area. Exposure levels were estimated by following Equation 2 and using the EU levels measured directly in the cell wells following each test. Settled PM>10 ranged from 0.1–0.2, 0.4–0.5, to 1–1.2 μg/cm2. Endotoxin content by mass was approximately 14.5 fold greater in the PM10 fraction than in the PM>10 μm size fraction (Supplementary Figure 3). Muramic acid content varied from 0.057 (± 0.003) to 0.044 (± 0.006) ng/μg in the PM10 and PM>10 dust fractions, respectively.
Figure 1.
Particle mass fraction distributions measured during PM10 and PM>10 cell exposures.
Cell Exposure Levels
Levels of endotoxin measured in cell wells after PM10 cell exposures ranged from 0.7 (± 0.12) EU/ml and 2.8 (± 0.15) EU/ml in the low and high exposure groups, respectively. These levels corresponded to an endotoxin loading that varied significantly between exposure groups with approximately 0.3 (± 0.07) EU/cm2 and 1.1 (± 0.1) EU/cm2 deposited during low and high exposures, respectively. Endotoxin deposition measurements resulted in exposure levels of 0.1–0.2 μg/cm2 and 0.37–0.41 μg/cm2 in low and high exposure groups, respectively.
Levels of endotoxin measured in the cell wells after PM>10 exposure ranged from 0.11 (± 0.03), to 0.19 (± 0.05), to 0.52 (± 0.12) EU/ml for low, mid, and high exposure groups, respectively. These levels corresponded to endotoxin loadings that ranged from 0.04 (± 0.01), to 0.08 (± 0.03), to 0.2 (± 0.04) EU/cm2 for low, mid, and high exposures, respectively. Levels of endotoxin deposited varied significantly between high and low PM>10 exposure groups. EU deposition measurements resulted in PM>10 mass exposure estimates of 0.1–0.2, 0.4–0.5, and 0.8–1.3 μg/cm2 for low, mid, and high exposure groups, respectively.
Muramic acid content did not vary significantly between the two particle size fractions. In PM10 exposed cells, muramic acid exposure levels varied from approximately 0.006 to 0.01 and 0.02 to 0.023 ng/cm2 in low and high exposure groups, respectively. In PM>10 exposed cells, muramic acid exposure levels varied from 0.005 to 0.01, to 0.015 to 0.02, and 0.045 to 0.05 ng/cm2 in low, mid, and high exposure groups, respectively.
Cellular Response after Exposure to Dairy Parlor Dust
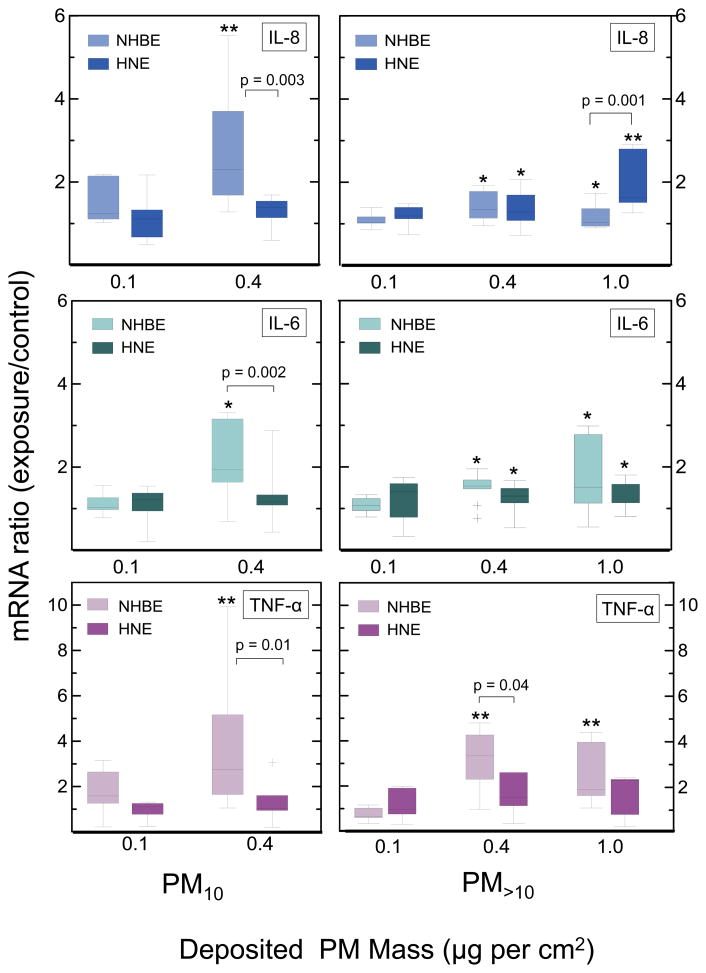
Differences between lower airway (NHBE) and upper airway (HNE) cell responses were observed after exposure to each dairy dust size fraction. In cells exposed to the smaller particle size fraction (PM10), a mass exposure of 0.4 μg/cm2 produced significant increases in transcripts coding for IL-8, IL-6, and TNF-α in NHBE cells, but not HNE cells (Figure 2). As a result, significant differences were observed between NHBE and HNE cells for IL-8, IL-6, and TNF-a at this exposure level. For the larger PM size fraction (PM>10), a 0.4 and 1 μg per cm2 exposure resulted in greater elevation in IL-8 transcription by HNE cells than observed NHBE cells (Figure 2). These same exposure levels resulted in enhanced accumulation of IL-6 transcripts in both NHBE and HNE cells (Figure 2) and increases in transcripts coding for TNF-α in NHBE but not in HNE cells.
Figure 2.
Box-whisker plots of transcript production in ALI NHBE and HNE cells exposed to PM10 (left panel) and PM>10 (right panel) size fractions. All transcript levels are normalized to HEPA-air controls. (+) signifies outliers. * signifies p<0.05, ** signifies p<0.0001, when compared with controls. P-values shown in plots are p-values for cell type differences.
Exposure to each particle size fraction elicited significantly different responses in upper airway cells. Exposure to PM>10 significantly increased transcripts for IL-8 and IL-6 in HNE cells whereas exposure to PM10 did not. Differences in the response of NHBE cells exposed to each particle size fraction were not as marked.
An exposure response was noted for IL-8 and IL-6 transcript production in both cell types and particle size fractions (Figure 2). No significant changes in IL-8, IL-6, or TNF-α transcript production were observed in the lowest exposure group (0.1 μg of PM10 or PM>10 per cm2) in either cell type or particle size exposure group. A 4-fold increase in PM mass exposure (0.4 μg PM10 or PM>10 per cm2) corresponded with elevated transcript production of (1) IL-8 and IL-6 in both airway cell types and particle size fractions and (2) TNF-α in NHBE cells exposed to either particle size fraction. A similar pattern of enhanced transcript production was noted in the highest exposure group (1 μg PM>10/cm2). Increases in transcript production were similar in the 0.4 and 1 μg PM>10/cm2 exposure groups, with the exception of IL-8 production in HNE cells, which was elevated significantly in HNE cells exposed to 1 μg PM>10/cm2.
Cytotoxicity, as estimated by measured LDH release, was not significantly altered in any exposure group (Figure 3).
Figure 3.
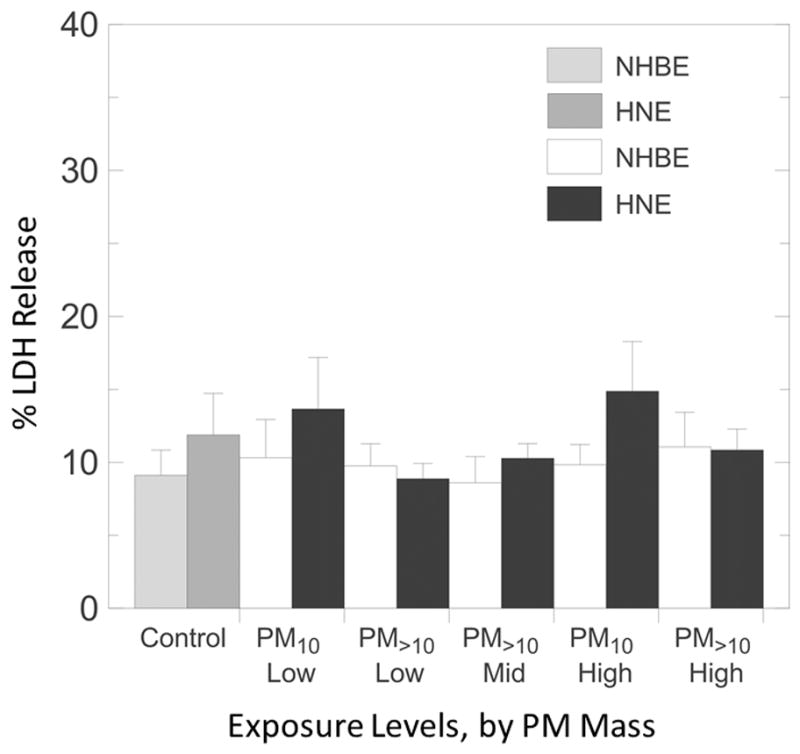
Percent LDH release from ALI NHBE and HNE cells exposed to PM10 nd PM>10 size fractions. Error bars indicate one standard deviation.
Discussion
Significant differences were observed in the accumulation of pro-inflammatory transcripts within NHBE and HNE cells following exposure to dairy dust (Figure 2). The responses of each cell type were significantly different and varied with particle size and by transcript type. In PM>10 exposed cells, IL-8 was increased in HNE cells but not in NHBE cells, whereas TNF-α was elevated in NHBE cells, but not HNE cells (Figure 2, right panel). These results suggest that IL-8 transcript production in NHBE and HNE cells may not be driven via the same stimuli and/or pathways, and moreover, if IL-8 is a chemokine of interest, HNE cells may not be a reliable surrogate for NHBE cellular responses. Further, TNF-α transcript production was different between NHBE and HNE cells. NHBE cells produced significantly higher levels of TNF-α than HNE cells after exposure to PM10 and PM>10 mass loadings of 0.4 μg per cm2 (Figure 2).
Our results differ from those reported by McDougall et al. (2008), who concluded that nasal epithelial cultures represent an acceptable surrogate for studies of lower airway inflammation. Some obvious differences are noteworthy between our study presented here and that of McDougall et al. (2008). For example, in our study, cells were exposed to an exogenous stressor (dairy dust) known to generate a proinflammatory response in bronchial airway epithelia (Poole et al., 2010), whereas McDougall et al. (2008) used endogenous cytokines (IL-1β and TNF-α) as a stimulant. Alternatively, Comer et al. (2012) exposed NHBE and HNE cells from the same COPD donor to an exogenous stressor (cigarette smoke extract) and observed differences in the response of HNE and NHBE cells after exposure. Although McDougall et al. (2008) found that human nasal and bronchial airway epithelial cells respond similarly to cytokine stimulation, our study, and Comer et al. (2012), suggest that similarities observed by McDougall et al. (2008) do not transfer to human bronchial and nasal epithelial cell responses to exogenous stimuli like agricultural dusts or cigarette smoke extract. Our results suggest that HNE and NHBE cells display distinctly different responses to dairy parlor dust.
The bimodal mass concentrations (Figure 1) used for cell exposures here are similar to inhalable mass concentrations noted in dairy farming environments (Schaeffer et al., 2013, Reynolds et al., 2012, 2013, Garcia et al., 2013b). The particle size fractions used here appear to play a role in eliciting differential responses among HNE and NHBE cells. PM10 exposure produced a significant increase in IL-8 transcript levels in NHBE cells, whereas PM>10 did not (Figure 2). Alternatively, IL-8 and IL-6 transcript levels in HNE cells rose with increased PM>10 mass loadings while IL-8 transcript levels did not change after PM10 exposure (Figure 2). Overall, NHBE cells responded more strongly to PM10, whereas HNE cells responded more strongly to the PM>10 size fraction. In vivo, PM>10 deposits primarily in the extrathoracic region (20–40% of inhaled PM>10 is deposited in the human nasal, mouth, larynx, and pharynx, versus 0–1% deposition in the bronchial region of the lungs (ICRP, 1994)). To that end, primary nasal cells from human donors may be more sensitized to PM>10 size fractions (as opposed to PM10 size fractions). Similarly, NHBE cells may be more sensitive to PM10 due to increased deposition of these particles in the conducting airways. Anywhere from 1–20% of inhaled PM10 is deposited in the human bronchial airways, whereas 5% or less of PM>10 is deposited in this same region (James et al., 1991). The mechanism for sensitizing HNE and NHBE cells to specific particle sizes is unclear but may involve epigenetic or immunogenic factors. Previously Holloway et al (2012) found that exposure to PM10 air pollutants induced gene-specific and global methylation in the lungs.
Because muramic acid levels did not vary significantly between particle size fractions, muramic acid is not believed to be responsible for the differences between PM10 and PM<10 exposed cells. The higher endotoxin content in PM10 (Supplementary Figure 3) suggests that higher endotoxin content in PM10 played a role in the increased NHBE IL-8 response to PM10. However, Poole et al. (2010) showed that endotoxin removal did not eliminate NHBE production of IL-8. Because endotoxin exposure levels were not explicitly controlled, one can not conclude that the higher endotoxin content in PM10 was responsible for NHBE IL-8 response to PM10. Further, because HNE cells responded significantly to PM>10 but not to PM10, the higher endotoxin content in PM10 exerted no observable effect on HNE cell response (Parsanejad et al., 2008).
Kullman et al. (1998) reported that agricultural dust exposures involve particle sizes that extend into the inhalable size fraction (i.e., PM>10;). Historically, PM>10 size fraction has been largely ignored in previous epidemiologic or toxicological contexts (Rask-Andersen et al., 1989, Kateman et al., 1990); however, efforts to measure the inhalable size fraction have increased in recent years (Burch et al., 2010, Garcia et al., 2013, Basinas et al., 2012, Reynolds et al., 2013). Our results support the inclusion of large, inhalable particles in future studies of dairy dust toxicity, given the results presented here.
Our exposure model is limited to human airway epithelium response, which does not fully mimic nasal or bronchial epithelia. Future studies need to consider whether these results are reproducible with (1) immune cells (e.g. mast cells, eosinophils, lymphocytes) present in co-culture and (2) a larger number of donor phenotypes. It is noteworthy that our sample size was also limited; however, our study was powered (successfully) to detect significant differences in responses by cell type and particle size. Further, the effect of donor was tested in PM10 and PM>10 exposed cells and no significant effect due to donor was found. Strengths of this study include (1) use of a direct, air-to-cell exposure system (2) use of primary cells and (3) application of distinct (and previously unstudied in airway cells) particle sizes at environmentally-relevant exposure levels. Many traditional in vitro studies rely on the use of particle extracts (or suspensions) with unrealistic doses (Frampton et al., 1999;Veranth et al., 2004; Romberger et al.; 2002, Wyatt et al., 2007). Our results suggest, for the first time, differences in the pro-inflammatory (1) response of human airway cells to two different particle size fractions across the inhalable size range of agricultural dusts and (2) response of two airway cell populations after exposure to agricultural dust.
Conclusions
Our results offer preliminary insight into the relatively unstudied toxicity of two different particle size fractions present within agricultural dusts. Human airway cell pro-inflammatory responses varied with cell type, particle size fraction, and particle mass loading. Similar to the results observed by Comer et al. (2012), significant differences in the response of NHBE and HNE cells to an exogenous stressor were noted. Our results suggest that HNE cells would not be a reliable surrogate for NHBE cellular response in future work with agricultural dusts. Our results also suggest that when selecting a ‘screening’ cell type for evaluating the pro-inflammatory response of airway cells to agricultural dusts, NHBE cells offer greater sensitivity to PM10 than HNE cells. Alternatively, both HNE and NHBE cells were sensitive to PM>10 agricultural dusts. Further, because significant responses were observed in cells exposed to PM>10, our findings suggest that particles in the inhalable size fraction need to continue to be considered alongside particles in the respirable and thoracic size fractions in future agricultural dust studies.
Supplementary Material
Acknowledgments
The authors would like to thank Robert Devlin and Lisa Dailey at the U.S. EPA for their donation of the NHBE cells used for this work. We also thank Margaret Davidson for her assistance with dust collection and cell culture. This work was supported by the High Plains Intermountain Center for Agricultural Health and Safety and grant number U54OH008085 from the National Institute of Occupational Safety and Health. This work was also supported, in part, by a grant from the National Institute of Environmental Health Sciences (R01ES019325).
Footnotes
Competing Interests
The authors declare that they have no competing interests.
References
- Adler KB, Li Y. Airway epithelium and mucus. Am J Respir Cell Mol Biol. 2001;25:397–400. doi: 10.1165/ajrcmb.25.4.f214. [DOI] [PubMed] [Google Scholar]
- Allan MJ, Rushton N. Use of the Cytotox(96) in routine biocompatibility testing in vitro. Promega Notes Magazine. 1994:45. [Google Scholar]
- ATS. Respiratory Health Hazards in Agriculture. American Journal of Respiratory and Critical Care Medicine, 1998/11/01 1998. American Thoracic Society – AJRCCM. :S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- Barber R, Harmer D, Coleman R, Clark B. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genom. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Basinas I, Sigsgaard T, Heederik D, Takai H, Omland Ø, Andersen NT, Wouters IM, Bønløkke JH, Kromhout H, Schlünssen V. Exposure to inhalable dust and endotoxin among Danish livestock farmers: Results from the SUS cohort study. Environ Monitor. 2012;14:604–614. doi: 10.1039/c1em10576k. [DOI] [PubMed] [Google Scholar]
- Burch JB, Svendsen E, Siegel PD, Wagner SE, von Essen S, Keefe T, Mehaffy J, Martinez AS, Bradford M, Baker L, Cranmer B, Saito R, Tessari J, Linda P, Andersen C, Christensen O, Reynolds SJ. Endotoxin exposure and inflammation markers among agricultural workers in Colorado and Nebraska. J Toxicol Environ Health A. 2010;73:5–22. doi: 10.1080/15287390903248604. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cleave J, Willson PJ, Town J, Gordon JR. Fractionation of swine barn dust and assessment of its impact on the respiratory tract following repeated airway exposure. J Toxicol Environ Health A. 2010;73:1090–1101. doi: 10.1080/15287394.2010.482916. [DOI] [PubMed] [Google Scholar]
- Collett J, Iovinelli Rl, Demoz B. A three-stage cloud impactor for size-resolved measurement of cloud drop chemistry. Atmos Environ. 1995;29:1145–1154. [Google Scholar]
- Comer DM, Elborn JS, Ennis M. Comparison of nasal and bronchial epithelial cells obtained from patients with COPD. PLoS ONE. 2012;7:e32924. doi: 10.1371/journal.pone.0032924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham KJ. Hazardous agents in agricultural dusts and methods of evaluation. Am J Indust Med. 1986;10:205–220. doi: 10.1002/ajim.4700100305. [DOI] [PubMed] [Google Scholar]
- Donham KJ, Cumro D, Reynolds SJ, Merchant JA. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: Recommendations for exposure limits. J Occup Environ Med. 2000;42:260–269. doi: 10.1097/00043764-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Duquenne P, Marchand G, Duchaine C. Measurement of endotoxins in bioaerosols at workplace: A critical review of literature and a standardization issue. Ann Occup Hyg. 2012;57:137–172. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB. Effects of aqueous extracts of PM10 filters from the Utah Valley on human airway epithelial cells. Am J Physiol-Lung Cell Mol Physiol. 1999;277:L960–L967. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- Gainet M, Thaon I, Westeel V. Twelve-year longitudinal study of respiratory status in dairy farmers. Eur Respir J. 2007;30:97–103. doi: 10.1183/09031936.00150405. [DOI] [PubMed] [Google Scholar]
- Garcia J, Bennett DH, Tancredi D, Schenker MB, Mitchell D, Reynolds SJ, Mitloehner FM. Occupational exposure to particulate matter and endotoxin for California dairy workers. Int J Hyg Environ Health. 2013;216:56–62. doi: 10.1016/j.ijheh.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol-Lung Cell Mol Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- Harting JR, Gleason A, Romberger DJ, Von Essen SG, Qiu F, Alexis N, Poole JA. Chronic obstructive pulmonary disease patients have greater systemic responsiveness to ex vivo stimulation with swine dust extract and its components versus healthy volunteers. J Toxicol Environ Health A. 2012;75:1456–1470. doi: 10.1080/15287394.2012.722186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D, Douwes J. Towards an occupational exposure limit for endotoxins. Ann Agric Environ Med. 1997;4:17–19. [Google Scholar]
- Holloway JW, Savarimuthu Francis S, Fong KM, Yang IA. Genomics and the respiratory effects of air pollution exposure. Respirology. 2012;17:590–600. doi: 10.1111/j.1440-1843.2012.02164.x. [DOI] [PubMed] [Google Scholar]
- Smith H, editor. ICRP. Annals of the ICRP: Human Respiratory Tract Model for Radiological Protection. I. Tarrytown: International Commission on Radiological Protection; 1994. p. 110. [PubMed] [Google Scholar]
- James AC, Stahlhofen W, Rudolf G, Egan MJ, Nixon W, Gehr P, Briant JK. The respiratory tract deposition model proposed by the ICRP task group. Radiat Protect Dosim. 1991;38:159–165. [Google Scholar]
- Kateman E, Heederik D, Pal TM, Smeets M, Smid T, Spitteler M. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health. 1990;16:428–433. doi: 10.5271/sjweh.1764. [DOI] [PubMed] [Google Scholar]
- Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indicators. 2008;8:1–13. [Google Scholar]
- Kullman GJ, Thorne PS, Waldron PF, Marx JJ, Ault B, Lewis DM, Siegel PD, Olenchock SA, Merchant JA. Organic dust exposures from work in dairy barns. Am Ind Hyg Assoc J. 1998;59:403–413. doi: 10.1080/15428119891010668. [DOI] [PubMed] [Google Scholar]
- Linaker C, Smedley J. Respiratory illness in agricultural workers. Occup Med. 2002;52:451–459. doi: 10.1093/occmed/52.8.451. [DOI] [PubMed] [Google Scholar]
- May S, Romberger DJ, Poole JA. Respiratory health effects of large animal farming environments. J Toxicol Environ Health, B. 2012;15:524–541. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsanejad R, Fields WR, Morgan WT, Bombick BR, Doolittle DJ. The time course of expression of genes involved in specific pathways in normal human bronchial epithelial cells following exposure to cigarette smoke. Exp Lung Res. 2008;34:513–530. doi: 10.1080/01902140802271826. [DOI] [PubMed] [Google Scholar]
- Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health, A. 2010;73:684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promega. CytoTox96 Non-Radioactive Cytotoxicity Assay: Instructions for Use of Product G1780. 2012 Available: http://www.promega.com/~/media/Files/Resources/Protocols/Technical%20Bulletins/0/CytoTox%20NonRadioactive%20Cytotoxicity%20Assay%20Protocol.pdf.
- Rask-Andersen A, Malmberg P, Lundholm M. Endotoxin levels in farming: Absence of symptoms despite high exposure levels. Br J Indust Med. 1989;46:412–416. doi: 10.1136/oem.46.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SJ, Clark ML, Koehncke N, von Essen S, Prinz L, Keefe TJ, Mehaffy J, Bradford M, Carnmer B, Davidson ME, Yang IV, Burch JB. Pulmonary function reductions among potentially susceptible subgroups of agricultural workers in Colorado and Nebraska. J Occup Environ Med. 2012;54:632–641. doi: 10.1097/JOM.0b013e31824d2e1c. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Donham KJ, Whitten P, Merchant JA, Burmeister LF, Popendorf WJ. Longitudinal evaluation of dose-response relationships for environmental exposures and pulmonary function in swine production workers. Am J Indust Med. 1996;29:33–40. doi: 10.1002/(SICI)1097-0274(199601)29:1<33::AID-AJIM5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Milton DK, Heederik D, Thorne PS, Donham KJ, Croteau EA, Kelly KM, Douwes J, Lewis D, Whitmer M, Connaughton I, Koch S, Malmberg P, Larsson BM, Deddens J, Saraf A, Larsson L. Interlaboratory evaluation of endotoxin analyses in agricultural dusts--comparison of LAL assay and mass spectrometry. J Environ Monit. 2005;7:1371–1377. doi: 10.1039/b509256f. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Nonnenmann MW, Basinas I, Davidson M, Elfman L, Gordon J, Kirychuck S, Reed S, Schaeffer JW, Schenker MB, Schlünssen V, Sigsgaard T. Systematic review of respiratory health among dairy workers. Agromedicine. 2013;18:219–243. doi: 10.1080/1059924X.2013.797374. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Thorne PS, Donham KJ, Croteau EA, Kelly KM, Lewis D, Whitmer M, Heederik DJ, Douwes J, Connaughton I, Koch S, Malmberg P, Larsson BM, Milton DK. Comparison of endotoxin assays using agricultural dusts. Am Indust Hyg Assoc J. 2002;63:430–438. doi: 10.1080/15428110208984731. [DOI] [PubMed] [Google Scholar]
- Romberger D, Bodlak V, Von Essen S, Mathisen T, Wyatt T. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93:289–296. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- Ross A, Dailey L, Brighton L, Devlin R. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2007;37:169–185. doi: 10.1165/rcmb.2006-0466OC. [DOI] [PubMed] [Google Scholar]
- Schaeffer J, Van Dyke-Gonnerman A, Davidson M, Reynolds S. Particle size characterization of agricultural aerosols in two Colorado dairies using a high-volume cloud impact. Poster: AIHce; 2013; Montreal, Canada. 2013. [Google Scholar]
- Schenker M. Exposures and health effects from inorganic agricultural dusts. Environ Health Persp. 2000;108(Suppl 4):661–664. doi: 10.1289/ehp.00108s4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Hinderliter PM, Orr G, Thrall BD, Pounds JG. Particokinetics In Vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci. 2007;95:300–312. doi: 10.1093/toxsci/kfl165. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Perry SS, Saito R, O’Shaughnessy PT, Mehaffy J, Metwali N, Keefe T, Donham KJ, Reynolds SJ. Evaluation of the Limulus amebocyte lysate and recombinant factor C assays for assessment of airborne endotoxin. Appl Environ Microbiol. 2010;76:4988–4995. doi: 10.1128/AEM.00527-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckens J, Dailey L, Walters G, Devlin RB. Direct particle-to-cell deposition of coarse ambient particulate matter increases the production of inflammatory mediators from cultured human airway epithelial cells. Environ Sci Technol. 2009;43:4595–4599. doi: 10.1021/es900698a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of culture respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, DeVasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cε in human bronchial epithelial cells. Am J Physiol - Lung Cell Mol Physiol. 2007;293:L1163–L1170. doi: 10.1152/ajplung.00103.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.