Abstract
Exposure to rotenone in vivo results in selective degeneration of dopaminergic neurons and development of neuropathological features of Parkinson's disease. As rotenone acts as an inhibitor of mitochondrial respiratory complex I, we employed oxidative lipidomics to assess oxidative metabolism of a mitochondria-specific phospholipid, cardiolipin, in substantia nigra of exposed animals. We found a significant reduction of oxidizable PUFA-containing cardiolipin molecular species. We further revealed increased contents of mono-oxygenated cardiolipin species at late stages of the exposure. Notably, linoleic acid in sn-1 position was the major oxidation substrate yielding its mono-hydroxy- and epoxy-derivatives whereas more readily “oxidizable” fatty acid residues (arachidonic, docosahexaenoic acids) – remained non-oxidized. Elevated levels of PUFA cardiolipins were detected in plasma of rats exposed to rotenone. Characterization of oxidatively modified cardiolipin molecular species in substantia nirga and detection of PUFA-containing cardiolipin species in plasma may contribute to better understanding of the Parkinson's disease pathogenesis and lead to the development of new biomarkers of mitochondrial dysfunction associated with this disease.
Keywords: Parkinson's disease, cardiolipin, oxygenated cardiolipin species, oxygenated linoleic acid
Introduction
Parkinson's disease is a neurodegenerative disorder in the elderly characterized by the loss of dopaminergic neurons in substantia nigra (SN) [1]. Mitochondrial dysfunction and oxidative stress are believed to be important contributors to the neuronal loss and the pathogenesis of Parkinson's disease [2-6]. Decreased activity of mitochondrial complex I [7], reduced amounts of GSH [8], protein modification [9], DNA damage [10] and lipid oxidation [11] have been documented in the substantia nigra compacta of patients with Parkinson's disease in many studies. While polyunsaturated phospholipids are the major substrates for oxidative modifications [12], evaluation of lipid peroxidation products has been restricted to detection of secondary oxidation products such as 4-hydroxy-2-nonenal [13, 14] and essential information on molecular targets, particularly specific polyunsaturated molecular species of phospholipids undergoing oxidation and leading to mitochondrial dysfunction and their association with Parkinson's disease, is lacking.
Given that oxidation products formed from polyunsaturated molecular species of a mitochondria-specific phospholipid, cardiolipin (CL) have been identified as important cell death signals [12, 15], their systematic analysis in PD-relevant samples may be particularly important. However, reliable identification and quantitation of these products even using sensitive contemporary LC/MS protocols, is challenging due to the inherent instability, fast metabolic conversions as well as high diversification of oxidized species resulting in their low steady-state concentrations [16]. We have developed several advanced techniques of oxidative lipidomics that allowed physical separation of oxidized and non-oxidized phospholipids as well as accurate identification and quantitative analysis of oxygenated molecular species of phospholipids by using liquid chromatography (LC) or high-performance thin-layer chromatography (HPTLC) protocols [17-19] along with different versions of mass spectrometry (MS) combined with enzymatic hydrolysis of fatty acid residues from modified phospholipids [20]. Here, using a rat rotenone model of Parkinson's disease [21] and oxidative lipidomics approach we were able, for the first time, to identify and quantitatively characterize oxygenated molecular species of CL formed in dysfunctional mitochondria in SN as well as in CL in plasma.
Methods
Rat rotenone model
The Institutional Animal Care and Use Committee of the University of Pittsburgh approved all experiments utilizing animals. Male Lewis rats (7-9 months old, Charles River) were injected intraperitoneally with vehicle or 3.0 mg/kg/day rotenone (Sigma-Aldrich) either once, for five daily injections or treated to parkinsonian endpoint. We chose to evaluate one and five daily injection paradigms because we have previously detected mitochondrial DNA damage in the substantia nigra and peripheral tissues following rotenone treatment at these time points [22, 23]. Animals treated with rotenone to parkinsonsian endpoint recapitulate many of the key pathological features of PD [24, 25]. Endpoint rotenone treated animals (10-14 days) were sacrificed when animals displayed behavioral features including bradykinesia, postural instability/gait disturbances and rigidity. Rat brains were first removed from the skull and rinsed in cold 1X PBS to remove any surface blood. Brains were placed on a cold petri dish and cut in half into the right and left hemisphere. Using a blade and forceps precise micro-dissection of the ventral midbrain was performed and the tissue was flash frozen in liquid nitrogen and stored at -80°C.
Extraction of lipids
Lipids were extracted from SN and plasma using the Folch and Bligh Dyer procedures, respectively [26, 27]. Lipid phosphorus was determined by a micro-method [28].
Analysis of esterified fatty acids
To release esterified fatty acids, total lipids were treated with either phospholipase A1 (PLA1) from Thermomyces lanuginosus (10 μl/μmol of phospholipids) (Sigma-Aldrich, St. Louis, MO) or phospholipase A2 (PLA2) from porcine pancreas (10 U/μmol of phospholipids) (Sigma-Aldrich, St. Louis, MO) in 0.5 M borate buffer, pH 9.0 containing 20 mM cholic acid, 2 mM CaCl2 and 100 μM DTPA for 60 min at 37°C. Under these conditions, almost 99% of phospholipids were hydrolyzed. Liberated oxygenated and non-oxygenated fatty acids were separated from lipids and lyso-lipids by solid phase extraction using phospholipid removing plates (Phenomenex, Torrance, CA) and analyzed by LC/MS as described [29]. Briefly, LC/MS in negative mode was performed using a Dionex Ultimate™ 3000 HPLC coupled on-line to a Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA). Fatty acids were separated on a reverse phase column (C18 Luna, 3 μm, 150 × 2 mm, Phenomenex, Torrance, CA) with flow rate 0.2 mL/min using gradient solvents containing 5 mM ammonium acetate (A: tetrahydrofuran/methanol/water/CH3COOH, 25:30:50:0.1 (v/v/v/v) and B: methanol/water 90:10 (v/v)). The column was eluted first 3 min isocratically at 50% B, from 3 to 23 min with a linear gradient from 50% solvent B to 98% solvent B, then 23-40 min isocratically using 98% solvent B, 40-42 min with a linear gradient from 98% solvent B to 50% solvent B, 42-60 min isocratically using 50% solvent B for equilibration of the column. Standards of oxygenated fatty acids were purchased from Cayman Chemical Co. (Ann Arbor, MI).
Analysis of CL
LC/MS was performed as previously described [29]. Briefly, LC/MS in negative mode was performed using a Dionex Ultimate™ 3000 HPLC coupled on-line to a linear ion trap mass spectrometer (LXQ, ThermoFisher Scientific, San Jose, CA). Thus, m/z values for CL molecular species were presented to 1 decimal place. Total lipids were separated on a normal phase column (Silica Luna 3 μm, 100A, 150×2 mm, (Phenomenex, Torrance CA)) with flow rate 0.2 mL/min using gradient solvents containing 5 mM CH3COONH4 (A – n-hexane:2-propanol:water, 43:57:1 (v/v/v) and B - n-hexane:2-propanol:water, 43:57:10 (v/v/v). Tetra- myristoyl – CL (TMCL) (Avanti polar lipids, Alabaster, AL) was used as an internal MS standard.
Analysis of oxygenated CL
CL was separated by two-dimension high-performance thin-layer chromatography (2D-HPTLC) [30] and CL and oxygenated CL were analysed by LC/MS as described [18]. To prevent lipid oxidation during separation, chromatography was performed under N2 conditions on diethylenetriaminepentaacetic acid (DTPA) treated silica plates (5×5cm, Whatman). LC/MS in negative mode was performed using a Dionex UltimateTM 3000 RSLCnano system coupled online Q-Exactive hybrid quadrupole-orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA) using a C8 column (Luna 3 μm, 100 Å, 150 × 2mm, Phenomenex, Torrance, CA) with flow rate 0.15 mL/min using an isocratic solvent system consisting of 2-propanol: water: triethylamine: acetic acid, 45:5:0.25:0.25, v/v. The resolution was set up at 140000 which corresponds to 5 ppm in m/z measurement error. Thus, m/z values for CLs and their oxidation species were presented to 4 decimal places. TMCL (Avanti polar lipids, Alabaster, AL) was used as an internal MS standard. TMCL molecular species is not usually present in the brain (and other tissues) and does not interfere with the endogenous CLs and widely used as an internal standard [31]. The ionization efficiencies of individual molecular species of CLs, particularly of those with differing fatty acid chains, may be different. To minimize the potential inaccuracies, the tuning of mass spectrometers was performed using TLCL, (C18:2)4-CL. In addition, TLCL was also utilized as a reference standard to build calibration curves employed for quantitative assessments of CLs in the brain. Finally, we were able to compare the total amounts of CLs in SN samples based on LC-MS analysis and summation of individual molecular species with that obtained from the direct determinations after 2D-HPTLC separation of total phospholipid extracts. These comparisons showed good co-incidence of the CL amounts determined in two independent ways.
Statistics
The results are presented as mean ± S.D. values from at least three experiments, and statistical analyses were performed by either paired/unpaired Student's t-test or one-way ANOVA. The statistical significance of differences was set at p< 0.05
Results
Rotenone is a highly lipophilic compound that can crosses the blood-brain barrier [32, 33]. Its toxicity mechanisms are mostly associated with the binding to and inhibiting electron transport at the level of complex I and generation of superoxide radicals [15, 34, 35]. These mitochondria-related effects lead to selective degeneration of dopaminergic neurons and produce neuropathological features of Parkinson's disease [21]. Therefore, our oxidative lipidomics efforts to detect mitochondria-specific modification of lipids were focused on the analysis of CLs in SN of rats exposed to rotenone. The flow-chart representing our analytical approach is shown in Fig. 1.
Figure 1.
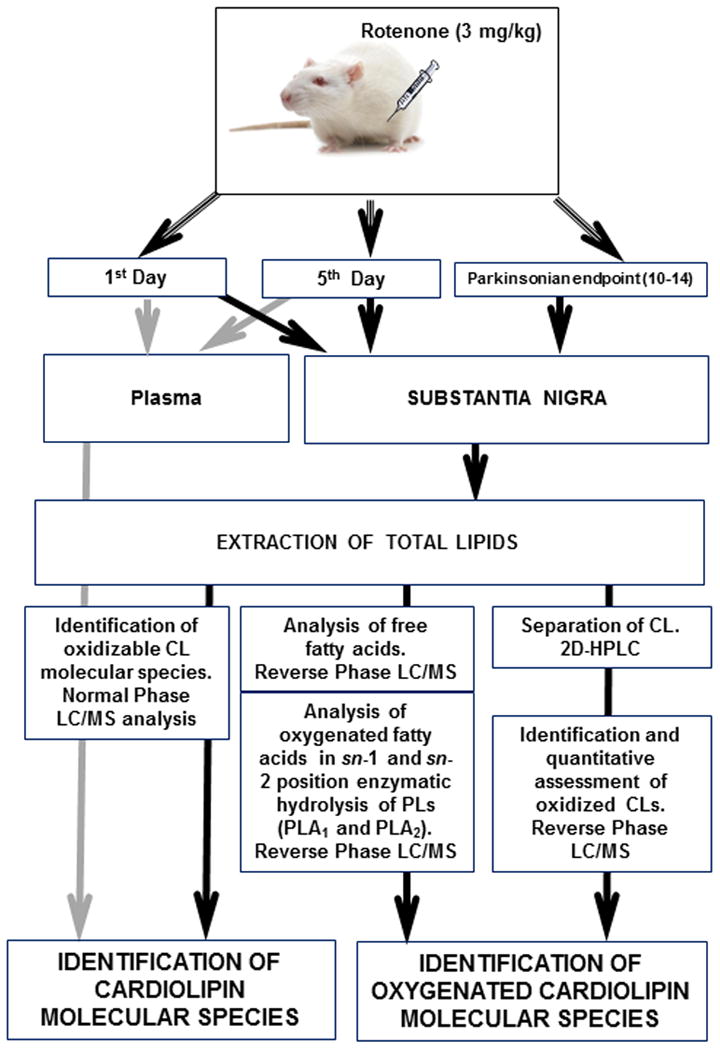
The flowchart representing analytical approach to assess CL and its oxygenated species in plasma and SN.
Because of high diversification of oxidatively modified phospholipids with potentially low content of each of them, we chose to simplify the analytical task by reducing the number of oxygenated molecular species. To this end, we treated the total lipid extracts with either PLA1 or PLA2 to release fatty acid residues from sn-1 and sn-2 position of phospholipids, respectively. This allowed the detection of oxidation products in a limited number of molecular species of oxidizable polyunsaturated fatty acids (PUFA). PUFA were mainly represented by linoleic (C18:2), arachidonic (C20:4), and docosahexaenoic (C22:6) acids and predominantly localized in sn-2 position (Fig. 2). Surprisingly, while the content of PUFA in sn-1 position was significantly lower than in sn-2, oxygenated fatty acid species were released almost exclusively upon PLA1 treatment (Fig. 3). Furthermore, among the PUFAs released, linoleic acid (C18:2, with 2 double bonds) underwent oxidative modification to mono-hydroxy- (HODE) and epoxy- (EpOME) derivatives (Fig. 3), whereas more polyunsaturated, hence more readily “oxidizable” fatty acid residues such as C20:4, and C22:6 – remained non-oxidized. A substantial increase of epoxy-molecular species of C18:2 was detected on day 5 and parkinsonsian endpoint (Fig. 3); however the changes in the content of hydroxy-species of C18:2 were significant only at parkinsonsian endpoint. No accumulation of oxygenated C18:2 was observed at day 1 of exposure (Fig. 3). No oxygenated PUFAs were detected in samples obtained from rats exposed to rotenone and treated with PLA2 (data not shown). In major classes of phospholipids including phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine, oxidizable polyunsaturated fatty acids (C18:2, C20:4 and C22:6) occupy predominantly the sn-2 position whereas in CL they can be equally distributed between sn-1 and sn-2 positions [18]. This suggests that oxygenated esterified C18:2 detected in SN of rotenone treated rats likely originated from the sn-1 position of CL.
Figure 2.
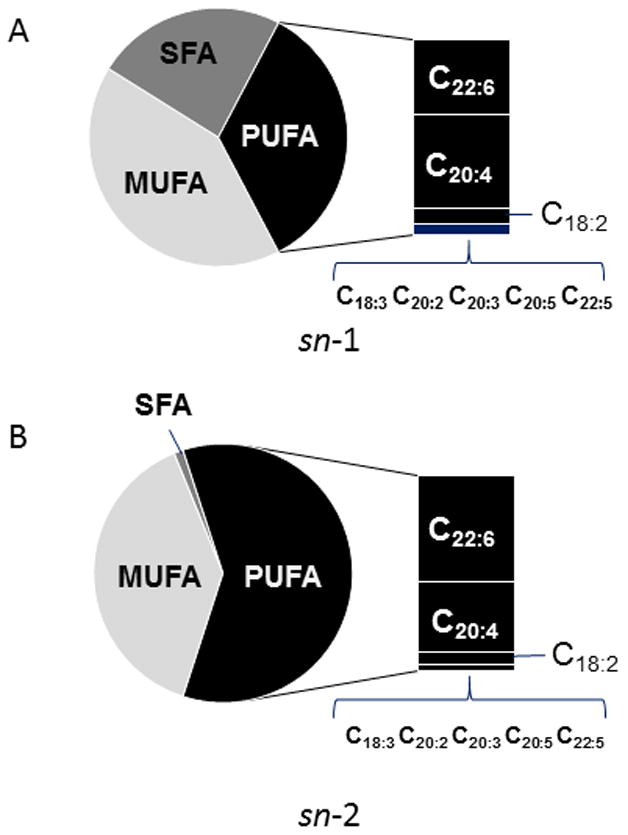
Profile of fatty acids liberated from total phospholipids extracted from SN by either PLA1 (A, sn-1 position) or PLA2 (B, sn-2 position). SFA – saturated fatty acids, MUFA – monounsaturated fatty acids, PUFA – polyunsaturated fatty acids; C18:2 - octadecadienoic acid (linoleic acid); C18:3 - octadecatrienoic acid, C20:2 – eicosadienoic acid, C20:3 - eicosatrienoic acid, C20:4 - eicosatetraenoic acid (arachidonic acid), C20:5 - eicosapentaenoic acid, C22:5 - docosapentaenoic acid, C22:6 - docosahexaenoic acid.
Figure 3.
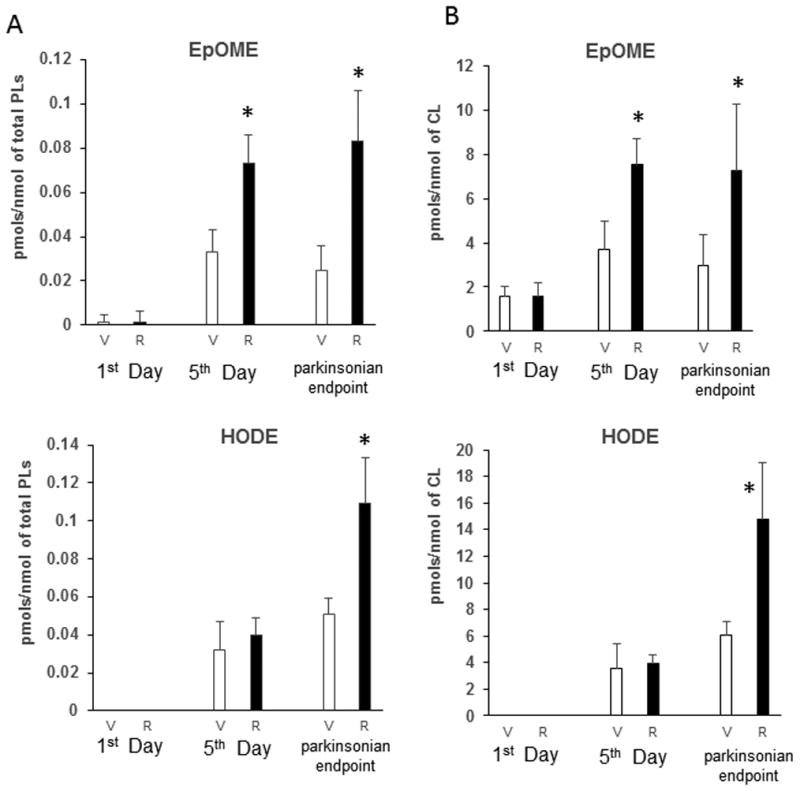
Quantitative assessment of oxygenated C18:2 (octadecadienoic acid) liberated by PLA1 from SN total phospholipids. HODE – hydroxy-species of C18:2, EpOME- epoxy-species of C18:2. Released oxygenated fatty acids were analyzed by reverse phase LC/MS after solid phase extraction. Data are normalized (A) per nmol of total phopsholipids (PLs) and (B) per nmol of cardiolipin (CL).
Therefore, we further specifically focused on the analysis of CLs in SN. Using normal phase LC followed by full MS and MS2 analysis, we identified oxidizable CL molecular species containing fatty acids with two – six double bonds (Table 1, Figs. 4A, 4B). Quantitative assessments revealed a substantial reduction of these species in SN of rotenone exposed rats at all-time points studied compared to control animals (Fig. 4C). Notably, all these polyunsaturated CL species contained at least one C18:2 residue. No changes in the CL content in from cortex of the same rats were detected (data not shown). Therefore, we suggested that decrease in the content of oxidizable CL was linked to rotenone-induced damage of mitochondria in SN.
Table 1.
Major CL and CL ox species detected in substantia nigra and plasma from rats exposed to rotenone.
| m/z | CN:DB | CL molecular species | Mono-oxygenated CL species in substantia nigra (m/z) | CL species detected in plasma (m/z) |
|---|---|---|---|---|
|
| ||||
| 1425.9771 | 70:5 | 16:1/18:1/18:1/18:2 | 1441.9735 | 1425.9832 |
| 16:0/18:2/18:1/18:2 | ||||
|
| ||||
| 1427.9966 | 70:4 | 16:0/18:1/18:1/18:2 | 1443.9903 | 1427.9962 |
| 16:1/18:1/18:1/18:1 | ||||
| 16:0/18:2/18:1/18:1 | ||||
|
| ||||
| 1430.0077 | 70:3 | 16:0/18:2/18:1/18:0 | 1446.0038 | 1430.0088 |
| 16:0/18:1/18:1/18:1 | ||||
| 16:1/18:1/18:1/18:0 | ||||
|
| ||||
| 1454.0059 | 72:5 | 18:1/18:1/18:1/18:2 | 1470.0074 | 1454.0051 |
|
| ||||
| 1456.0260 | 72:4 | 18:1/18:1/18:0/18:2 | 1472.0189 | 1456.0249 |
|
| ||||
| 1475.9943 | 74:8 | 18:1/18:1/18:2/20:4 | 1491.9891 | 1475.9922 |
| 18:0/18:2/18:2/20:4 | ||||
|
| ||||
| 1478.0067 | 74:7 | 18:0/18:1/18:2/20:4 | 1494.0033 | 1478.015 |
| 18;1/18:1/18:1/20:4 | ||||
| 18:1/18:2/18:0/20:4 | ||||
| 16:0/18:1/18:2/22:4 | ||||
| 16:1/18:0/18:2/22:4 | ||||
| 16:0/18:1/18:0/22:6 | ||||
| 16:1/18:0/18:0/22:6 | ||||
|
| ||||
| 1499.9929 | 76:10 | 18:1/20:4/18:1/20:4 | 1515.9889 | ND |
| 18:2/18:0/18:2/22:6 | ||||
| 18:1/18:1/18:2/22:6 | ||||
|
| ||||
| 1502.0061 | 76:9 | 18:1/18:2/18:0/22:6 | 1518.0026 | ND |
| 18:0/18:2/18:1/22:6 | ||||
| 18:1/18:1/18:1/22:6 | ||||
| 18:1/20:2/18:0/20:4 | ||||
| 18:2/20:3/18:0/20:4 | ||||
| 18:1/20:3/18:1/20:4 | ||||
CL – cardiolipin; CN and DB refer to the total carbon atoms in the fatty acid chains and total number double bonds, respectively; ND – not detected
Figure 4.
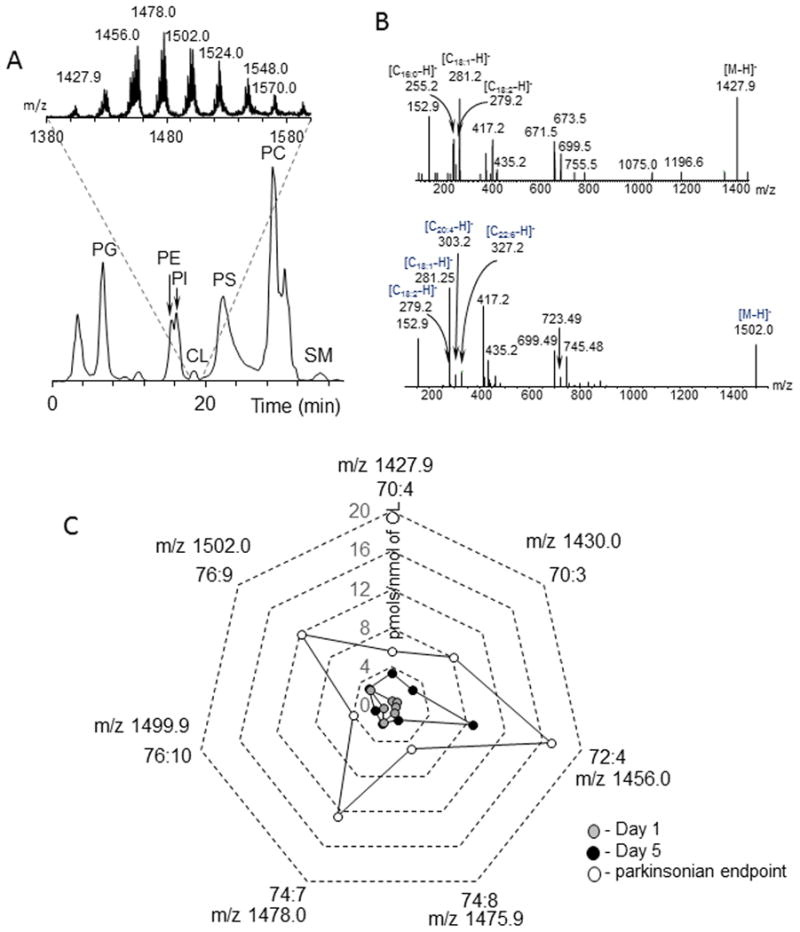
Assessment of CL in SN by normal phase LC/MS. A. Typical normal phase LC/MS chromatogram of phospholipids and full mass spectrum of CL (insert) extracted from SN obtained from control rats. Data were acquired in negative mode. CL - cardiolipin, PA - phosphatidic acid, PC - phosphatidylcholine, PE - phosphatidylethanolamine , PG - phosphatidylglycerol, PI - phosphatidylinositol, PS - phosphatidylserine, SM - sphingomyelin. B. MS2 fragmentation of CL molecular ions with m/z 1427.9 (upper panel) and m/z 1502.0 (lower panel). C. Quantitative assessments of CL in SN of rotenone exposed rats. The data are presented as rotenone-induced decreases in the amounts of oxidizable CL molecular species.
Assuming that lipid peroxidation can contribute to the depletion of oxidizable CL we further characterized oxygenated CL species in SN. CLs were pre-separated by HPTLC and subjected to reverse phase LC to resolve non-oxidized vs oxidized CL species after which high mass accuracy MS was employed to identify CL oxygenated species (Fig. 5). We detected significantly increased content of mono-oxygenated CL species at parkinsonsian endpoint (Fig. 6). Quantitative analysis revealed accumulation of several mono-oxygenated CL species (m/z 1443.9903, m/z 1446.0038, m/z 1472.0189, m/z 1491.9891, m/z 1494.0033, m/z 1515.9889 and m/z 1518.0026) (Fig.6) which originated from oxidizable CL species (m/z 1427.9966, m/z 1430.0077, m/z 1456.0260, m/z 1475.9943, m/z 1478.0067, m/z 1499.9929 and m/z 1502.0061). Notably, all of these species were reduced by rotenone exposure and contained at least one C18:2 residue (Table 1, Fig. 3). No accumulation of oxygenated CL in SN on day 1 was detected (data not shown).
Figure 5.
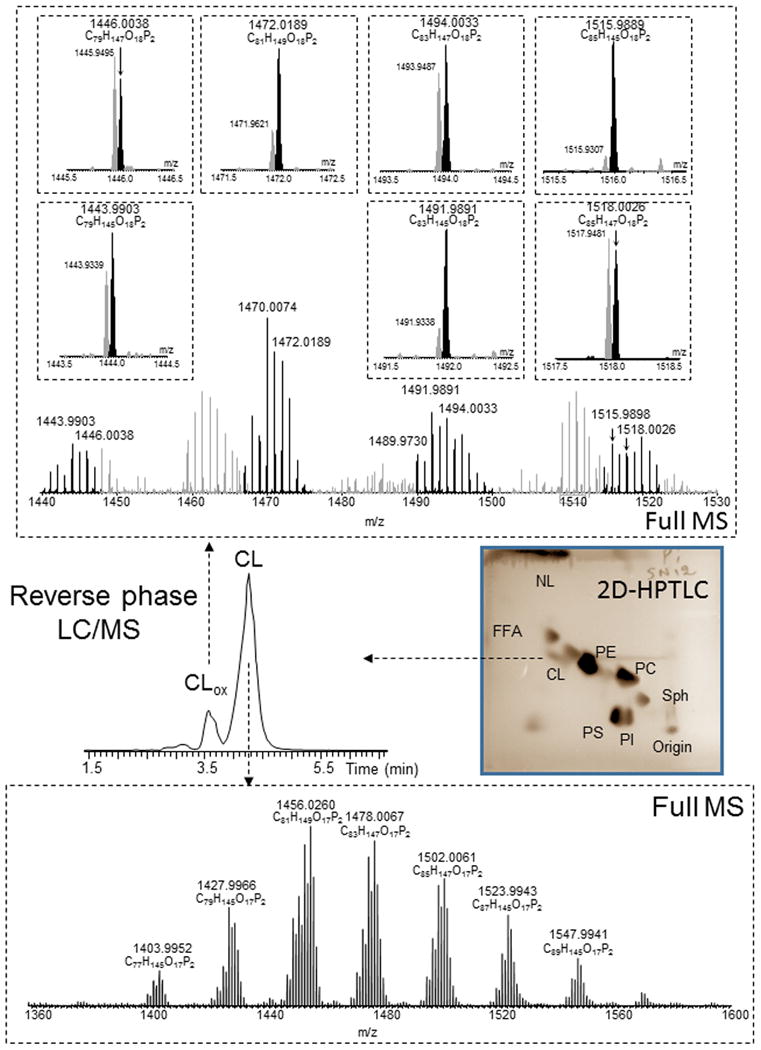
Detection and identification of CL oxygenated species in rat SN. Typical HPTLC of total lipids, LC/MS profile and mass spectra of CL and its oxygenated species obtained from SN of rat exposed to rotenone (parkinsonian endpoint). CL was isolated by 2D-HPTLC. The CL fraction was then subjected to reverse phase LC/MS analysis using a C8 column (4.6 mm × 15 cm). CL and oxygenated CL were separated using an isocratic solvent system (see method section). Under these conditions, oxygenated CL eluted prior to CL. Molecular species of oxygenated CL were identified as mono-oxygenated species based on exact m/z ratios. CL - cardiolipin, FFA – free fatty acids, NL – neutral lipids, PA - phosphatidic acid, PC - phosphatidylcholine, PE - phosphatidylethanolamine, PG - phosphatidylglycerol, PI - phosphatidylinositol, PS - phosphatidylserine, SM - sphingomyelin.
Figure 6.
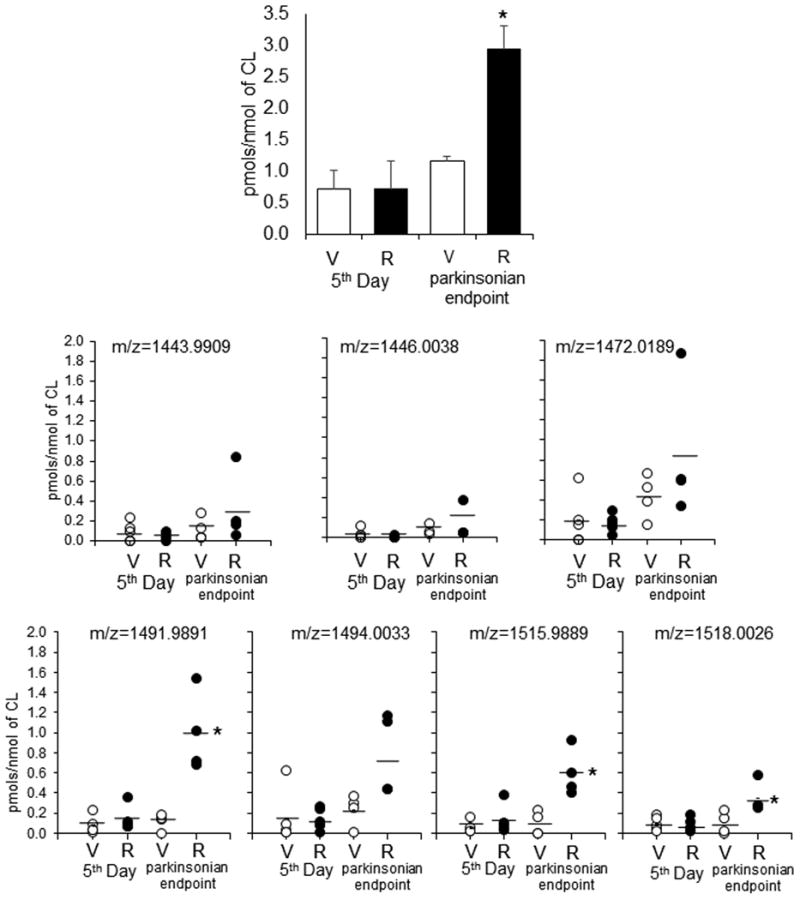
Quantitative assessment of total (A) and individual oxygenated molecular species of CL (B) in SN of control and rotenone treated rats. V-vehicle; R-rotenone. Molecular species with m/z 1443.9903, m/z 1446.0038, m/z 1472.0189, m/z 1491.9891, m/z 1494.0033, m/z 1515.9889, m/z 1518.0026 are originated from molecular species with m/z 1427.9966, m/z 1430.0077, m/z 1456.0260, m/z 1475.9943, m/z 1478.0067, m/z 1499.9929, m/z 1502.0061, respectively, after addition of one oxygen. Data are mean ± SD, n=4-5. *p<0.05 vs respective control (vehicle).
Assuming that mitochondria with their CLs and oxidized CLs can be released from damaged cells into extracellular environments and act as damage associated molecular patterns (DAMPs) [36] we performed analysis of CL in plasma of rats at two time points (day 1 and day 5) after the exposure to rotenone. MS analysis revealed the increase of relative intensity for CL molecular species with m/z 1425.9832, m/z 1427.9962, m/z 1430.0088, m/z 1454.0051, m/z 1456.0249, m/z 1475.9922 and m/z 1478.0150 (Fig. 7). Relative intensity for several individual CL molecular species as well as their total content were significantly higher on days 1 and 5 compared to respective controls (Fig 7B, C). All CL in plasma were represented by non-oxygenated molecular species. The sensitivity of LC/MS assay for oxygenated CL is approximately 10 nM. Given that the observed levels of oxygenated CL in rotenone-treated rats constituted only 0.2% of its total content in the brain, the expected increased levels in plasma would be on the order of 0.3 nM. Assuming that metabolic conversions of oxygenated CL (for example by lipoprotein associated Lp-PLA2 [18]) would result in its hydrolysis, the contents of oxygenated CL would be even lower.
Figure 7.
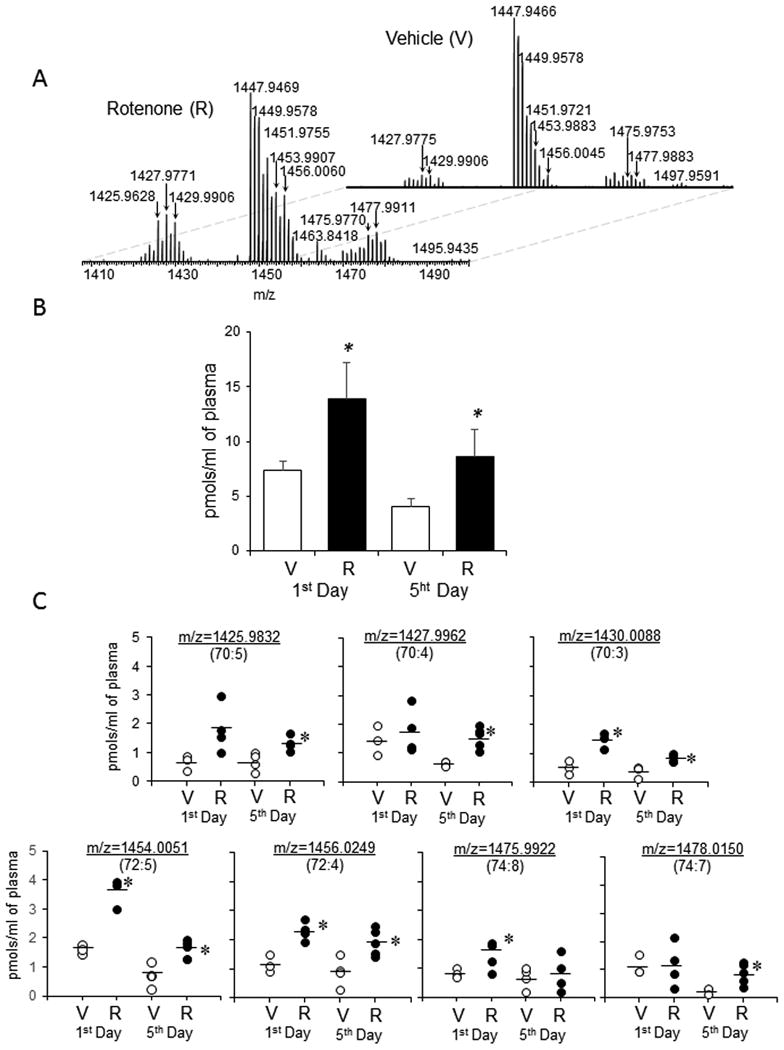
Detection, identification and quantification of CL in rat plasma. Plasma lipids were separated by normal phase LC and detected by using orbitrap Q-Exactive. A. Typical mass spectra of CL obtained from control rats and rats exposed to rotenone. Quantitative assessment of CL (B) and its individual molecular species (C) in rat plasma. V-vehicle; R-rotenone. Data are means ± SD, n=3-5. *p<0.05 vs respective control (vehicle). Mass spectra of CL were acquired using a Q-Exactive orbitrap mass spectrometer. Thus, m/z values for CL species were presented to 4 decimal places. Arrows indicate the CL species that accumulated in plasma.
Discussion
Oxidatively modified phospholipids have been recognized as important signals in acute injury and chronic diseases [37-39]. CL is a negatively charged phospholipid with four fatty acid residues [40]. In normal conditions CL is found exclusively in the inner-mitochondrial membrane where it accounts for 25% of all phospholipids [41] and essential for normal functions of many proteins, including the activity of respiratory complexes [42-45]. Accumulation of oxygenated CL molecular species and their hydrolysis products has been demonstrated for acute brain injury caused by trauma and cardiac arrest [46, 47], hyperoxic and nanoparticle induced lung injury [48, 49] as well as acute irradiation syndrome [18, 29, 50]. Polyunsaturated CLs in the brain have been considered not only as oxidation substrates [12] but also as a source of oxygenated lipid mediators [18 , 46, 51].
Rotenone - a known inhibitor of complex I in mitochondria – has been used to mimic clinical features of Parkinson's disease [1]. The rotenone model is characterized by slow and progressive loss of dopaminergic neurons and formation of Lewy bodies in SN [1]. Degeneration of dopaminergic neurons in SN has been long associated with mitochondrial dysfunction. Given that disrupted electron transport may be linked to the enhanced ROS (reactive oxygen species) production in mitochondria [52], we sought to test whether this would result in oxidative modifications of a susceptible phospholipid target, CL, highly concentrated in close proximity to complex I in the inner mitochondrial membrane [53, 54]. Using the rat rotenone model of Parkinson's disease, here, we demonstrate, for the first time, the accumulation of CL oxygenated species in SN.
Two major pathways triggered in dysfunctional mitochondria - mitophagy and apoptosis – may be involved in responses of dopaminergic neurons to rotenone in SN [55-57]. For both pathways CLs have been recognized as signaling molecules [16, 58]. In the case of pro-survival mitophagy CL is externalized to the outer leaflet of the outer mitochondrial membrane but does not undergo oxidation [58]. The externalized CL acts as a signal recognized by LC3 to facilitate the elimination of damaged mitochondria, and rescue the cell [58]. In contrast, accumulation of oxygenated CL species has been identified as an early event in the execution of apoptotic program [15] resulting in the release of cyt c into the cytosol and, activation of caspases and cell death [15]. Our oxidative lipidomics protocols revealed the early loss of oxidizable linoleic acid-containing CL species (on day 1 and 5 after exposure to rotenone) and accumulation of CL oxidation products in the same molecular species in SN on the parkinsonsian endpoint. Of note, in this model animals received rotenone daily, thus the total dose of rotenone was increasing during the exposure. This is compatible with our previous observations in vitro demonstrating that small doses of rotenone stimulated mitophagy in primary cortical neurons without CL oxidation [58]. However, at higher doses, rotenone caused CL oxidation and activation of the apoptotic cell death pathway. It is tempting to speculate that at the early time point, the mitophageal pathway was activated in SN neurons as a rescue mechanism which transitioned to triggering the apoptotic death as the damage was enhanced by increasing doses of rotenone at later time points.
Analysis of CL oxidation products in SN revealed unusual features of rotenone-associated oxidation: i) exclusive accumulation of oxygenated C18:2 in sn-1 rather than sn-2 position and ii) selective generation of mono-oxygenated CL species. In line with this, our previous studies identified C18:2, located in sn-1 position of CL as the major oxidation substrate in rotenone-treated lymphocytes [20]. Notably, mono-oxygenated-CL species were the major products detected in rotenone-exposed lymphocytes [20]. While the mechanisms of this unusual specificity towards C18:2 species of CLs remain to be elucidated, they are indicative of enzymatic rather than random stochastic free radical oxidation pathways. One of the candidate catalysts in oxidation of CL is cytochrome c with its established role during apoptosis [15]. Oxidizing equivalents (O2•- -->H2O2) formed in mitochondria during apoptosis feed the peroxidase activity of cytochrome c/CL complexes resulting in the selective depletion of oxidizable CL species in mitochondria and the accumulation of oxygenated CLs [15]. Alternatively, 12/15-lipoxygenase driven mechanisms may be enacted in damaged mitochondria to cause CL oxidation [59]. In the latter case, however, the selectivity to phospholipid substrates (CLs) should be less pronounced. Following oxidation, the hydrolysis reactions – possibly catalyzed by phospholipase A2 – can be involved in depletion of oxidizable CL species in SN of rotenone-treated rats. Recently, we discovered a new biosynthetic pathway for lipid mediators activated in vivo after acute tissue injury realized by oxidation and hydrolysis of oxygenated CLs species by mitochondrial Ca2+-independent iPLA2γ [18]. Several reports indicate that iPLA2-Vi, a calcium-independent PLA2, may be implicated in the pathogenesis of Parkinson's disease [60-62]. Mutation of PLA2G6, encoding calcium-independent PLA2, is characteristic of PD and results in the suppression of the enzymatic activity [60]. There are only few publications on the effect of rotenone on iPLA2 activity in mitochondria. In studies on isolated lung and liver mitochondria, rotenone treatment caused the release of PUFA which was partially sensitive to R-BEL [63, 64]. Our in vivo studies, however, did not document higher levels of free fatty acids or oxygenated free fatty acids in SN of rotenone-treated rats vs control rats. Accordingly, with the exception of lyso-phosphatidylcholine, no accumulation of other lyso-phospholipids have been observed.
While apoptosis-driven CL oxidation may be accountable for the observed accumulation of oxidized CL, it is likely that non-apoptotic cell death pathways are also triggered in rotenone-treated animals resulting in the release of damaged mitochondria. This may explain the detected higher levels of PUFA CL species in plasma. Indeed, execution of necroptotic program has been shown to trigger release of mitochondria into extra-cellular compartments [65].
In conclusion, we demonstrated that exposure of rats to rotenone causes depletion of PUFA CL and accumulation of mono-oxygenated CL species in SN and the emergence of elevated levels of CL in plasma. These metabolic CL changes could reflect rotenone-induced mitochondrial dysfunction including mitophageal response at the early time points and enzymatic CL oxidation during execution of programmed cell death pathways (apoptosis, necroptosis) at the later stages after the exposure. Characterization of oxidatively modified CL molecular species in SN and detection of PUFA-containing CL species in plasma may contribute to better understanding of the Parkinson's disease pathogenesis and lead to the development of new biomarkers of mitochondrial dysfunction associated with this disease.
Acknowledgments
Supported by NIH: ES020693, ES020718, NS076511, NS061817, U19AIO68021, PO1 HL114453, NIOSH OH008282, HFSP-RGP0013/2014.
Footnotes
Declaration of Interests: The authors report no declarations of interest.
References
- 1.Sanders LH, Greenamyre JT. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radical Bio Med. 2013;62:111–20. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Z, Wood NW. Cell death pathways in Parkinson's disease: role of mitochondria. Antioxid Redox Signal. 2009;11:2135–49. doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- 3.Sherer TB, Greenamyre JT. Oxidative damage in Parkinson's disease. Antioxid Redox Signal. 2005;7:627–9. doi: 10.1089/ars.2005.7.627. [DOI] [PubMed] [Google Scholar]
- 4.Mogi M, Harada M, Kondo T, Mizuno Y, Narabayashi H, Riederer P, et al. The soluble form of Fas molecule is elevated in parkinsonian brain tissues. Neurosci Lett. 1996;220:195–8. doi: 10.1016/s0304-3940(96)13257-2. [DOI] [PubMed] [Google Scholar]
- 5.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–9. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 6.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. Journal of Parkinson's disease. 2013;3:461–91. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–7. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 8.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett. 1992;142:128–30. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 9.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998;57:338–42. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 11.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 12.Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, Florida: CRC Press; 1988. [Google Scholar]
- 13.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2696–701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Molecular and cellular biochemistry. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 15.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–32. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 16.Kagan VE, Chu CT, Tyurina YY, Cheikhi A, Bayir H. Cardiolipin asymmetry, oxidation and signaling. Chemistry and physics of lipids. 2014;179:64–9. doi: 10.1016/j.chemphyslip.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyurina YY, Domingues RM, Tyurin VA, Maciel E, Domingues P, Amoscato AA, et al. Characterization of cardiolipins and their oxidation products by LC-MS analysis. Chemistry and physics of lipids. 2014;179:3–10. doi: 10.1016/j.chemphyslip.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthu TS, et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nature chemistry. 2014;6:542–52. doi: 10.1038/nchem.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samhan-Arias AK, Ji J, Demidova OM, Sparvero LJ, Feng W, Tyurin V, et al. Oxidized phospholipids as biomarkers of tissue and cell damage with a focus on cardiolipin. Biochimica et biophysica acta. 2012;1818:2413–23. doi: 10.1016/j.bbamem.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyurina YY, Winnica DE, Kapralova VI, Kapralov AA, Tyurin VA, Kagan VE. LC/MS characterization of rotenone induced cardiolipin oxidation in human lymphocytes: implications for mitochondrial dysfunction associated with Parkinson's disease. Molecular nutrition & food research. 2013;57:1410–22. doi: 10.1002/mnfr.201200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre T. Rotenone model of Parkinson disease - Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–35. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 22.Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ, et al. Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson's disease. Neurobiology of disease. 2014;70:214–23. doi: 10.1016/j.nbd.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders LH, Howlett EH, McCoy J, Greenamyre JT. Mitochondrial DNA Damage as a Peripheral Biomarker for Mitochondrial Toxin Exposure in Rats. Toxicological sciences : an official journal of the Society of Toxicology. 2014 doi: 10.1093/toxsci/kfu185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez TN, Greenamyre JT. Toxin models of mitochondrial dysfunction in Parkinson's disease. Antioxid Redox Signal. 2012;16:920–34. doi: 10.1089/ars.2011.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG. Lessons from the rotenone model of Parkinson's disease. Trends in pharmacological sciences. 2010;31:141–2. doi: 10.1016/j.tips.2009.12.006. author reply 2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 28.Boettcher C, Pries C, Vangent CM. A Rapid and Sensitive Sub-Micro Phosphorus Determination. Anal Chim Acta. 1961;24:203. [Google Scholar]
- 29.Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, Epperly MW, et al. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiation research. 2011;175:610–21. doi: 10.1667/RR2297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–6. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 31.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. Journal of lipid research. 2005;46:1196–204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:484–94. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. BioEssays : news and reviews in molecular, cellular and developmental biology. 2002;24:308–18. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- 34.Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson's disease. IUBMB Life. 2001;52:135–41. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 35.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends in immunology. 2011;32:157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Aldrovandi M, O' Donnell VB. Oxidized PLs and vascular inflammation. Current atherosclerosis reports. 2013;15:323. doi: 10.1007/s11883-013-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–59. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashraf MZ, Kar NS, Podrez EA. Oxidized phospholipids: biomarker for cardiovascular diseases. The international journal of biochemistry & cell biology. 2009;41:1241–4. doi: 10.1016/j.biocel.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–3. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daum G, Lees ND, Bard M, Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 42.Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–80. [PubMed] [Google Scholar]
- 43.Sharpley MS, Shannon RJ, Draghi F, Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–8. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]
- 44.Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–63. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- 45.Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1990;265:19434–40. [PubMed] [Google Scholar]
- 46.Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, et al. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Annals of neurology. 2007;62:154–69. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 47.Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nature neuroscience. 2012;15:1407–13. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, Li J, et al. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyurina YY, Kisin ER, Murray A, Tyurin VA, Kapralova VI, Sparvero LJ, et al. Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS nano. 2011;5:7342–53. doi: 10.1021/nn202201j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free radical biology & medicine. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Kiebish MA, Han X, Seyfried TN. Examination of the brain mitochondrial lipidome using shotgun lipidomics. Methods in molecular biology. 2009;579:3–18. doi: 10.1007/978-1-60761-322-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochimica et biophysica acta. 2014;1837:408–17. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Bogdanov M, Mileykovskaya E, Dowhan W. Lipids in the assembly of membrane proteins and organization of protein supercomplexes: implications for lipid-linked disorders. Sub-cellular biochemistry. 2008;49:197–239. doi: 10.1007/978-1-4020-8831-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Celardo I, Martins LM, Gandhi S. Unravelling mitochondrial pathways to Parkinson's disease. British journal of pharmacology. 2014;171:1943–57. doi: 10.1111/bph.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palikaras K, Tavernarakis N. Mitophagy in neurodegeneration and aging. Frontiers in genetics. 2012;3:297. doi: 10.3389/fgene.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. Journal of Alzheimer's disease : JAD. 2014;42:S125–52. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 58.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nature cell biology. 2013;15:1197–205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coffa G, Brash AR. A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15579–84. doi: 10.1073/pnas.0406727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nature genetics. 2006;38:752–4. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu CS, Lai SC, Wu RM, Weng YH, Huang CL, Chen RS, et al. PLA2G6 mutations in PARK14-linked young-onset parkinsonism and sporadic Parkinson's disease. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2012;159B:183–91. doi: 10.1002/ajmg.b.32012. [DOI] [PubMed] [Google Scholar]
- 62.Kauther KM, Hoft C, Rissling I, Oertel WH, Moller JC. The PLA2G6 gene in early-onset Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2011;26:2415–7. doi: 10.1002/mds.23851. [DOI] [PubMed] [Google Scholar]
- 63.Jaburek M, Jezek J, Zelenka J, Jezek P. Antioxidant activity by a synergy of redox-sensitive mitochondrial phospholipase A2 and uncoupling protein-2 in lung and spleen. The international journal of biochemistry & cell biology. 2013;45:816–25. doi: 10.1016/j.biocel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Rauckhorst AJ, Broekemeier KM, Pfeiffer DR. Regulation of the Ca(2+)-independent phospholipase A2 in liver mitochondria by changes in the energetic state. Journal of lipid research. 2014;55:826–36. doi: 10.1194/jlr.M043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-alpha-induced necroptosis act as danger signals. Cell death & disease. 2014;5:e1312. doi: 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


