Abstract
Aim
Opioids are the most prescribed analgesics for moderate and severe pain management; however chronic use impairs host innate immune response and increases susceptibility to infection. Recently, autophagy has been shown to be an innate defense mechanism against bacterial infection. The effect of autophagy induced bacterial clearance following morphine treatment has not been previously investigated.
Methods
Autophagosomes were visualized by confocal microscopy following GFP-LC3 transfection and also by transmission electron microscopy. The relative protein levels were analyzed by Western blot. Macrophages were transfected with GFP-mcherry-LC3 simultaneously to monitor autolysosome formation and subsequent events that lead to degradation.
Results
Morphine treatment potentiated LPS-induced vesicular translocation of GFP-LC3 with a concurrent increase in LC3-II levels. In addition, morphine up-regulated LPS-induced Beclin1 level, but down-regulated Bcl-2 level. We further show that p38 MAP kinase signaling is required for autophagy activation. In contrast, morphine inhibited LPS-induced autophagosome maturation and autophagolysosomal fusion as indicated by the failure to recruit LAMP1 into autophagosome and reduced degradation of SQSTM1/p62 protein level. Morphine modulation of LPS-induced autophagosome maturation visualized using co-localization of GFP-mcherry-LC3 was TLR4 independent, but mediated through μ opioid receptor signaling. Correspondingly, morphine and LPS co-treatment significantly increased Streptococcus pneumoniae load, when compared with LPS treatment alone.
Conclusion
These observations imply that although morphine treatment facilitates LPS-induced autophagy, it inhibits autophagolysosomal fusion leading to decreased bacterial clearance and increased bacterial load. These observations support the increased susceptibility to infection and the prevalence of persistent infection in the drug abuse population.
Keywords: autophagosome, LPS, macrophage, maturation, morphine, TLR4
Introduction
Autophagy is a catabolic process that degrades cytoplasmic components within the lysosome. It serves as an essential cytoprotective response to pathologic stressors that occur during diseases such as cancer, ischemia, and infection(Murrow and Debnath, 2013). Three distinct types of autophagy have been described, macroautophagy, microautophagy and chaperone-mediated autophagy(Kaushik et al., 2008). Macroautophagy (herein referred to as autophagy) involves the formation of a double-membrane structure, called the autophagosome, and its subsequent maturation and fusion with lysosomes to form degradative autolysosomes, in which the sequestrated cytosolic contents and the autophagosomal inner membrane are degraded(Yang and Zhang, 2014). Recently, autophagy was found to participate in the host defense elimination of bacterial pathogens including group A Streptococcus (Nakagawa et al., 2004), Shigella flexneri (Kayath et al., 2010), and Mycobacterium tuberculosis(Deretic et al., 2009). Autophagy can be induced upon bacterial infection and involves the formation of double-membrane compartments (known as autophagosomes) around target bacteria and their transport to lysosomes for degradation(Shahnazari and Brumell, 2011). Macrophages, dendritic cells, and epithelial cells have a set of transmembrane receptors that recognize different types of pathogen-associated molecular patterns (PAMPs) called Toll-like receptors (TLRs). TLRs represent a conserved family of innate immune recognition receptors that play key roles in detecting microbes, initiating innate immune responses, and linking innate and adaptive immunity. Engagement of TLR signaling during phagocytosis recruits the autophagy microtubule-associated protein 1A/1B-light chain 3 (LC3) to phagosomes and promotes their maturation and microbial killing(Sanjuan et al., 2007). Previous studies have demonstrated the role of TLR4 as a sensor for autophagy associated with innate immunity. TLR4 is the receptor for lipopolysaccharide (LPS) which is a major component of the outer membrane of Gram-negative bacteria (Park and Lee, 2013).
Opioid compounds, such as morphine, produce powerful analgesia that is effective in treating various types of pain(Sacerdote, 2006). Our previous studies show that morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae (S. pneumoniae) lung infection(Wang et al., 2008). However, the mechanism by which morphine treatment modulates LPS-induced autophagolysosomal formation leading to bacterial killing is not known. The primary goal of the current study was to investigate whether modulation of the autophagic pathway in BMDMs leading to microbial activity through the induction of autophagy is a potential mechanism by which pathogen elimination is inhibited following morphine treatment. We show that morphine increases LPS-induced autophagy initiation and execution, through a mechanism that involves TLR4-p38 signaling. However, morphine decreased LPS-induced maturation of autophagosomes into degradative autolysosomes. These studies suggest that morphine treatment induce defective autophagic bacterial killing, leading to a compromised innate immune response.
Materials and Methods
Antibodies and Reagents
LPS(lipopolysaccharide), MTT(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), pepstatin A, E-64d, SB203580, SP600125 and beta-actin antibody were purchased from Sigma (St Louis, MO,USA). DyLight TM488-conjugated donkey anti-rabbit IgG was from Jackson Immuno Research Laboratories, Inc (West Grove, PA, USA). SuperFect Transfection Reagent was from QIAGEN (Valencia, CA, USA). Bcl-2 and Beclin1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). LC3, SQSTM1/p62 and Lysosome-associated membrane glycoprotein 1 (LAMP1) antibodies were from Cell Signaling (Danvers, MA, USA). Green fluorescent protein (GFP)-mCherry-LC3 construct was from Addgene Inc. (Cambridge, CA, USA).
Bone Marrow-Derived Macrophages (BMDMs) culture
Pathogen-free C57BL/6J wild-type (WT), C57BL/10ScNJ-Tlr4lps-del (TLR4 knockout) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). μ-opioid receptor knockout (MORKO) mice (C57BL/6129/Ola genetic background) were generated by Loh and colleagues(Roy et al., 1998). Briefly, a XhoI/XbaI fragment, which spans exons 2 and 3, was replaced with a Neor cassette, followed by the ligation of a thymidine kinase expression cassette to the 3′ end of this segment. All animals were maintained under specific pathogen-free (SPF) barrier conditions. All animal experiments were done in accordance with the Institutional Animal Care and Use Committee’s guidelines at the University of Minnesota. The protocol was approved by Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota. BMDMs were obtained by growing bone marrow cells from male mice (7-8 weeks) at 37°C in 5%CO2 for 7 days in Dulbecco Modified Eagle Medium (DMEM) with L-glutamine (Life Technologies, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT,USA), and 1% penicillin/ streptomycin (pen/strep) antibiotic solution(Life Technologies, Grand Island, NY, USA) in the presence of mouse macrophage colony stimulating factor recombinant protein(M-CSF, eBioscience, San Diego, CA, USA). Acquisition was determined on at least 100 phagosomes for each condition and expressed as a percentage of recruitment. Three independent experiments were performed and the bars show the standard deviations of one representative triplicate.
Western Blot
Cell lysates were prepared in Radioimmunoprecipitation assay buffer (RIPA buffer) (Sigma, St Louis, MO, USA) and stored at −80°C. Total protein concentration was determined by Bradford assay. Whole cell extracts (20 μg) were electrophoresed on a sodium dodecyl sulfate/polyacrylamide gel (SDS-PAGE), and electrotransferred onto a nitrocellulose membranes (Amersham Biosciences, Pittsburgh, PA, USA). Blots were blocked by Tris-buffered saline solution containing 5% non-fat milk. Primary and secondary antibody-HRP conjugates were prepared in blocking buffer. Bands were visualized by the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Pittsburgh, PA, USA) according to manufacturer’s protocol. To verify equal loading of proteins, membranes were stripped and reprobed with anti-beta-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA)(Ma et al., 2014). BMDMs were pretreated with either 20μM p38 mitogen-activated protein kinases (MAPK) inhibitor SB203580 or 20μM c-Jun N-terminal protein kinases (JNK) inhibitor SP600125 for 2 hours before overnight morphine (1μM) incubation. SB203580 or SP600125 was maintained overnight, along with morphine, followed by LPS treatment for 24 hours.
Confocal microscopy
Immunofluorescence staining was carried out using antibody against LC3 (Cell Signaling, Danvers, MA, USA). DyLight TM488-conjugated donkey anti-rabbit IgG (Jackson Immuno Research Laboratories Inc, West Grove, PA, USA) was used as the secondary antibody. BMDMs were transfected using Amaxa’s Nucleofector device and a Mouse Macrophage Nucleofector kit (Lonza, Walkersville, MD, USA). Cells (1 × 106 cells per sample) were centrifuged at 200×g for 10 minutes at room temperature. 100 μl of cell suspension was combined with 2 μg of plasmid GFP-LC3, GFP-LAMP1 or GFP-mCherry-LC3. Cell/DNA suspension was transferred into certified cuvette. The appropriate Nucleofector® Program Y-001 was selected. 500 μl of the pre-equilibrated DMEM was added to the cuvette and gently transferred into 12-well plate. The cells were incubated in a humidified incubator with 5% CO2 at 37°C for 24h and used for subsequent experiments. GFP-LC3 and GFP-LAMP1 constructs are generous gifts from Dr. Sundaram Ramakrishnan (University of Minnesota, USA). 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies, Grand Island, NY, USA) was used to visualize the nucleus. Fields were chosen randomly from various regions to ensure objectivity of sampling. Digital images at 400×magnification were acquired using a Nikon confocal microscope. Quantitation of autophagy was performed based on the percentage of GFP-LC3-positive autophagic vacuoles or cells with LC3 and LAMP1 punctate dots. In all experiments, a minimum of 50 cells per sample were counted, and duplicate or triplicate samples were counted per experimental condition.
Transmission electron microscopy (TEM)
Transmission Electron Microscopy (TEM) of BMDMs was carried out as previously described (Smith et al., 2010) on cellular pellets obtained after centrifugation at 1000g for 5 min. Cell pellets were fixed in 2.5% glutaraldehyde for 40 min. After three washes with 0.1M sodium cacodylate, samples were post-fixed with 1% osmium tetroxide (OsO4) for 30 min. After three rinses, samples were dehydrated through a graded series of ethanol. Samples were subsequently embedded into Epon 812 resin. Thin sections (65 nm) were cut with an ultramicrotome (Leica UC6) and stained with uranyl acetate and lead citrate, then examined using a JEOL 1200EX electron microscope (JEOL, Peabody, MA, USA)(Ma et al., 2014).
Bactericidal Assay
BMDMs were plated in 6-well plates at a density of 2×105 cells per well with DMEM (supplemented with 10% FBS, 1% penicillin/streptomycin). BMDMs were treated with morphine (1μM) overnight and then LPS (0.25μg/ml) for another 24 hours. Cells were then washed and media was replaced with fresh antibiotic-free DMEM (supplemented with 10% FBS), 1μM morphine and 0.25μg/ml LPS added in the corresponding wells. The virulence of S. pneumoniae was maintained by subculturing bacteria obtained from the spleens of mice rendered bacteremic 24h following an intratracheal challenge with 106 CFUs of this organism.For expansion and growth of bacteria, S. pneumoniae serotype 3 (ATCC 6303; Rockville, MD) were streaked onto a blood agar plate (Becton, Dickinson and Co.) and grown at 37°C in 5% CO2 to mid-log phase. Typical colonies were picked and inoculated into brain heart infusion (BHI) broth. The bacteria were pelleted by centrifugation (16,000 × g for 5 min) and washed twice in endotoxin-free phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA). The concentration of bacteria was determined spectrophotometrically (OD590) and confirmed by plating serially diluted bacteria onto blood agar plates(Difco, Detroit, MI) (Ma et al., 2010). BMDMs were infected with S. pneumoniae (multiplicity of infection [MOI], 20:1) for 60 mins. BMDMs were then washed in PBS and incubated overnight in DMEM containing 10% FBS and 1% penicillin/streptomycin to kill non internalized bacteria. Cells were then washed, scraped off and plated onto blood agar plates.
Statistical analysis
Data were collected from three independent experiments and expressed as the mean ± standard error of the mean (SEM). Where appropriate, mean values were compared using a paired Student’s t test. A P value of 0.05 was considered statistically significant.
Results
Morphine potentiates LPS-induced LC-3 recruitment to phagosomes
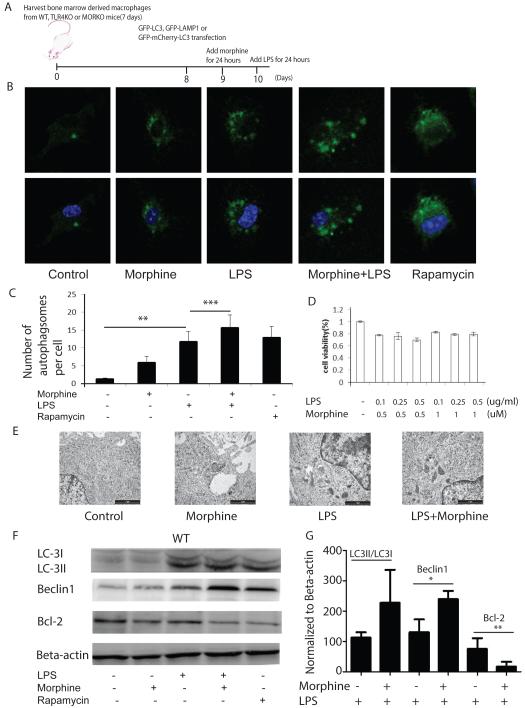
Using LC3 as an autophagosomal marker(Al-Younes et al., 2011), we first determined if morphine treatment modulates LPS-induced autophagy. BMDMs were transfected with GFP-LC3, and treated with morphine (1μM) for 24 hours before activation with LPS for 24 hours. Cells were then visualized using confocal microscopy. The experimental design is summarized in Figure1A. LPS treatment resulted in punctate LC3 distribution indicative of autophagosome formation (Figure1B). Morphine treatment alone also demonstrated punctate LC3 distribution which was further augmented in the presence of LPS treatment. Cells treated with rapamycin were used as positive control for autophagy induction. Morphine and LPS treated cells showed significant increase in autophagosome formation compared to samples that were treated with LPS alone (Figure 1C). MTT assay was performed to confirm that the concentration of morphine (1μM) and LPS (0.25μg/ml) used did not affect cell viability (Figure 1D). Autophagosome formation was also evaluated using transmission electron microscopy (TEM) at the ultrastructural level (Kirkegaard et al., 2004). Vehicle treated BMDMs exhibited normal ultrastructural morphology of cytoplasm, organelles and nuclei (Figure 1E). In contrast, exposure to morphine and LPS revealed double-membrane vacuolar structures. The number of double membrane vacuolar structures was also visible in samples that were treated with morphine alone (Figure 1E).
Figure 1. Morphine potentiates LPS-induced autophagy initiation.
(A) Schematic diagram of experimental design. (B) Upper panel: BMDMs were transfected with GFP-LC3, and treated with morphine in the absence or presence of LPS for 24 hours. Nuclei were labeled with DAPI. Rapamycin was used as a positive control. Representative images are shown. (C) Quantification of the number of autophagosomes in a single cell. Confocal images were obtained at 600×magnification. **, P<0.05. ***, P<0.05. (D) Cell viability analysis was determined using a MTT assay. (E) BMDMs were treated with morphine 24 hours before LPS treatment for 24 hours. Cells were then analyzed by TEM. Images were obtained at 40,000× magnification using a JEOL 1200 EX transmission electron microscope (JEOL, Peabody, MA, USA). Scale bar: 1 μm. (F) BMDMs were treated with morphine for 24 hours and then treated with LPS for an additional 24 hours. Cell lysates were separated on 10% SDS-PAGE and levels of LC3II/I, Beclin1 and Bcl-2 were determined by Western blot. (G) Densitometric analysis of LC3II/I, Beclin1 and Bcl-2 expression levels. Values represent percentage changes from control of LC3II/LC3I, Beclin1 or Bcl-2 normalized against beta-actin. *, **, P<0.05. Replicated experiments gave similar statistical significances. The data demonstrate that morphine potentiates LPS-induced autophagy initiation indicated by autophagosome formation, increase in LC3II/LC3I conversion and Beclin1 expression.
Autophagy can be divided into three stages: initiation, execution and maturation. The initiation of autophagy can be triggered by a variety of extracellular signals, including nutrient starvation and treatment with hormones. Beclin1 is a novel Bcl-2 homology 3 (BH3) domain-only protein that plays an important role in both autophagosome formation and autolysosomal fusion(Castillo et al., 2013). Beclin1 and the class III phosphatidylinositide 3-kinase (PI3K) Vps34 form two distinct complexes. Bcl-2 inhibits autophagy by interacting with Beclin1, disrupting the formation of Beclin1/hVps34 complex, a critical step for autophagy initiation (Pattingre et al., 2005)(Pattingre et al., 2008). Thus, accumulation of Beclin1 protein can be used as an indicator of autophagy induction(Ma et al., 2014). Our results further confirm that morphine treatment modulates LPS-induced autophagy, since up-regulation of Beclin1 in LPS-treated BMDMs was further augmented with morphine treatment (Figure 1F and 1G). Interestingly, we observed a reciprocal decrease in Bcl-2 levels when BMDMs were treated with either LPS alone or in the presence of morphine (Figure 1F and 1G).
The key stages of autophagosomal execution are mediated by two very interesting covalent-conjugation pathways: the covalent linkage of Atg5 and Atg12, and the covalent lipidation of Atg8 by phosphatidylethanolamine. The second conjugation pathway results in the covalent addition of the lipid phosphatidylethanolamine to the newly generated carboxyl terminus of microtubule-associated-protein light-chain 3 (LC3), the human homologue of S. cerevisiae autophagy-related protein 8 (Atg8) (Kirkegaard et al., 2004). During autophagy, LC3-I is converted to LC3-II through lipidation and is associated with autophagic vesicles (Ohsumi, 2014). The amount of LC3-II in the presence and absence of saturating levels of inhibitors can be used to examine the transit of LC3-II through the autophagic pathway(Ma et al., 2014). If autophagic flux is occurring, the levels of LC3-II will be higher in the presence of the inhibitors. Lysosomal protein degradation can be prevented through the use of protease inhibitors (e.g., pepstatin A and E-64d)(Klionsky and Abdalla, 2012). To evaluate that the increase in autophagosome number was associated with conversion of LC3-I to LC3-II, cells were treated as described above and subjected to western blot analysis. The data demonstrates that there was an increase in LC3-I to LC3-II conversion in the LPS treated samples which was further augmented in cells that were treated with morphine in the presence of the protease inhibitors pepstatin A and E-64d (Figure 1F). Rapamycin was used as the positive control.
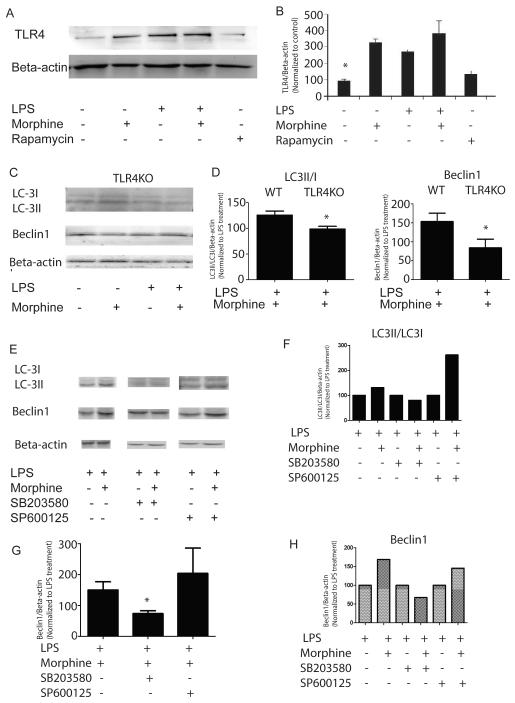
Morphine modulates LPS-induced autophagy initiation through TLR4 signaling
To determine the mechanism by which morphine modulates LPS-induced autophagy initiation, we explored the effect of morphine on TLR4 expression in BMDMs. Morphine and LPS treatment further enhanced TLR4 expression compared with control (Figure 2A and 2B). Morphine and LPS induced LC3 I/II conversion and Beclin1 expression were abolished in the TLR4KO mice (Figure 2C and 2D). These results illustrate that morphine modulates LPS-induced autophagosome execution through TLR4 signaling. Previous work suggested a critical role for p38 MAPK in mediating downstream signaling of the TLR4 pathway(Xu et al., 2007). To evaluate if the down-stream mechanism of morphine modulation of LPS-induced autophagy in BMDMs is mediated through MAPK signaling pathway, we examined the effects of inhibition of JNK or p38 MAPK on LPS-induced autophagy related genes in BMDMs (Figure 2E). Inhibition of JNK did not have marked effects on morphine modulation of LPS-induced LC3 I/II conversion and Beclin1 expression (Figure 2E, 2F, 2G and 2H). In contrast, p38 MAPK inhibition abolished those effects (Figure 2E, 2F, 2G and 2H). These data suggested that p38 MAPK signaling plays a crucial role in morphine modulation of LPS-induced autophagy.
Figure 2. Morphine potentiates LPS-induced autophagy initiation through TLR4-p38 signaling.
(A) Morphine increased LPS-induced TLR4 expression in WT BMDMs. (B) Densitometric analysis of TLR4 expression. Values represent percentages of TLR4 normalized against beta-actin and compared with untreated control. *, P<0.05, Shown are representative results from one of three independent experiments. (C) LC3, Beclin1 and Bcl-2 expressions in BMDMs derived from TLR4KO mice following treatment with morphine in the presence or absence of LPS. (D) Densitometric analysis of LC3II/I and Beclin1 expression levels in BMDMs harvested from WT and TLR4KO mice. Values were normalized against beta-actin and represented as percentage of LPS treatment. (E) WT BMDMs were incubated with p38 mitogen-activated protein kinases(MAPK) inhibitor SB203580 (20μM) or c-Jun N-terminal kinases (JNK) inhibitor SP600125 (20μM) for 2 hours before treatment with morphine for 24 hours. Cells were then treated with LPS for 24 hours. LC3 and Beclin1 protein levels were evaluated using Western blot. (F and H) Densitometric analysis of LC3II/I and Beclin1 expressions in the representative blot. (G) Statistical analysis of Beclin1 levels from three independent blots in the presence or absence of MAP kinase inhibitors SB203580 (20μM) or c-Jun N-terminal kinases (JNK) inhibitor SP600125 (20μM). Values were normalized against beta-actin and represented as percentage of LPS treatment. The data suggested that p38 MAPK signaling plays a crucial role in morphine modulation of LPS-induced autophagy.
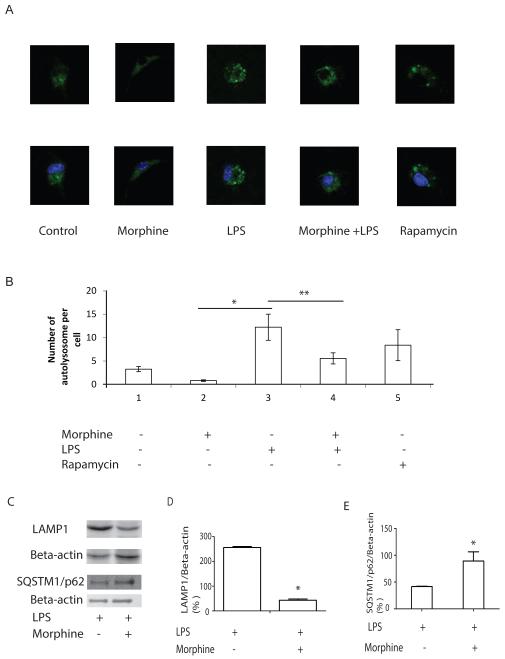
Morphine decreased LPS-induced LAMP1 expression
During the final stage of maturation, autophagosomes fuse with lysosomes to form phagolysosomes which are degradative compartments where the sequestered cytoplasmic cargo becomes exposed to and degraded by the hydrolytic enzymes. The two structurally related, major lysosomal membrane proteins LAMP1 and LAMP2 is essential for the protection of the lysosomal membrane. We transfected GFP-LAMP1 in LPS activated BMDMs treated with or without morphine. GFP-LAMP1 vesicles in single cell were observed by confocal images and counted. Morphine treatment significantly inhibited LPS-induced GFP-LAMP1 recruitment to vesicles (Figure 3A and 3B) suggesting inhibition of autophagosomal maturation. We further demonstrated using western blot analysis that morphine treatment inhibited LPS-induced LAMP1 protein expression (Figure 3C and 3D). p62 (also known as sequestosome-1/SQSTM1) is a multidomain adaptor protein transporting ubiquitinated proteins during autophagy (Mathew et al., 2009); as such, p62 was identified as one of the specific substrates that are degraded through the autophagy–lysosomal pathway (Komatsu and Ichimura, 2010). Impaired autophagy is accompanied by p62 accumulation, resulting in large p62/ubiquitinated protein aggregates (Komatsu and Ichimura, 2010). To further validate that morphine treatment inhibits phagolysosomal fusion we determined LPS-induced p62 levels using western blot analysis. Consistent with the finding that morphine inhibits phagolysomal maturation, morphine treatment prevented the degradation of p62 (Figure 3C and 3E).
Figure 3. Morphine inhibits LPS-induced maturation of autophagosomes.
(A) Upper panel: BMDMs were transiently transfected with GFP-LAMP1 and cultured for 24 hours. Cells were then treated with morphine for 24 before LPS treatment for 24 hours. Images were visualized by fluorescence microscope at 600×magnification. Lower panel: Nuclei was labeled with DAPI. (B) Quantification of the number of autophagosomes in a single cell. GFP-LAMP1 vesicles in a single cell was counted. GFP-LAMP1-labeled cells from different treatment groups were merged with DAPI. *, P<0.05. **, P<0.05. (C) LAMP1 and SQSTM1/p62 protein levels were evaluated using Western blot. (D) Densitometric analysis of LAMP1 expression. Values were normalized against beta-actin. *, P<0.05, replicated experiments gave similar statistical significances. (E) Densitometric analysis of SQSTM1/p62 expression. Values were normalized against beta-actin. *, P<0.05, Representative data are from three independent experiments. Morphine inhibited phagolysomal maturation indicated by inhibition of LPS-induced LAMP1 expression and prevention of p62 degradation.
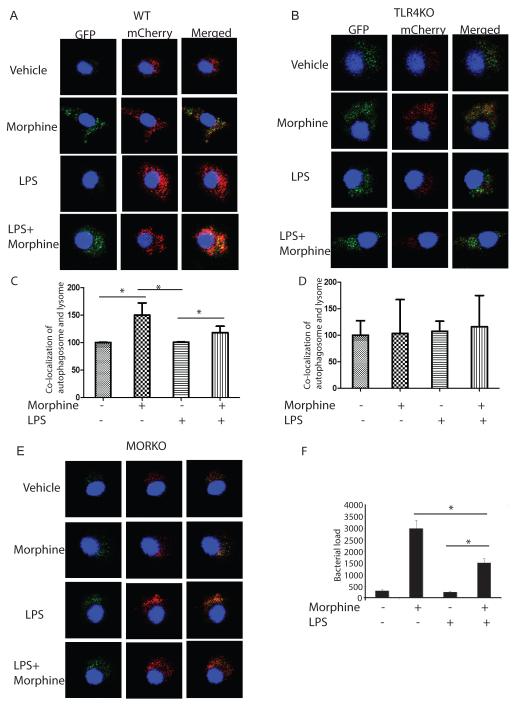
Morphine inhibited LPS-activated colocalization of autophagosome and lysosome through TLR4 independent but μ opioid receptor signaling pathway
Autophagosome matures into autolysosome, where the captured cargo is degraded. Maturation occurs through fusion with late endosomal/lysosomal organelles or delivery of trafficking intermediates carrying H+ ATPase components and lysosomal hydrolytic enzymes. The maturing autophagosome becomes acidified and is converted into a degradative organelle (autolysosome) with single delimiting membrane (the inner of the two membranes is dissolved) containing degraded material including internal membranes originating from the capture. To evaluate the effect of morphine on LPS-induced autophagosomal maturation, autophagic flux was evaluated. The method is based on the concept of lysosomal quenching of GFP in GFP-labeled autophagic substrate such as LC3. GFP signal is sensitive to the acidic and/or proteolytic conditions of the lysosome lumen. The low pH inside the lysosome quenches the GFP fluorescent signal, which makes it difficult to trace the delivery of GFP-LC3 to lysosomes. In contrast, mCherry fluorescence persists in acidic compartments, and mCherry-LC3 can readily be detected in autolysosomes. The colocalization of both mCherry and GFP fluorescence signal, stains a vesicular compartment that has not fused with lysosomes (yellow puncta, i.e., phagophores or autophagosomes), whereas a mCherry vesicle without GFP fluorescence emission corresponds to autolysosome (red puncta)(Castillo et al., 2013). Therefore, autophagic flux can be morphologically traced with a novel mCherry-GFP-LC3 tandem construct. If autophagic flux is activated, both red and yellow punctate are increased; however, if autophagosomal maturation to autolysosomes is blocked, only yellow punctate are increased without increasing the red signals concomitantly (Mizushima et al., 2010). BMDMs from WT, TLR4KO and MORKO mice were transfected with mCherry-GFP-LC3, separately. In WT derived BMDMs, most of LC3-positive autophagic vacuoles are only red, suggesting that autolysosomes constitute the majority of autophagic vacuoles upon LPS treatment (Figures 4A). However, in TLR4KO derived BMDMs, LPS treatment resulted in the appearance of mostly green LC3 puncta (Figures 4B) with very little red-only LC3-positive vacuoles suggesting that LPS-induced phagolysosomal maturation and fusion is abolished in the TLR4KO mice (Figures 4B). In morphine treated WT derived BMDMs, the LC3 puncta appears yellow (convergence of green and red signals), suggesting that morphine inhibits LPS-induced autophagolysomal formation therefore resulting in the stabilization of the green fluorescence (Figure 4C). The inhibitory effect of morphine on LPS-induced autophagolysosomal fusion persisted in TLR4KO BMDMs suggesting morphine’s effect was mediated probably through a TLR4 independent pathway (Figure 4D). As expected, morphine’s effects were abolished in BMDMs derived from MORKO mice (Figure 4E).
Figure 4. Morphine inhibits LPS induced autophagolysosome fusion.
(A, B and E) BMDMs from WT, TLR4KO and MORKO were transfected by GFP-mCherry-LC3, separately. Twenty-four hours after transfection, cells were treated with morphine for 24 hours before LPS treatment. Cells were then fixed before being subjected to confocal microscopy. (C) Quantification of co-localization of autophagosome and lysosome in WT mice BMDMs. *, P<0.05. (D) Quantification of co-localization of autophagosome and lysosome in TLR4KO mice BMDMs. (F) BMDMs were treated with morphine overnight and then treated with LPS for 24 hours. BMDMs were then infected with S. pneumoniae for 60 mins. Bacterial killing was allowed to occur for another 24 hours. Viable internalized bacteria within the cells were enumerated by a colony forming unit (CFU) assay. Results from three independent experiments are presented. *, P<0.05. In the presence of LPS, morphine treatment significantly increased S. pneumoniae load compared to LPS treatment.
We next investigated if lack of autophagolysosomal fusion increased bacterial viability and load. A S. pneumoniae bactericidal assay was used to determine bacterial clearance. In the presence of LPS, morphine treatment significantly increased S. pneumoniae load compared to LPS treatment (Figure 4F). This data supports our hypothesis that morphine treatment facilitates LPS induced autophagy but inhibits autophagosome maturation and autophagolysosomal fusion, events that are essential for efficient removal of internalized pathogens thus leading to increased survival of pathogens within macrophages.
Discussion
Autophagy has been recently shown, to be a mechanism by which host cells capture and eliminate intracellular pathogens. Toll- like receptor 4 (TLR 4) serves as an environmental sensor for autophagy(Xu et al., 2007). LPS is the biologically active constituent of endotoxins derived from the cell wall of Gram-negative bacteria, which is a potent inducer of autophagy in many cell lines, including macrophages. Not surprisingly, successful pathogens have evolved numerous ways to protect themselves from autophagic degradation. A wealth of information now clearly demonstrates that chronic morphine use or abuse increases susceptibility to both bacterial and viral opportunistic infection. However, the role of modulation of autophagy in morphine mediated inhibition of bacterial clearance has not been previously demonstrated in BMDMs. The objective of the present study was to investigate if morphine modulates autophagy induced by LPS in BMDMs as a mechanism for bacterial clearance. The focus of the studies was to determine whether LPS induced autophagosome maturation, the ultimate step in bacterial degradation and clearance, was modulated by morphine.
We demonstrate that morphine increased LPS-induced LC-3 recruitment to phagosomes suggesting potentiation of LPS induced autophagy by morphine. However, morphine inhibited LPS-induced LAMP1 expression and LPS induced autophagosomal maturation and auotphagolysosomal fusion suggesting that although autophagy was potentiated the subsequent maturation steps were inhibited. Interestingly, morphine mediated increase in LPS induced autophagy initiation was TLR4 dependent and mediated through activation of the p38 MAP kinase pathway. In contrast, morphine induced inhibition of autophagosomal maturation persisted in BMDMs harvested from TLR4KO mice.
These observations imply that although morphine treatment facilitates LPS induced autophagy leading to increased survival of macrophages, it inhibits autophagosome maturation and autophagolysosomal fusion which is essential for efficient removal of internalized pathogens such as S.pneumoniae thereby causing an accumulation of viable bacteria in the degradative late endosomal compartment. We further demonstrate that although morphine induced autophagy is mediated through a TLR4 dependent pathway, morphine induced inhibition of autophagolysosomal fusion is mediated through TLR4 independent pathways.
Acidification of the phagolysosomal compartment is an essential step for the elimination of internalized bacteria. Macrophages acidify the phagosomal lumen by recruiting V-ATPase, a multisubunit protein-pump complex that actively transports protons across membranes using energy from ATP hydrolysis(Sun-Wada et al., 2009). Delivery of the V-ATPase to the phagosome results in a pH decrease from 6.5 to 5.0 within minutes of phagosome maturation(Mukherjee S, 1997). The acidic environment inhibits bacterial growth by enhancing activities of antimicrobial hydrolases. Acidic pH is also essential for proper vesicular trafficking, directing the fusion of phagosomes with lysosomes or vesicles harboring antimicrobial molecules. The underlying molecular mechanisms of transporter translocation are not understood but experimental evidence implicates the involvement of protein phosphorylation in this process. Activation of PKA has been shown to mediate accumulation and activity of V-ATPase. We have previously shown that morphine modulates the PKA pathway independent of TLR activation (Ninkoviæ and Roy, 2012). We speculate that morphine modulation of phagolysosomal acidity through inhibition of PKA and p38 MAP kinase could be a potential mechanism by which morphine disrupts lysosomal acidity and phagolysosomal fusion. Future studies will focus on the downstream pathways that are involved in morphine modulation of phagolysosomal acidity and the role of V-ATPase in this process.
This study provides new insight into the possible mechanism(s) underlying the observed increase in the susceptibility to infection and the prevalence of persistent infection in the drug abusing population.
Acknowledgements
This work was supported by National Institutes of Health grants RO1 DA 12104, RO1 DA 022935, RO1 DA031202, K05DA033881 and 1R01DA034582 (S.R.). We are grateful to Ms. Fang Zhou for the assistance of TEM. TEM was carried out in the Characterization Facility, University of Minnesota, and a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org) via the MRSEC program.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Reference
- Al-Younes HM, Al-Zeer M. a., Khalil H, Gussmann J, Karlas A, Machuy N, Brinkmann V, Braun PR, Meyer TF. Autophagy-independent function of MAP-LC3 during intracellular propagation of Chlamydia trachomatis. Autophagy. 2011;7:814–828. doi: 10.4161/auto.7.8.15597. [DOI] [PubMed] [Google Scholar]
- Castillo K, Valenzuela V, Matus S, Nassif M, Oñate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court F. a, Schneider BL, Armentano D, Hetz C. Measurement of autophagy flux in the nervous system in vivo. Cell Death Dis. 2013;4:e917. doi: 10.1038/cddis.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Delgado M, Vergne I, Master S, Haro S. De. Autophagy in Infection and Immunity. Current Topics in Microbiology and Immunology. 2009;335:1–18. doi: 10.1007/978-3-642-00302-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol. Biol. Cell. 2008;19:2179–92. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayath CA, Hussey S, El hajjami N, Nagra K, Philpott D, Allaoui A. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 2010;12:956–66. doi: 10.1016/j.micinf.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2004;2:301–14. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D, Abdalla F. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;4:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–8. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Ma J, Wan J, Meng J, Banerjee S, Ramakrishnan S, Roy S. Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis. 2014;5:e1099. doi: 10.1038/cddis.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang J, Wan J, Charboneau R, Chang Y, Barke R. a, Roy S. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect. Immun. 2010;78:830–7. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen H-Y, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Murrow L, Debnath J. Autophagy As A Stress Response And Quality Control Mechanism—Implications for Cell Injury and Human Disease. Annu. Rev. Pathol. 2013:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–40. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Ninković J, Roy S. Morphine decreases bacterial phagocytosis by inhibiting actin polymerization through cAMP-, Rac-1-, and p38 MAPK-dependent mechanisms. Am. J. Pathol. 2012;180:1068–79. doi: 10.1016/j.ajpath.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice : role of m - opioid receptor in morphine mediated immune functions. 1998:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Sacerdote P. Opioids and the immune system. Palliat. Med. 2006;20(Suppl 1):s9–15. [PubMed] [Google Scholar]
- Sanjuan M, Dillon C, Tait S. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Shahnazari S, Brumell JH. Mechanisms and consequences of bacterial targeting by the autophagy pathway. Curr. Opin. Microbiol. 2011;14:68–75. doi: 10.1016/j.mib.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–81. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun-Wada G-H, Tabata H, Kawamura N, Aoyama M, Wada Y. Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J. Cell Sci. 2009;122:2504–13. doi: 10.1242/jcs.050443. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke R. a., Charboneau R, Schwendener R, Roy S. Morphine Induces Defects in Early Response of Alveolar Macrophages to Streptococcus pneumoniae by Modulating TLR9-NF- κB Signaling. J. Immunol. 2008;180:3594–3600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, Liu X-D, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Zhang H. You are what you eat: multifaceted functions of autophagy during C. elegans development. Cell Res. 2014;24:80–91. doi: 10.1038/cr.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]