Abstract
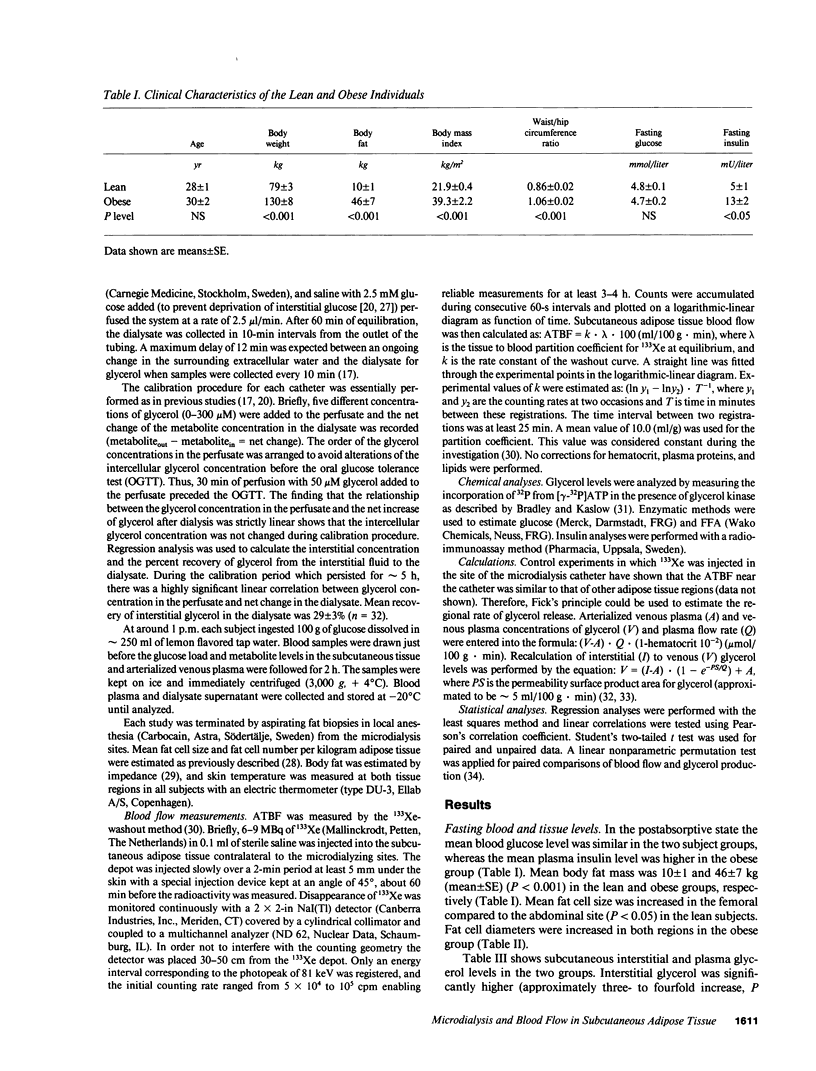
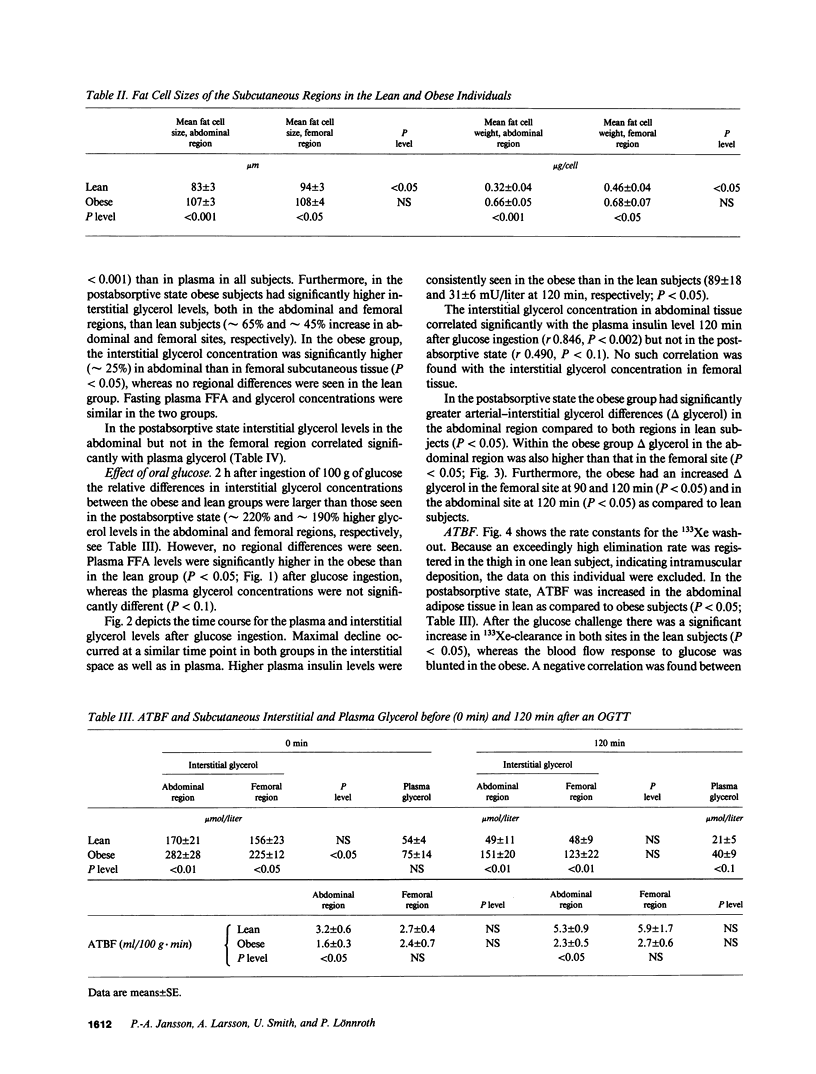
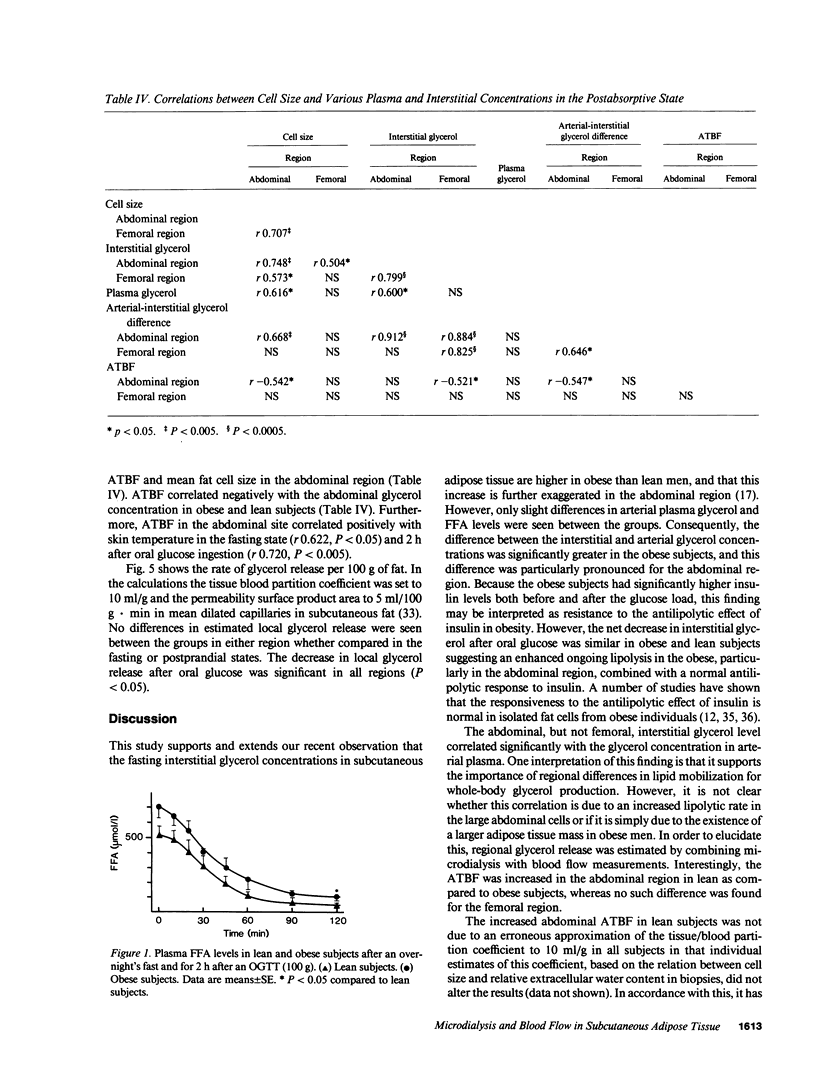
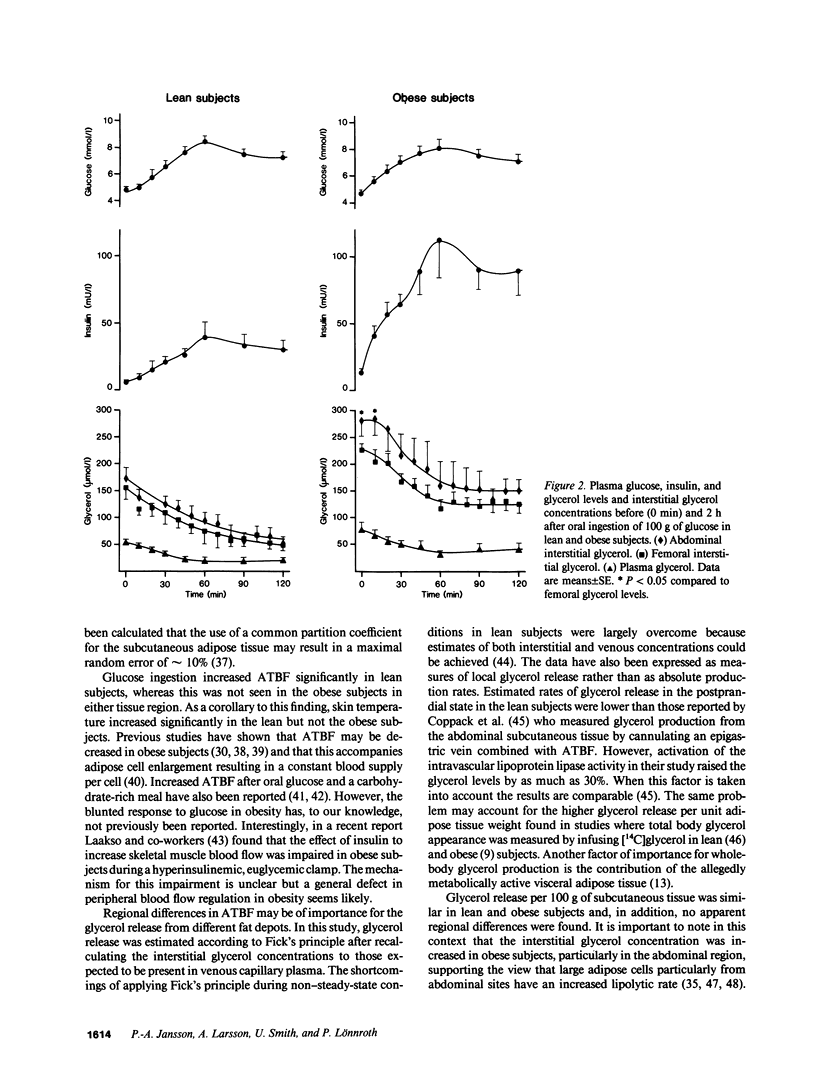
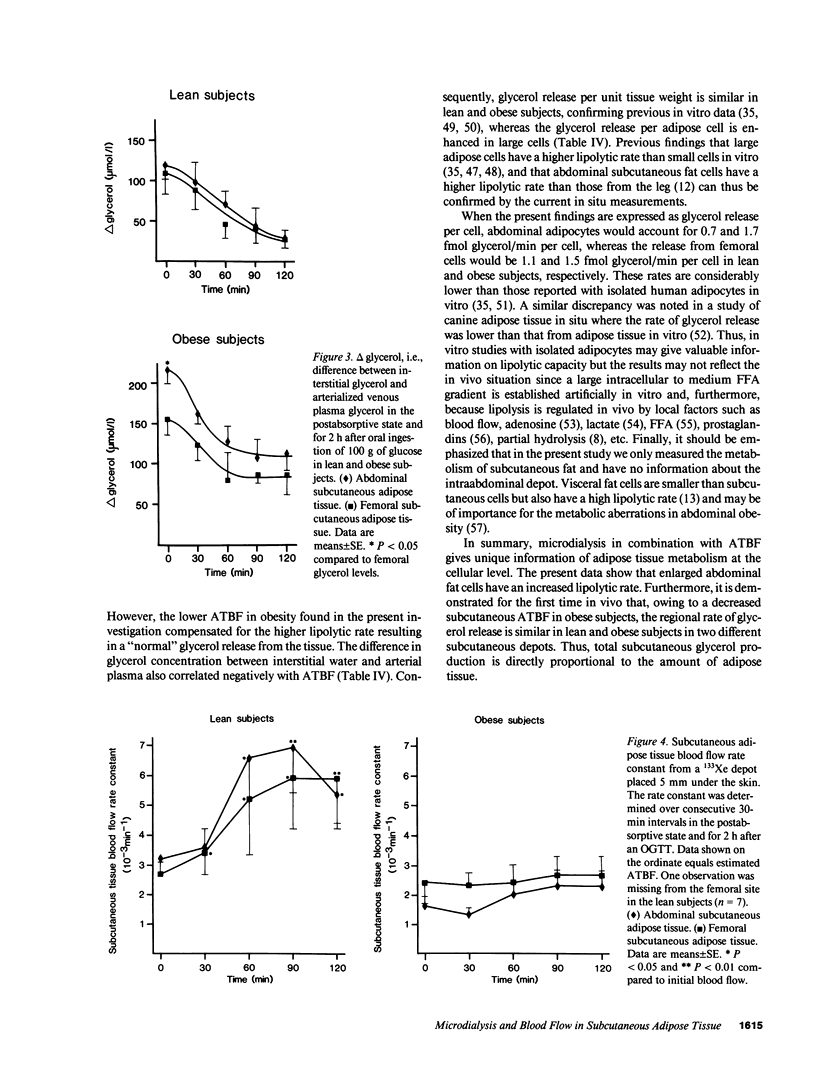
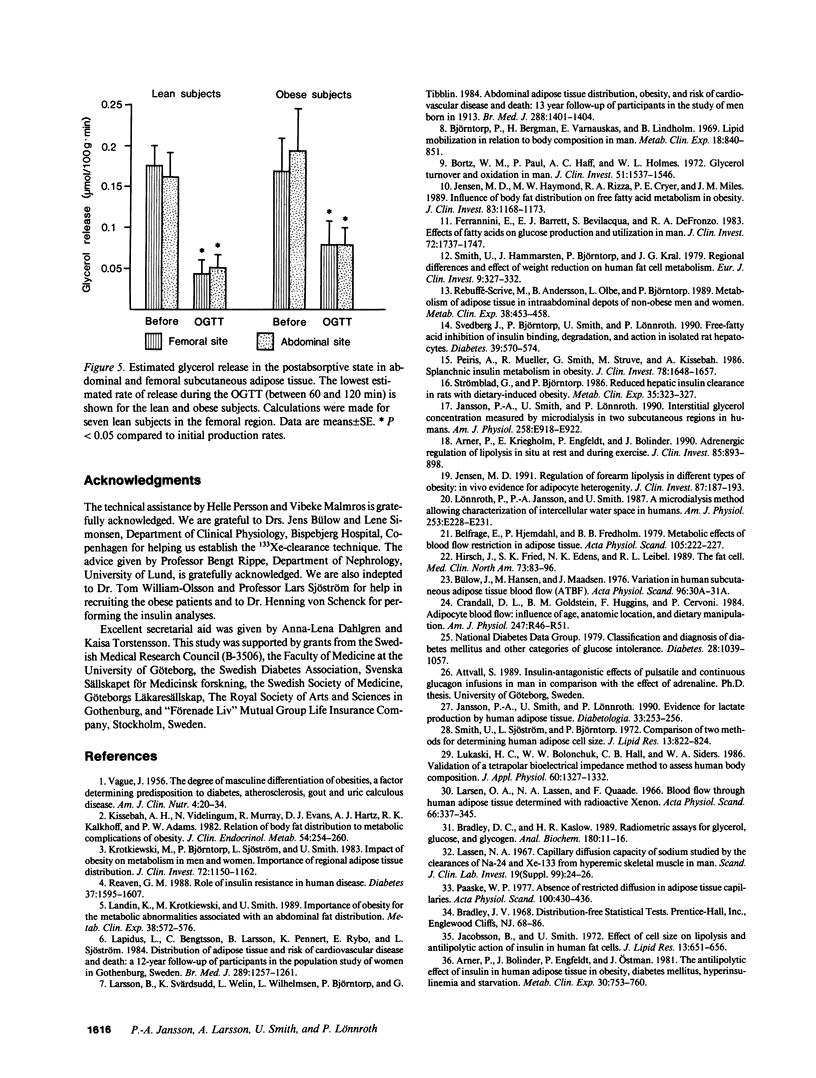
To estimate the regional subcutaneous glycerol production rate in normal and obese humans, the venous arterialized plasma glycerol, interstitial glycerol in the subcutaneous adipose tissue together with adipose tissue blood flow (ATBF, ml/100 g.min) were measured in the postabsorptive state and for 2 h after ingestion of 100 g of oral glucose. Eight lean and eight obese men with normal oral glucose tolerance tests were investigated with the subcutaneous microdialysis technique and 133Xe clearance. In the postabsorptive state, the interstitial glycerol concentrations in lean and obese subjects were 170 +/- 21 vs. 282 +/- 28 microM (P less than 0.01) and 156 +/- 23 vs. 225 +/- 12 microM (P less than 0.05) in the abdominal and femoral subcutaneous adipose tissue, respectively. The corresponding arterial glycerol levels were 54 +/- 4 vs. 75 +/- 14 microM (NS). Abdominal ATBF was greater in lean subjects (3.2 +/- 0.6 vs. 1.6 +/- 0.3; P less than 0.05), whereas femoral ATBF was similar in both groups (2.7 +/- 0.4 vs. 2.4 +/- 0.7). Estimated mean local glycerol release (mumol/100 g.min) was similar in the lean and obese group (0.16 +/- 0.03 vs. 0.20 +/- 0.05 and 0.18 +/- 0.02 vs. 0.17 +/- 0.04) in the abdominal and femoral site, respectively. We conclude that glycerol production from the subcutaneous tissue is increased in obesity, irrespective of adipose tissue distribution. This enhancement is due to the increased adipose tissue mass.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Bolinder J., Engfeldt P., Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981 Aug;30(8):753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage E., Hjemdahl P., Fredholm B. B. Metabolic effects of blood flow restriction in adipose tissue. Acta Physiol Scand. 1979 Feb;105(2):222–227. doi: 10.1111/j.1748-1716.1979.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Björntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990 Jul-Aug;10(4):493–496. [PubMed] [Google Scholar]
- Björntorp P., Bergman H., Varnauskas E., Lindholm B. Lipid mobilization in relation to body composition in man. Metabolism. 1969 Oct;18(10):840–851. doi: 10.1016/0026-0495(69)90059-6. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. The composition and metabolism in vitro of adipose tissue fat cells of different sizes. Eur J Clin Invest. 1972 Jan;2(2):78–84. doi: 10.1111/j.1365-2362.1972.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. C., Kaslow H. R. Radiometric assays for glycerol, glucose, and glycogen. Anal Biochem. 1989 Jul;180(1):11–16. doi: 10.1016/0003-2697(89)90081-x. [DOI] [PubMed] [Google Scholar]
- Burns T. W., Langley P. E., Terry B. E., Robinson G. A. The role of free fatty acids in the regulation of lipolysis by human adipose tissue cells. Metabolism. 1978 Dec;27(12):1755–1762. doi: 10.1016/0026-0495(78)90261-5. [DOI] [PubMed] [Google Scholar]
- Bülow J., Astrup A., Christensen N. J., Kastrup J. Blood flow in skin, subcutaneous adipose tissue and skeletal muscle in the forearm of normal man during an oral glucose load. Acta Physiol Scand. 1987 Aug;130(4):657–661. doi: 10.1111/j.1748-1716.1987.tb08189.x. [DOI] [PubMed] [Google Scholar]
- Coppack S. W., Fisher R. M., Gibbons G. F., Humphreys S. M., McDonough M. J., Potts J. L., Frayn K. N. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 1990 Oct;79(4):339–348. doi: 10.1042/cs0790339. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Skinner N. S., Jr, Hanley H. G., Sachs R. G. Relationship of adipose tissue blood flow to fat cell size and number. Am J Physiol. 1971 Apr;220(4):932–937. doi: 10.1152/ajplegacy.1971.220.4.932. [DOI] [PubMed] [Google Scholar]
- DiGirolamo M., Howe M. D., Esposito J., Thurman L., Owens J. L. Metabolic patterns and insulin responsiveness of enlarging fat cells. J Lipid Res. 1974 Jul;15(4):332–338. [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Karlsson J. Metabolic effects of prolonged sympathetic nerve stimulation in canine subcutaneous adipose tissue. Acta Physiol Scand. 1970 Dec;80(4):567–576. doi: 10.1111/j.1748-1716.1970.tb04824.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B. The effect of lactate in canine subcutaneous adipose tissue in situ. Acta Physiol Scand. 1971 Jan;81(1):110–123. doi: 10.1111/j.1748-1716.1971.tb04881.x. [DOI] [PubMed] [Google Scholar]
- Gries F. A., Berger M., Neumann M., Preiss H., Liebermeister H., Hesse-Wortmann C., Jahnke K. Effects of norepinephrine, theophylline and dibutyryl cyclic AMP on in vitro lipolysis of human adipose tissue in obesity. Diabetologia. 1972 Apr;8(2):75–83. doi: 10.1007/BF01235630. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Fried S. K., Edens N. K., Leibel R. L. The fat cell. Med Clin North Am. 1989 Jan;73(1):83–96. doi: 10.1016/s0025-7125(16)30693-9. [DOI] [PubMed] [Google Scholar]
- Jacobsson B., Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972 Sep;13(5):651–656. [PubMed] [Google Scholar]
- James R. C., Burns T. W., Chase G. R. Lipolysis of human adipose tissue cells: influence of donor factors. J Lab Clin Med. 1971 Feb;77(2):254–266. [PubMed] [Google Scholar]
- Jansson P. A., Smith U., Lönnroth P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia. 1990 Apr;33(4):253–256. doi: 10.1007/BF00404805. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Smith U., Lönnroth P. Interstitial glycerol concentration measured by microdialysis in two subcutaneous regions in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E918–E922. doi: 10.1152/ajpendo.1990.258.6.E918. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Haymond M. W., Rizza R. A., Cryer P. E., Miles J. M. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989 Apr;83(4):1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. D. Regulation of forearm lipolysis in different types of obesity. In vivo evidence for adipocyte heterogeneity. J Clin Invest. 1991 Jan;87(1):187–193. doi: 10.1172/JCI114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Simon B. Biphasic effects of prostaglandin E2 on the human fat cell adenylate cyclase. J Clin Invest. 1979 Aug;64(2):609–612. doi: 10.1172/JCI109500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Vydelingum N., Murray R., Evans D. J., Hartz A. J., Kalkhoff R. K., Adams P. W. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982 Feb;54(2):254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- Krotkiewski M., Björntorp P., Sjöström L., Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983 Sep;72(3):1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin K., Krotkiewski M., Smith U. Importance of obesity for the metabolic abnormalities associated with an abdominal fat distribution. Metabolism. 1989 Jun;38(6):572–576. doi: 10.1016/0026-0495(89)90219-9. [DOI] [PubMed] [Google Scholar]
- Lapidus L., Bengtsson C., Larsson B., Pennert K., Rybo E., Sjöström L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984 Nov 10;289(6454):1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen O. A., Lassen N. A., Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand. 1966 Mar;66(3):337–345. doi: 10.1111/j.1748-1716.1966.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Larsson B., Svärdsudd K., Welin L., Wilhelmsen L., Björntorp P., Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984 May 12;288(6428):1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen N. A. Capillary diffusion capacity of sodium studied by the clearances of Na-24 and Xe-133 from hyperemic skeletal muscle in man. Scand J Clin Lab Invest Suppl. 1967;99:24–26. [PubMed] [Google Scholar]
- Lesser G. T., Deutsch S. Measurement of adipose tissue blood flow and perfusion in man by uptake of 85Kr. J Appl Physiol. 1967 Nov;23(5):621–630. doi: 10.1152/jappl.1967.23.5.621. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Foley J., Bogardus C., Mott D., Howard B. V. Free fatty acid metabolism and obesity in man: in vivo in vitro comparisons. Metabolism. 1986 Jun;35(6):505–514. doi: 10.1016/0026-0495(86)90006-5. [DOI] [PubMed] [Google Scholar]
- Lukaski H. C., Bolonchuk W. W., Hall C. B., Siders W. A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol (1985) 1986 Apr;60(4):1327–1332. doi: 10.1152/jappl.1986.60.4.1327. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Fredholm B. B., Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am J Physiol. 1989 Feb;256(2 Pt 1):E250–E255. doi: 10.1152/ajpendo.1989.256.2.E250. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L. Adipose tissue blood flow determined by the washout of locally injected 133 Xenon. Scand J Clin Lab Invest. 1972 Feb;29(1):31–36. doi: 10.3109/00365517209081052. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L., Larsen O. A. Relationship of subcutaneous adipose tissue blood flow to thickness of subcutaneous tissue and total body fat mass. Scand J Clin Lab Invest. 1973 Jun;31(4):383–388. doi: 10.3109/00365517309084321. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Campbell P. J., Kennedy F. P., Miles J. M., Gerich J. E. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986 Dec;35(12):1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- Paaske W. P. Absence of restricted diffusion in adipose tissue capillaries. Acta Physiol Scand. 1977 Aug;100(4):430–436. doi: 10.1111/j.1748-1716.1977.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Peiris A. N., Mueller R. A., Smith G. A., Struve M. F., Kissebah A. H. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986 Dec;78(6):1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rebuffé-Scrive M., Andersson B., Olbe L., Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989 May;38(5):453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- Simonsen L., Bülow J., Astrup A., Madsen J., Christensen N. J. Diet-induced changes in subcutaneous adipose tissue blood flow in man: effect of beta-adrenoceptor inhibition. Acta Physiol Scand. 1990 Jun;139(2):341–346. doi: 10.1111/j.1748-1716.1990.tb08932.x. [DOI] [PubMed] [Google Scholar]
- Smith U., Hammersten J., Björntorp P., Kral J. G. Regional differences and effect of weight reduction on human fat cell metabolism. Eur J Clin Invest. 1979 Oct;9(5):327–332. doi: 10.1111/j.1365-2362.1979.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Smith U., Sjöström L., Björnstorp P. Comparison of two methods for determining human adipose cell size. J Lipid Res. 1972 Nov;13(6):822–824. [PubMed] [Google Scholar]
- Strömblad G., Björntorp P. Reduced hepatic insulin clearance in rats with dietary-induced obesity. Metabolism. 1986 Apr;35(4):323–327. doi: 10.1016/0026-0495(86)90148-4. [DOI] [PubMed] [Google Scholar]
- Svedberg J., Björntorp P., Smith U., Lönnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990 May;39(5):570–574. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- VAGUE J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956 Jan-Feb;4(1):20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- Zierler K. L. THEORY OF THE USE OF ARTERIOVENOUS CONCENTRATION DIFFERENCES FOR MEASURING METABOLISM IN STEADY AND NON-STEADY STATES. J Clin Invest. 1961 Dec;40(12):2111–2125. doi: 10.1172/JCI104437. [DOI] [PMC free article] [PubMed] [Google Scholar]



