Abstract
BACKGROUND
Medicare reimbursement cuts have been associated with declining Gonadotropin-releasing Hormone (GnRH) agonist overuse in localized prostate cancer. Medical school affiliation and foreign training have been associated with persistent overuse. However, physician-level prescribing changes and the practice type of persistent overusers have not been examined. We sought to describe physician-level changes in GnRH agonist overuse and test the association of time in practice and solo practice type with GnRH agonist overuse.
METHODS
We matched American Medical Association physician data for 2,138 urologists to SEER–Medicare data for 12,943 men diagnosed with early stage and lower grade adenocarcinoma of the prostate between 2000 and 2007. We conducted a population-based, retrospective study using multi-level modeling to control for patient and provider characteristics.
RESULTS
Three distinct patterns of GnRH agonist overuse were observed. Urologists’ time in practice was not associated with GnRH agonist overuse (OR 0.89; 95% CI 0.75–1.05).However, solo practice type (OR 1.65; 95% CI 1.34–2.02), medical school affiliation (OR 0.65; 95% CI 0.55–0.77), and patient race were. Compared to non-Hispanic whites, non-Hispanic blacks (OR 1.76; 95% CI 1.37–2.27), Hispanics (OR 1.41; 95% CI 1.12–1.79) and men of “other” race (OR 1.44; 95% CI 1.04–1.99) had greater odds of receiving unnecessary GnRH agonists.
CONCLUSIONS
GnRH agonist overuse remains high among some urologists who may be professionally isolated and difficult to reach. These urologists treat more vulnerable populations, which may contribute to health disparities in prostate cancer treatment quality. Nonetheless, these findings provide guidance to develop interventions to address overuse in prostate cancer.
Keywords: Provider Characteristics, Physician Time in Practice, Prostate Cancer, Patterns of Care, Overuse
Introduction
Prostate cancer is a prevalent, costly disease for which guideline-recommended treatment options are equivocal for most men (1–3). Although primary gonadotropin-releasing hormone (GnRH) agonists are recommended for men with metastatic disease and adjuvant to radiation for some men with localized disease, use of GnRH agonists alone in localized disease was recommended only for a small group of patients from 2000–2003, making most use in absence of radiation overuse (4–7).
Despite scant data supporting clinical effectiveness, GnRH agonist use in localized disease grew steadily from the 1990s, peaking in 2003 (8). Although clinical characteristics and patient preferences were thought to influence overuse, most variation in treatment has been attributed to the practice style of urologists, who prescribe over 90% of all GnRH agonists used in prostate cancer (9). In 2003, the Medicare Modernization Act (MMA) targeted wide variation in physician reimbursement of drugs covered under Medicare Part B (including GnRH agonists). The standardization, implemented incrementally in 2004 and 2005, decreased reimbursement for GnRH agonists 65% (10).
Whereas oncologists’ response to MMA cuts was to increase the use of recommended chemotherapies (11, 12), urologists maintained GnRH agonist use in patients for whom it was recommended but decreased overuse in patients for whom it was non-recommended (8). By 2005, GnRH agonist overuse declined 34%; however, 25.7% of men for whom it was not recommended still received this treatment in 2005 (13). Understanding characteristics of urologists who persistently overuse GnRH agonists—despite reimbursement change—is essential to improving quality of care. Whether response within urology was uniform is unknown. Urologists are delaying retirement (14). Physicians who have practiced longer may be least receptive to reimbursement pressures to change practice (11) and contribute to persistent overuse. However, results of previous studies exploring time in practice and quality of care conflict (15). In addition, more urologists practice as solo practitioners than any other surgical specialty (16). Solo practice type is associated with poorer quality of care in primary care (17), but to our knowledge this has not been tested in specialty care.
We describe physician-level patterns of overuse and the effect of time in practice and solo practice type on persistent GnRH agonist overuse. We hypothesize urologists with greater time in practice and those in solo practice are less responsive to reimbursement change.
Materials and Methods
We conducted a retrospective, longitudinal analysis using a national population-based sample of elderly prostate cancer patients. The study was approved by the Institutional Review Board of the University of North Carolina.
Data Sources
We linked Surveillance, Epidemiology and End Results (SEER)-Medicare data to the American Medical Association physician Masterfile. SEER includes 17 population-based cancer registries (18). Medicare administrative claims cover medical services for more than 97% of the U.S. population ≥65 years, approximately 81% of whom are covered under fee-for-service (19, 20). The Masterfile includes training and certification records supplemented by data from regulatory agencies and physician self-report for approximately 800,000 member and non-member U.S. physicians (21).
Cohort Definition
Patients
We identified men diagnosed with their first and only cancer as incident adenocarcinoma of the prostate between January 1, 2000 and December 31, 2007. We excluded patients whose comorbidities and/or initial treatment could not be ascertained with Medicare data: age <66 years, lacking a complete year of claims; diagnosed at autopsy, death certificate, or at nursing/convalescent facility; not enrolled in fee-for-service (defined as continuous Part A and B coverage and not in an HMO for at least 12 months post diagnosis); died within 12 months of diagnosis; and/or diagnosed in Louisiana (due to disruptions in health services caused by Hurricane Katrina). The Tumor Node Metastasis staging system was used to restrict the cohort to patients for whom GnRH agonists are not National Comprehensive Cancer Network (NCCN) guideline-recommended across the study period: no evidence of nodal or metastatic involvement and no greater than unilateral, stage T2 tumors and World Health Organization grades 1–2. We mapped Extent of Disease-1988 3rd edition variables for patients diagnosed from 2000–2003 and Collaborative Staging variables for patients diagnosed 2004–2007 to American Joint Committee on Cancer staging categories used by NCCN (4). Because of changes in staging over the study period, the cohort excluded men diagnosed with 1) T1 or T2 cancers with Gleason scores 8–10; 2) T2b tumors before 2002 when the staging definition changed; 3) T2c tumors after 2002 when the category was added; or, 4) T3a tumors(8). Guideline-recommended use of GnRH agonists in men receiving external beam radiation is dependent on D’Amico risk for recurrence (7). Prior to 2004, SEER did not collect prostate-specific antigen levels, one of three indicators used to determine D’Amico risk. Therefore, because we could not determine whether or not receipt of GnRH agonists constituted overuse, men receiving external beam radiation therapy were also excluded from the cohort.Physicians. Urologists prescribed 94.6% of GnRH agonists observed. The urologist responsible for the majority of prostate cancer-related initial treatment claims was considered the treating urologist.
Measures
Dependent Variable
GnRH agonist overuse was defined as having an initial treatment claim for a HealthCare Common Procedure Coding System code for a GnRH agonist administered within 1 year from date of diagnosis without another non-surveillance prostate treatment administered within the treatment window. Non-surveillance treatments included orchiectomy (not subject to reimbursement changes), radical prostatectomy, all forms of radiation therapy planned or delivered, chemotherapy, and cryotherapy (Appendix Table 1). Any primary use of GnRH agonists in localized disease was considered inappropriate use by the American Urological Association and NCCN clinical practice guidelines in effect during the study period and is thus considered overuse in our cohort (4, 7, 22, 23). The binary dependent variable compares men receiving primary GnRH agonists with men receiving prostate cancer treatments other than external beam radiation.
Explanatory Variables
Time in practice was calculated as the difference between SEER diagnosis date (averaged as the 15th day of month) and date of the urologists’ medical degree. We dichotomized time in practice (<20 versus ≥20 years). This specification best fit our data reflecting urology practice patterns, varying slightly from other studies (24). Practice type was taken from the AMA Masterfile in which physicians self-report their practice as solo or group. We also included a category for missing.
Control Variables
We controlled for changes due to MMA and other temporal factors by including indicator variables for diagnosis in pre-MMA (2000–2003); MMA implementation (2004–2005); and post-MMA (2006–2007) periods.
We controlled for: (1) physician gender; (2) medical professionalization defined as a binary indicator of board certification and degree of affiliation with an academic institution (none/some/missing);(25, 26) and (3) training location (U.S./non-U.S.). Practice factors included panel size, (tertile of Medicare fee-for-service prostate cancer patients/year/urologist) (27); and tertile of proportion of minority Medicare patients (28).
At the patient level, we controlled for grade (WHO categories 1/2/missing), stage (T1/T2), age, comorbidities (NCI Comorbidity Index with uniform weights (29, 30)) and marital status (married or living with partner/single, widowed, or divorced/missing). Missing categories were created because these data have been shown to be missing in a non-random manner (31). Thus, their exclusion may bias results. We also assessed proclivity to seek care (any primary care physician claim in the 12 months prior to diagnosis (32)) and men’s use of consultations in the prostate cancer treatment decision (33). A primary care consultation was >1 visit to the same primary care physician occurring in both 1) the 12 months prior to diagnosis; and, 2) the window between diagnosis and treatment (33). Specialist Care included three binary variables indicating the presence of ≥1 prostate-related claim filed by a radiation oncologist, urologist, or medical oncologist between diagnosis and the earlier of first treatment or 12 months (33). Geographic indicators included: SEER region; rurality of the patient’s community at diagnosis (<2,500 residents/≥2,500 residents); and community deprivation measured by quartile of median income of the patients’ residential zip code and quartile of proportion of adults without high-school education in the patients’ residential zip code.
Analysis
Unadjusted analyses included t-tests and analysis of variance (continuous variables) and Pearson chi-squared tests (binary/categorical variables). Because prior studies demonstrate high intraclass correlation among prostate cancer providers (9), we used multilevel mixed effects logistic regression models (34) to calculate odds ratios (OR), 95% confidence intervals (CI) and differential effects. We calculated the fixed portion of marginal effects in each MMA period using mean and modal values of covariates. Interaction terms were constructed to test differential effects of time in practice by MMA period.
Model fit was tested in a 50% random sample. Likelihood ratio tests determined appropriateness of inclusion of model constructs and allowing both intercepts and slopes to vary randomly by urologist. We compared random slope and random intercept model fit with the Bayesian Information Criterion (BIC). Stata/SE 12.1 was used for all analyses (35).
Sensitivity Analysis
Some men may have been eligible for GnRH agonists. Because we could not exclude men based on prostate-specific antigen (PSA) levels (1 of 3 criteria for stratification) (36), analyses were repeated among men with <5 years actuarial life expectancy at diagnosis: age <88 for all years, except 2004 (age 89) and 2005 (age 87) (37–43).
Results
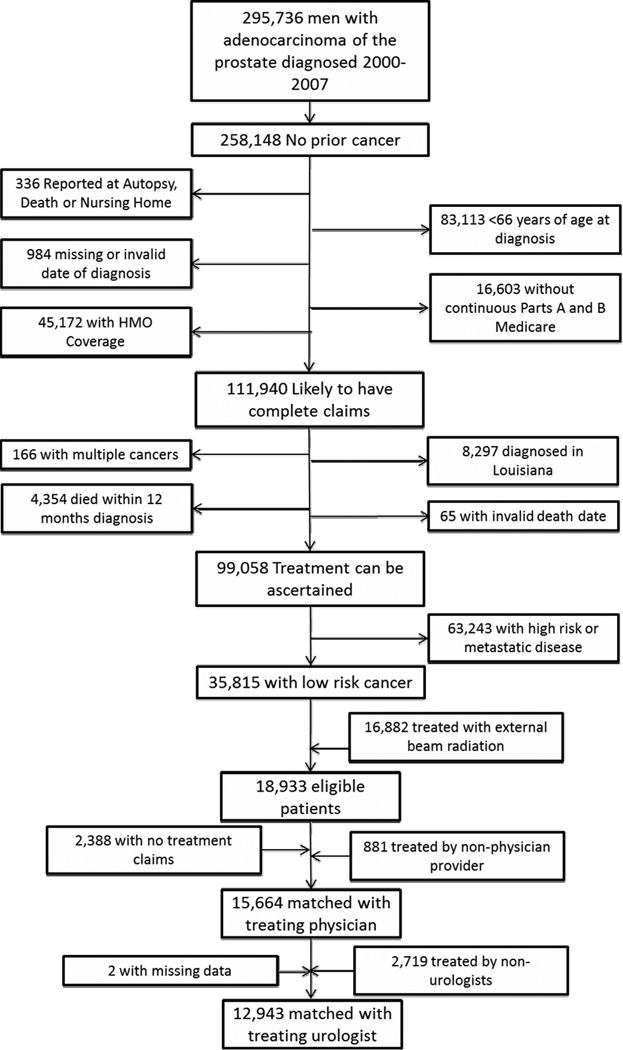
The final sample included 12,943 men diagnosed with T1 and T2 well- or moderately-differentiated prostate cancer from 2000 through 2007, and treated through 2008 by 2,138 urologists (Figure 1).
Figure 1.
Cohort Exclusions
GnRH Agonist Overuse
Among men treated 2000–2008, 18.5% received GnRH agonists without other therapy (Table 1). GnRH agonist overuse decreased from 21.0% before MMA implementation to 17.6% during the implementation phase, and to 13.6% following full policy deployment.
Table 1.
Patient Characteristics by Physician Practice Type
| Solo | Group | Missing | |||
|---|---|---|---|---|---|
| Mean (Standard Deviation) or n (%) | p-value | ||||
| N=2,749 | N=9,658 | N=536 | |||
| GnRH agonist overuse | 709 (25.8%) | 1,616(16.7%) | 75 (14.0%) | <0.001 | |
| MMA Implementation Period | <0.001 | ||||
| Pre-MMA | 1,601 (58.2%) | 5,202 (53.9%) | 221 (41.2%) | ||
| MMA Implementation | 590 (21.5%) | 2,261 (23.4%) | 139 (25.9%) | ||
| Post-MMA | 558 (20.3%) | 2,195 (22.7%) | 176 (32.8%) | ||
| T Stage | 0.09 | ||||
| T1 | 2,156 (78.4%) | 7,747 (80.2%) | 435 (81.2%) | ||
| T2 | 593 (21.6%) | 1,911 (19.8%) | 101 (18.8%) | ||
| Grade | <0.001 | ||||
| Well-differentiated, 2–4 | 188 (6.8%) | 441 (4.6%) | 21 (3.9%) | ||
| Moderately-differentiated, 5–7* | 2,496 (90.8%) | 9,023 (93.4)% | 506 (94.4%) | ||
| Missing | 65 (2.4%) | 194 (2.0%) | 9 (1.7%) | ||
| Comorbidities | <0.001 | ||||
| 0 | 1,754 (63.8%) | 6,569 (68.0%) | 385 (71.8%) | ||
| 1 | 633 (23.0%) | 2,041 (21.1%) | 99 (18.5%) | ||
| 2 | 197 (7.2%) | 643 (6.7%) | 30 (5.6%) | ||
| ≥3 | 165 (4.6%) | 405 (4.2%) | 22 (6.0%) | ||
| Mean Age (SD) | 74.9 (6.2) | 73.9 (6.1) | 73.7 (6.0%) | <0.001 | |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic White | 1,993 (72.5%) | 7,685 (79.6%) | 398 (74.3%) | ||
| Non-Hispanic Black | 179 (6.5%) | 632 (6.5%) | 48 (9.0%) | ||
| Hispanic | 310 (11.3%) | 618 (6.4%) | 40 (7.5%) | ||
| Other | 187 (6.8%) | 306 (3.2%) | 34 (6.3%) | ||
| Missing | 80 (2.9%) | 417 (4.3%) | 16 (3.0%) | ||
| Marital Status | <0.001 | ||||
| Not Married | 603 (19.0%) | 1,833 (19.7%) | 114 (21.9%) | ||
| Married | 1,791 (65.2% | 6,689 (69.3% | 374 (69.8%) | ||
| Missing | 355 (12.9%) | 1,136 (11.8%) | 48 (9.0%) | ||
| Visits in Previous Year | <0.001 | ||||
| 0–2 visits | 552 (20.1%) | 1,798 (18.6%) | 100 (18.7%) | ||
| 3–5 visits | 1,090 (39.7%) | 4,292 (44.4% | 257 (47.9%) | ||
| ≥6 visits | 1,107 (40.3% | 3,568 (36.9%) | 179 (33.4%) | ||
| Primary Care Consultation | 1,484 (54.0%) | 5,462 (56.6%) | 312 (58.2%) | 0.03 | |
| Radiation Oncology Consultation | 360 (13.1%) | 1,510 (15.6%) | 90 (16.8%) | 0.003 | |
| Medical Oncology Consultation | 80 (2.9%) | 367 (3.8%) | 25 (4.7%) | 0.04 | |
| Urology Consultation | 2,718 (98.9%) | 9,559 (99.0%) | 531 (99.1%) | 0.87 | |
| Rural Residence | 55 (2.0%) | 1484 (1.9%) | 8 (1.5%) | 0.73 | |
| SEER Region | <0.001 | ||||
| Seattle | 141 (5.1%) | 417 (4.3%) | 13 (2.4%) | ||
| Connecticut | 139 (5.1%) | 633 (6.6%) | 10 (1.9%) | ||
| Detroit | 192 (7.0%) | 686 (7.1%) | 30 (5.6%) | ||
| Hawaii | 29 (1.1%) | 83 (0.9%) | 10 (1.9%) | ||
| Iowa | 58 (2.1%) | 718 (7.4% | 23 (4.3%) | ||
| New Mexico | 132(4.8%) | 346 (3.6%) | 13 (2.4%) | ||
| California | 1,356 (49.3%) | 3,448 (35.7%) | 272 (50.7%) | ||
| Utah | 57 (2.1%) | 481 (5.0%) | 18 (3.4%) | ||
| Georgia | 52 (1.9%) | 267 (2.8%) | 25 (4.7%) | ||
| Kentucky | 267 (9.7%) | 964 (10.0%) | 29 (5.4%) | ||
| New Jersey | 326 (11.9%) | 1,615 (16.7%) | 93 (17.4%) | ||
| Median Income of Patients' Communities | <0.001 | ||||
| <$35,031 | 700 (25.5%) | 1,876 (19.4%) | 106 (19.8%) | ||
| $35,051–$46,079 | 674 (24.5%) | 2,432 (25.2%) | 108 (20.2%) | ||
| $46,084–$60,668 | 618 (22.5%) | 2,386 (24.7%) | 139 (25.9%) | ||
| $60,669–$200,008 | 632 (23.0%) | 2,556 (26.5%) | 157 (29.3%) | ||
| Missing | 125 (4.6%) | 408 (4.2%) | 26 (4.9%) | ||
| Proportion of Patient's Community w/o High-school Education | <0.001 | ||||
| 0%–9.7% | 584 (21.2%) | 2,589 (26.8%) | 156 (29.1%) | ||
| 9.7%–15.5% | 567 (20.6%) | 2,441 (25.3%) | 132 (24.6%) | ||
| 15.5%–25.2% | 652 (23.7%) | 2,233 (23.1%) | 100 (18.7%) | ||
| 25.2%–100% | 823 (29.9%) | 1,992 (20.6%) | 122 (22.8%) | ||
| Missing | 123 (4.5%) | 403 (4.2%) | 26 (4.9%) | ||
N=12,943
P-values by ANOVA for continuous variables, chi2 test for binary/categorical variables
Physician Practice Patterns
Although GnRH agonist overuse declined, unadjusted analysis showed three patterns of urologist response (Figure 2). Static users (n=1,478) had low levels of use at entry into the cohort and either continued to use primary GnRH agonists infrequently following MMA or contributed data in only one period. This group treated the most patients (n=5,809). Decreasing users (n=394), had highest levels of GnRH agonist overuse in 2000 (averaged 23.1% over the period), but decreased sharply in 2004 and, by 2008, were similar to static users. Increasing users (n=276) sharply increased GnRH agonist overuse in 2004 and maintained even higher levels of overuse after MMA implementation. Their average overuse was 32.6% among the 2,817 patients they treated over the study period. Non-responders, those with low rates of use remaining stable over time, differed from responders in both physician characteristics and patient panels (data not shown). Patient panels of increasing users were significantly different from those of decreasing users (Appendix Table 2), although urologists whose overuse increased were not different than urologists whose overuse decreased (Appendix Table 3). Increasing users’ patients were older, had more comorbid conditions, were more likely to be non-Hispanic black or “other” race, resided in communities with fewer resources, and were less likely to receive radiation oncology consultations.
Figure 2.
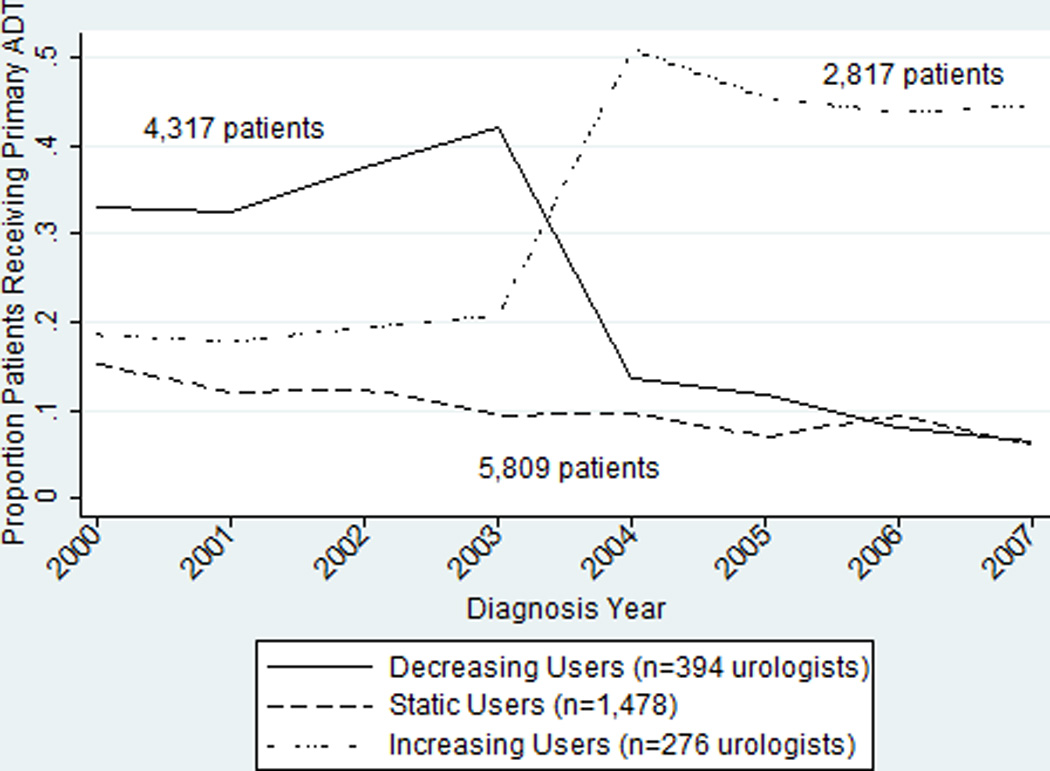
Unadjusted Change in GnRH Agonist Overuse by Year
Unadjusted Analysis
In unadjusted analysis, GnRH agonist overuse among urologists with greater time in practice declined less sharply compared to their less-practiced counterparts (Figure 3). Overall, 19.4% of patients of urologists with ≥20 years in practice received primary GnRH agonist whereas 16.9% of patients of urologists practicing <20 years received it. Compared to urologists in practice <20 years, patients of longer-practicing urologists were: slightly older, spread disproportionately across SEER regions, and more likely to be non-Hispanic black, Hispanic, unmarried, or live in communities with fewer resources. Urologists in practice ≥20 years also differed on physician characteristics. Across the study period GnRH agonist overuse among patients seen by a urologist practicing alone was higher than that among patients seen in a group practice or whose practice type was missing (25.8%, 16.7% and 14.0%,respectively, p<0.001; Table 1). Compared to patients seen in a group practice, patients of solo practitioners were: slightly older, spread disproportionately across SEER regions, more likely to be Hispanic or “Other” race/ethnicity, live in communities with fewer resources, and have well-differentiated tumors or missing grade information and have more comorbidities (Table 2).
Figure 3.
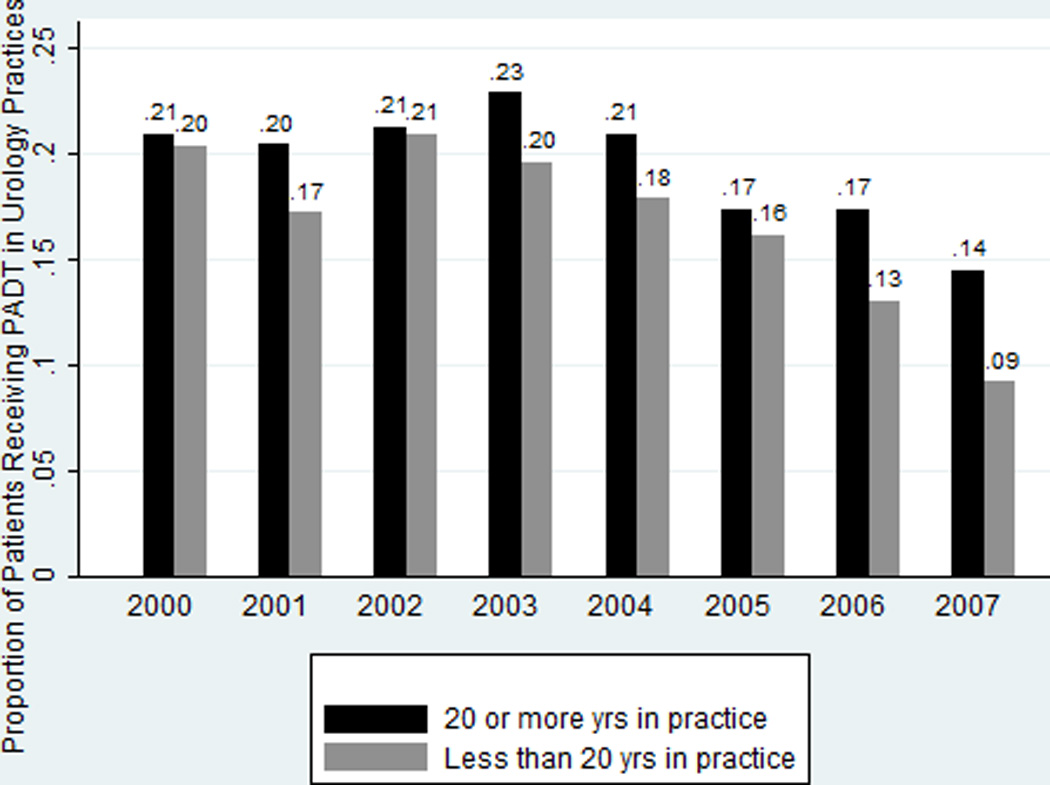
Unadjusted Change in GnRH Agonist Overuse by Time in Practice
Table 2.
Physician Characteristics by Physician Practice Type
| Solo | Group | Missing | |||
|---|---|---|---|---|---|
| Mean (Standard Deviation) or n (%) | p-value | ||||
| N=462 | N=1,545 | N=131 | |||
| Mean Primary GnRH agonist Use 2000–2007 (SD) | 0.3 (0.3) | 0.2 (0.3) | 0.1 (0.2) | <0.001 | |
| Time in Practice | 385 (29.5%) | 879 (67.4%) | 41 (3.1%) | <0.001 | |
| Male* | 1–5* (5–10%) | 41 (83.7%) | 5–10* (10–15%) | <0.001 | |
| Board Certified | 407 (20.4%) | 1,471 (73.8%) | 115 (5.8%) | <0.001 | |
| US Trained | 330 (18.4%) | 1,349 (75.0%) | 119 (6.6%) | <0.001 | |
| Medical School Affiliation | <0.001 | ||||
| None | 258 (27.2%) | 639 (67.4%) | 51 (5.4%) | ||
| Some | 192(16.6%) | 886 (76.7%) | 77 (6.7%) | ||
| Missing* | 10–15* (30–35%) | 20 (57.1%) | 1–5* (5–10%) | ||
| Physician Prostate Panel Size (prostate patients/year) | <0.001 | ||||
| 0–20 | 302 (26.2%) | 768 (66.6%) | 83 (7.2%) | ||
| 21–37 | 119 (18.3%) | 498 (76.5%) | 34 (5.2%) | ||
| ≥38 | 41 (12.3%) | 279 (83.5%) | 14 (4.2%) | ||
| Proportion Patients Minority | <0.001 | ||||
| 0%–6.1% | 146 (18.8%) | 582 (75.1%) | 47 (6.1%) | ||
| 6.2%–19.5% | 110 (18.4%) | 457 (76.6%) | 30 (5.0%) | ||
| ≥20.0% | 206 (26.9%) | 506 (66.1%) | 54 (7.1%) | ||
N=2,138
P-values by t-test for continuous variables, chi2 test for binary/categorical variables; time invariant physician measures described at first entry into cohort
Exact count masked to protect subject confidentiality, per Data Use Agreement
Adjusted Analysis
A multilevel random intercepts model best fit training data; coefficients were similar in validation data. The interaction of time in practice and MMA period was not jointly significant and therefore not included in the model (Table 3). After adjusting for patient and urologist characteristics and secular changes, time in practice was not associated with GnRH agonist overuse or increasing overuse (OR 0.89, 95% CI 0.75, 1.05). Being seen by a solo practitioner substantially increased the odds of GnRH agonist overuse compared to being seen in group practice (OR 1.65, 95% CI 1.34, 2.02). Having a medical school affiliation was associated with lower odds of overuse (OR 0.65, 95% CI 0.55, 0.77), compared to those with no affiliation.
Table 3.
Multilevel Logistic Regression Model of Time in Practice and Practice Type on GnRH Agonist Overuse
| OR | 95% CI | ||
|---|---|---|---|
| Time in Practice | 0.89 | 0.75, 1.05 | |
| Solo Practitioner (compared to group practice)* | |||
| Yes | 1.65 | 1.34, 2.02 | |
| Missing | 0.82 | 0.55, 1.23 | |
| Physician Gender | 0.92 | 0.45, 1.89 | |
| Board Certification | 1.00 | 0.71, 1.41 | |
| US Training | 0.78 | 0.62, 0.99 | |
| Medical School Affiliation (compared to none)* | |||
| Some | 0.65 | 0.55, 0.77 | |
| Missing | 0.82 | 0.39, 1.73 | |
| Prostate Patient Panel Size (compared to 0–20 patients/year) | |||
| 21–37 | 1.04 | 0.89, 1.21 | |
| ≥38 | 1.00 | 0.83, 1.22 | |
| Proportion Patients Minority (compared to 0%–6.1% minority)* | |||
| 6.2%–19.5% | 0.79 | 0.66, 0.93 | |
| ≥20.0% | 0.81 | 0.66, 1.00 | |
| MMA ImplementationPeriod (compared to Pre-MMA)* | |||
| MMA Period | 0.78 | 0.68, 0.91 | |
| Post-MMA | 0.54 | 0.46, 0.64 | |
| T Stage | 1.36 | 1.19, 1.57 | |
| Grade (compared to Well-differentiated) | |||
| Moderately-differentiated 5–7* | 3.12 | 2.33, 4.17 | |
| Missing | 4.90 | 3.15, 7.60 | |
| Comorbidities (Compared to None) | |||
| 1 | 1.29 | 1.13, 1.48 | |
| 2 | 1.25 | 1.01, 1.55 | |
| ≥3 | 1.46 | 1.14, 1.87 | |
| Patient Age (continuous) | 2.30 | 1.88, 2.82 | |
| Patient Age squared | 1.00 | 0.99, 1.00 | |
| Race/ethnicity (compared to Non-Hispanic White)* | |||
| Non-Hispanic Black | 1.76 | 1.37, 2.27 | |
| Hispanic | 1.41 | 1.12, 1.79 | |
| Other | 1.44 | 1.04, 1.99 | |
| Missing | 1.84 | 1.40, 2.41 | |
| Marital Status (compared to Unmarried) | |||
| Married | 0.91 | 0.79, 1.05 | |
| Missing | 1.64 | 1.34, 1.99 | |
| Pre-treatment Primary Care Use (compared to 0–2 visits in prior year)* | |||
| 3–5 | 1.64 | 1.38, 1.96 | |
| ≥6 | 1.73 | 1.44, 2.10 | |
| Primary Care Consultation | 0.42 | 0.37, 0.48 | |
| Radiation Oncology Consultation | 1.73 | 1.47, 2.05 | |
| Medical Oncology Consultation | 1.03 | 0.76, 1.40 | |
| Urology Consultation | 6.03 | 2.93, 12.41 | |
| Rural Residence | 0.94 | 0.62, 1.44 | |
| Region (compared to Seattle) | |||
| Connecticut | 4.45 | 2.56, 7.74 | |
| Detroit | 2.39 | 1.36, 4.19 | |
| Hawaii | 4.78 | 2.02, 11.30 | |
| Iowa | 3.38 | 1.90, 6.01 | |
| New Mexico | 2.63 | 1.37, 5.04 | |
| California | 2.54 | 1.56, 4.15 | |
| Utah | 1.46 | 0.76, 2.83 | |
| Georgia | 2.44 | 1.27, 4.70 | |
| Kentucky | 3.17 | 1.85, 5.44 | |
| New Jersey | 5.49 | 3.32, 9.08 | |
| Median Income of Patients' Communities (compared to <$35,031) | |||
| $35,051–$46,079 | 0.83 | 0.69, 1.00 | |
| $46,084–$60,668 | 0.83 | 0.66, 1.05 | |
| $60,669–$200,008 | 0.66 | 0.50, 0.88 | |
| Missing | 6.60 | 0.72, 60.11 | |
| Proportion of Patient's Community w/o High-school Education (compared to <9.7%) | |||
| 9.7%–15.5% | 1.18 | 0.98, 1.43 | |
| 15.5%–25.2% | 1.24 | 0.99, 1.55 | |
| 25.2%–100% | 1.17 | 0.89, 1.54 | |
| Missing | 0.11 | 0.01, 0.99 | |
| Constant | 0.00 | 0.00, 0.00 | |
N=12,943 patients; 2,138 urologists
- Solo practitioner— X2=574.55, p<0.001
- Medical school affiliation—X2=24.36, p<0.001
- Proportion patients minority— X2=7.90, p=0.019
- MMA implementation period — X2=59.46, p<0.001
- Race/ethnicity— X2=39.33, p<0.001
At the patient level, increased age, greater comorbidity, being in a racial/ethnic minority, high primary care utilization in the prior year, and receiving a radiation oncology consultation prior to treatment were associated with increased odds of GnRH agonist overuse (Table 3). Receiving a primary care consultation (OR 0.42; 95% CI 0.37–0.48) and being in the highest income category (OR 0.66; 95% CI 0.50–0.88) were associated with lower odds of GnRH agonist overuse.
In sensitivity analysis, removing 453 men guideline-ineligible for primary GnRH agonists by virtue of age and diagnosis year produced similar results. Differential effects based on covariates’ mean and modal values confirmed OR interpretations (Appendix Table 4).
Discussion
Consistent with other studies, we found that GnRH agonist overuse declined precipitously over the 2000s, coincident with reimbursement policy changes (8, 13). Rates of overuse found in this study were between other published estimates and reflected a blending of two previously published sampling strategies and outcome definitions (8, 13). Further, overuse continued on a downward trajectory in the two years following MMA implementation. Nonetheless, GnRH agonist overuse remained, with 14% of men with T1 or T2 well- or moderately-differentiated tumors prescribed primary GnRH agonists in the post-MMA period.
Although GnRH agonist overuse significantly declined in the aggregate, we identified three distinct responses to the reimbursement change: static users, decreasing users, and increasing users. We were unable to distinguish increasing users from decreasing users based on physician characteristics measured. Increasing users’ access to referral networks and urologist-level preferences for medical versus surgical intervention may explain these findings and deserve further study. Although we cannot distinguish whether urologists were responding to a changing patient population, these urologists were more likely to treat older patients and minority patients. No evidence suggests these patients benefit from GnRH agonists and they may have risk profiles making them more vulnerable to the harms of overuse. Future research should examine whether overuse in these populations, which remains over 30%, resulted from unmeasured frailty, incomplete disease severity reporting, patient preference, or physician bias. Moreover, future research should explore why urologists of these patients may be reluctant to encourage active surveillance (44).
Among all urologists, time in practice was not associated with GnRH agonist overuse, which is consistent with two earlier studies (27, 45), but not others (15). Reimbursement context may account for these mixed results. Studies finding an association of time in practice and poor quality of care were conducted in HMOs, HMO-penetrated states, and the Canadian health system, rather than in fee-for-service Medicare (15, 46). This suggests that more practiced physicians may succumb to clinical inertia or lapsed technical skill, but aligning financial incentives with quality of care may moderate this effect.
We found higher GnRH agonist overuse among solo practitioners than urologists in group practices (25.7% versus 17.1% of patients). Because more urologists (23%) practice as solo practitioners than any other surgical specialty (16), they are an important target for intervention. However, it will be important to understand why solo practitioners appear less responsive to MMA policy changes. Studies examining physician characteristics of GnRH agonist overuse have not considered practice type (27, 45), but solo practice has been identified as a barrier to innovation adoption (47). Whether the added financial vulnerability of urologists practicing on their own, isolation from timely and relevant knowledge, or lack of peer comparison discourages adoption of quality practice should be determined. Meaningful use provisions and efforts to reorganize practices into accountable care networks have placed pressure on smaller and solo practices to be subsumed into larger multispecialty and academic practices (48). These practice-level changes may result in new patterns of care; however, further study of environmental and/or culture change to promote quality is needed.
Urologists who overuse GnRH agonists also were more likely to lack affiliation with a medical school, which may make them hard to reach for intervention. Medical school-affiliated physicians may have resources (e.g., trainees, tumor boards) that encourage guideline-concordant practice; however, they may also lack motivation for financial gain (49). We cannot identify reasons for the association, but either could make it difficult to engage these urologists in traditional quality improvement efforts.
Our study has several limitations. First, generalizability may be limited because we: (1) excluded patients treated by non-physician providers; (2) restricted our study to urologists because they make most treatment decisions about GnRH agonist for patients with localized prostate cancer; and, (3) did not study men ≤65 years. Also limiting generalizability is that patients in SEER may not reflect the US. However the SEER population is comparable to the US along many demographic characteristics (18) and characteristics of urologists in our study mirror national trends (50). Second, administrative claims data are subject to errors in coding which can potentially lead to biased results (51). However, variables required to support payment of claims (e.g., treatment) have been shown to be highly reliable, especially those that are well remunerated such as GnRH agonists (52–55). Thus we would not expect the measurement of GnRH agonist overuse to be affected. Finally, we lacked PSA information necessary to exclude a small group of men with high-risk disease. However, misclassification should be small because only 14% of all prostate cancer patients (including those with advanced disease) are thought to have PSA levels >20 ng/mL—the cut point for high-risk disease and many men with high-risk disease had more than one risk factor. Moreover, when conducting a sensitivity analysis that removed patients with ≥5 years life expectancy, the results did not change.
Conclusions
Approximately 30–40% of healthcare spending in the U.S. has been attributed to overuse, the provision of unnecessary care for which harms outweigh benefits (56, 57). Overuse results in patient harms, health disparities, and waste in a healthcare system already stretched to capacity (57). Despite being designated a significant quality problem and national priority (58), relatively little research focuses on the problem of overuse or strategies to address it (56). We identified important nuances in response to reimbursement changes designed to limit overuse, consequences of persistent overuse, and a group of urologists who may need additional support in reducing GnRH agonist overuse, which remains high among professionally isolated urologists, despite a significant decline during and after MMA implementation.
Supplementary Material
Acknowledgements
Work on this study was supported by the Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund (UCRF) via the State of North Carolina. The American Medical Association is the source for the raw physician data. The National Cancer Institute provided the SEER-Medicare linked data and reviewed the manuscript for potential privacy violations. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The statistics, tables, ideas and opinions expressed herein are those of the author(s) and endorsement by the American Medical Association, State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
FUNDING
Dr. Ellis was supported by a National Cancer Institute training grant (R25CA116339) and a University of North Carolina Lineberger Comprehensive Cancer Center Dissertation Completion Award. Dr. Nielsen was supported in part by The American Cancer Society (grant number MRSG-13-154-01-CPPB) and The Urology Care Foundation / Astellas (Rising Stars in Urology Research Award). Dr. Weinberger was supported by a Senior Research Career Scientist Award (91–408) from the U.S. Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service.
Footnotes
This study was peer-reviewed and presented at the American Society of Clinical Oncology in June 2014.
Conflicts of Interest
The authors declare no conflict of interest in relation to this work.
References
- 1.Wilt TJ, Shamliyan T, Taylor B, MacDonald R, Tacklind J, Rutks I, et al. Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer. Rockville, MD: 2008. Feb, 2008. Report No.: 08-EHC010-EF Contract No.: 08-EHC010-EF. [PubMed] [Google Scholar]
- 2.National Cancer Institute. Bethesda, MD: National Cancer Institute; 2010. [cited 2010 October 21, 2010]. SEER Cancer Statistics Review, 1975–2007. Available from: http://seer.cancer.gov/csr/1975_2007/. [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahnson RR, Hanks GE, Huben RP, Kantoff P, Kozlowski JM, Kuettel M, et al. NCCN Practice Guidelines for Prostate Cancer. Oncology (Williston Park) 2000;14(11A):111–119. [PubMed] [Google Scholar]
- 5.Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schroder FH, Sternberg CN, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61(1):11–25. doi: 10.1016/j.eururo.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leape LL, Berwick DM. Five years after To Err Is Human: what have we learned? JAMA. 2005;293(19):2384–2390. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Prostate Cancer. 2004 [Google Scholar]
- 8.Elliott SP, Jarosek SL, Wilt TJ, Virnig BA. Reduction in physician reimbursement and use of hormone therapy in prostate cancer. J Natl Cancer Inst. 2010;102(24):1826–1834. doi: 10.1093/jnci/djq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98(12):839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating NL. Medicare reimbursement and prescribing hormone therapy for prostate cancer. J Natl Cancer Inst. 2010;102(24):1814–1815. doi: 10.1093/jnci/djq467. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson M, O'Malley AJ, Earle CC, Pakes J, Gaccione P, Newhouse JP. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25(2):437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson M, Earle CC, Price M, Newhouse JP. How Medicare's payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29(7):1391–1399. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary MP, Baum NH, Bohnert WW, Blizzard R, Bonney WW, Cooper TP, et al. 2003 American Urological Association Gallup survey: physician practice patterns, cryosurgery/brachytherapy, male infertility, female urology and insurance/professional liability. J Urol. 2004;171(6 Pt 1):2363–2365. doi: 10.1097/01.ju.0000127745.26501.5e. [DOI] [PubMed] [Google Scholar]
- 15.Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260–273. doi: 10.7326/0003-4819-142-4-200502150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Quessential Medical Market Research. Physician Outlook: Urology. Exeter, NH: Quessential Medical Market Research; 2013. Spring. 2013. Report No. [Google Scholar]
- 17.Damiani G, Silvestrini G, Federico B, Cosentino M, Marvulli M, Tirabassi F, et al. A systematic review on the effectiveness of group versus single-handed practice. Health Policy. 2013;113(1–2):180–187. doi: 10.1016/j.healthpol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. SEER-Medicare. Bethesda, MD: National Cancer Institute; 2011. [cited 2011 October 13, 2011]. Available from: http://healthservices.cancer.gov/seermedicare/. [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Services CfMaM, editor. Medicare and Medicaid Statistical Supplement, 2013. 2013 ed. Baltimore, MD: Centers for Medicare and Medicaid Services; 2013. Office of Information Products and Data Analysis (OIPDA) [Google Scholar]
- 21.Baldwin LM, Adamache W, Klabunde CN, Kenward K, Dahlman C, J LW. Linking physician characteristics and medicare claims data: issues in data availability, quality, and measurement. Med Care. 2002;40(8 Suppl):IV-82–IV-95. doi: 10.1097/00005650-200208001-00012. [DOI] [PubMed] [Google Scholar]
- 22.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Prostate Cancer. 2008 [Google Scholar]
- 24.Freiman MP. The rate of adoption of new procedures among physicians. The impact of specialty and practice characteristics. Med Care. 1985;23(8):939–945. doi: 10.1097/00005650-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Wright JD, Neugut AI, Wilde ET, Buono DL, Malin J, Tsai WY, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29(25):3408–3418. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid RO, Friedberg MW, Adams JL, McGlynn EA, Mehrotra A. Associations between physician characteristics and quality of care. Arch Intern Med. 2010;170(16):1442–1449. doi: 10.1001/archinternmed.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol. 2007;25(34):5359–5365. doi: 10.1200/JCO.2006.09.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack CE, Bekelman JE, Liao KJ, Armstrong K. Hospital racial composition and the treatment of localized prostate cancer. Cancer. 2011 doi: 10.1002/cncr.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of epidemiology. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 31.Elliott SP, Johnson DP, Jarosek SL, Konety BR, Adejoro OO, Virnig BA. Bias due to missing SEER data in D'Amico risk stratification of prostate cancer. The Journal of urology. 2012;187(6):2026–3031. doi: 10.1016/j.juro.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 32.Feldstein PJ. Health Policy Issues: An Economic Perspective. Fourth ed. Chicago: Health Administration Press; 2007. [Google Scholar]
- 33.Jang TL, Bekelman JE, Liu Y, Bach PB, Basch EM, Elkin EB, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170(5):440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raudenbush S, Bryk A. Hierarchical linear and nonlinear modeling. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 35.StataCorp LP. Stata/IC 12.1 for Windows. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 36.D'Amico AV, Whittington R, Malkowicz SB, Wu YH, Chen M, Art M, et al. Combination of the preoperative PSA level, biopsy gleason score, percentage of positive biopsies, and MRI T-stage to predict early PSA failure in men with clinically localized prostate cancer. Urology. 2000;55(4):572–577. doi: 10.1016/s0090-4295(99)00479-3. [DOI] [PubMed] [Google Scholar]
- 37.Arias E United States life tables, 2000. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2002;51(3):1–38. [PubMed] [Google Scholar]
- 38.Arias E United States life tables, 2002. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2004;53(6):1–38. [PubMed] [Google Scholar]
- 39.Arias E United States Life Tables, 2001. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2004;52(14):1–38. [PubMed] [Google Scholar]
- 40.Arias E United States life tables, 2003. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2006;54(14):1–40. [PubMed] [Google Scholar]
- 41.Arias E United States life tables, 2004. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2007;56(9):1–39. [PubMed] [Google Scholar]
- 42.Arias E United States life tables, 2006. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;58(21):1–40. [PubMed] [Google Scholar]
- 43.Arias E United States life tables, 2007. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;59(9):1–60. [PubMed] [Google Scholar]
- 44.Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156(8):591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eccles MP, Foy R, Sales A, Wensing M, Mittman B. Implementation Science six years on--our evolving scope and common reasons for rejection without review. Implementation science : IS. 2012;7:71. doi: 10.1186/1748-5908-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehrotra A, Reid RO, Adams JL, Friedberg MW, McGlynn EA, Hussey PS. Physicians with the least experience have higher cost profiles than do physicians with the most experience. Health Aff (Millwood) 2012;31(11):2453–2463. doi: 10.1377/hlthaff.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott SD, Plotnikoff RC, Karunamuni N, Bize R, Rodgers W. Factors influencing the adoption of an innovation: an examination of the uptake of the Canadian Heart Health Kit (HHK) Implementation science : IS. 2008;3:41. doi: 10.1186/1748-5908-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaughan A, Coustasse A. Accountable care organization musical chairs: will there be a seat remaining for the small group or solo practice? [corrected] Hospital topics. 2011;89(4):92–97. doi: 10.1080/00185868.2011.627814. [DOI] [PubMed] [Google Scholar]
- 49.Borges NJ, Navarro AM, Grover A, Hoban JD. How, when, and why do physicians choose careers in academic medicine? A literature review. Academic medicine : journal of the Association of American Medical Colleges. 2010;85(4):680–686. doi: 10.1097/ACM.0b013e3181d29cb9. [DOI] [PubMed] [Google Scholar]
- 50.Neuwahl S, Thompson K, Fraher E, Ricketts T. HPRI data tracks. Urology workforce trends. Bulletin of the American College of Surgeons. 2012;97(1):46–49. [PubMed] [Google Scholar]
- 51.Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2007;11(11):1488–1496. doi: 10.1007/s11605-007-0282-0. discussion 96–7. [DOI] [PubMed] [Google Scholar]
- 52.Virnig B, editor. SEER-Medicare Training Workshop. Bethesda, MD: National Institutes of Health; 2011. SEER-Medicare Training Workshop. [Google Scholar]
- 53.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 Suppl):IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 54.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 Suppl):IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 55.Lund JL, Sturmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, et al. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care. 2013;51(5):e27–e34. doi: 10.1097/MLR.0b013e31823ab60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171–178. doi: 10.1001/archinternmed.2011.772. [DOI] [PubMed] [Google Scholar]
- 57.National Priorities Partnership. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, D.C.: 2008. [Google Scholar]
- 58.National Priorities Partnership. Input to the Secretary of Health and Human Services on Priorities for the National Quality Strategy. Washington, D.C.: National Quality Forum; 2011. Sep 1, 2011. Report No. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.