Abstract
Objectives
Secondary caries is the most common reason for composite restoration replacement and usually forms between dentin and the filling. The objective of this study was to investigate the combined effect of cyclic loading and bacterial exposure on bacterial penetration into gaps at the interface between dentin and resin composite restorative material using a novel bioreactor system and test specimen design.
Methods
Human molars were machined into 3 mm thick disks with 2 mm deep × 5 mm diameter cavity preparations into which composite restorations were placed. A ∼15-30 micrometer (small) or ∼300 micrometer wide (large) dentin-restoration gap was introduced along half of the interface between the dentin and restoration. Streptococcus mutans UA 159 biofilms were grown on each sample prior to testing in a bioreactor both with and without cyclic loading. Both groups of samples were tested for 2 weeks and post-test biofilm viability was confirmed with a live-dead assay. Samples were fixed, mounted and cross-sectioned to reveal the gaps and observe the depth of bacterial penetration.
Results
It was shown that for large gap samples the bacteria easily penetrated to the full depth of the gap independent of loading or non-loading conditions. The results for all cyclically loaded small gap samples show a consistently deep bacterial penetration down 100% of the gap while the average penetration depth was only 67% for the non-loaded samples with only two of six samples reaching 100%.
Significance
A new bioreactor was developed that allows combining cyclic mechanical loading and bacterial exposure of restored teeth for bacterial biofilm and demineralization studies. Cyclic loading was shown to aid bacterial penetration into narrow marginal gaps, which could ultimately promote secondary caries formation.
Keywords: Resin Composite, Marginal Gap, Biofilm, Streptococcus mutans, Cyclic Loading, Secondary Caries
1 Introduction
Key driving forces behind the increased use of dental composite materials are their tooth-like appearance [1] and lack of the potentially toxic element mercury that is present in amalgam [2,3]. Additional clinical advantages include enhanced conservation of tooth structure [4], the ability to be bonded to enamel and dentin surfaces [5], convenient handling, and the ready availability of a wide range of tooth shades. However, annual failure rates up to 15% have been reported for composite restorations, depending on restoration class [6], and a review of the literature has suggested the average lifetime of posterior dental composites is only six years [7].
The most common reason for restoration replacement is secondary tooth decay (dental caries) at a dentin-restoration interface [8-12], while the second most observed reason is restoration fracture caused by occlusal loading combined with chemical and physical degradation of the restorative material in the oral environment [13]. Furthermore, it is known that the appearance of secondary caries is associated with bacterial biofilm formation on the dentin-restoration interface. Indeed, the biofilm byproduct lactic acid promotes demineralization of the adjacent dentin which can lead to tooth decay [14,15].
Although bacterial biofilm formation is considered a necessary ingredient, the presence of the biofilm alone does not guarantee secondary tooth decay [16]. Numerous studies have identified the presence of bacteria within marginal gaps between the restoration and the dentin [17-19], and the presence of such gaps is likely an important factor as well. No clinical correlation between marginal gap size and bacterial colonization has been found for resin based composite restorations [20]; however, clinical data is limited and recent in vitro evidence from microbial caries models shows demineralization along the cavity walls of composite restorations increasing in magnitude with larger gap sizes [21,22]. Overall, there is a need for further studies to better understand the factors that control secondary caries formation at the marginal interface between composite restorations and tooth dentin.
Another complication is that teeth are also subjected to cyclic loading during mastication, bruxism (grinding), etc. Cyclic loading may promote marginal gap formation and growth; indeed, some studies have shown the degradation of restoration margins during cyclic mechanical loading [23-27]. Furthermore, it has been demonstrated that the presence of bacteria and a marginal gap does not guarantee further caries formation [15,28,29]. Thus, there may be an additional role of cyclic mechanical loading beyond simply creating a marginal gap or growing it above a critical size to allow bacterial penetration. Based on a survey of the available literature, understanding the mechanism of recurrent decay at the margins of dental composite restorations likely requires the evaluation of the simultaneous effects of bacterial biofilm presence, marginal gaps, and cyclic loading. To date, no such studies have been reported.
Accordingly, the objectives of this paper are the following: 1) to describe a novel bioreactor based test method and test specimen design that has been developed to allow cyclic mechanical loading of simulated tooth restorations within a growing oral biofilm environment, and 2) to study the synergistic effects associated with bacteria and cyclic loading on the marginal penetration of bacteria around dental restorations.
2 Materials and Methods
2.1 Bioreactor Fatigue Test System
While bioreactor systems are commonly used for the in vitro study of oral biofilms in controlled laboratory settings [30-35], to date no such systems allow the simultaneous application of cyclic loading to the test sample. The goal was to develop a simulated tooth restoration sample that is practical to reproducibly manufacture and test to study dental restorations in a simulated oral environment with growing biofilms and without external contamination. The sealed bioreactor must provide nutrients to the bacteria in a 5% CO2 environment at 37°C, while applying cyclic stress to the restoration.
For the sample design, the physiological size of human teeth dictated the maximum possible dimensions. Based on a survey of typical human teeth it was determined 9 mm diameter disks could be readily machined from the crowns of human molars. Such disks allow the placement of a 5 mm diameter composite restoration on one side (Fig. 1a).
Fig 1.
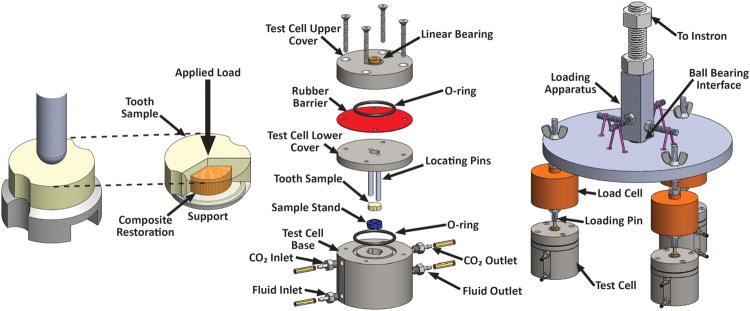
(a) Schematic of the simulated tooth filling sample and the loading configuration; at right, the dentin is made transparent to observe the composite disk and support ring. (b) Exploded view of a bioreactor test cell. (c) Schematic of the load distribution system for loading three bioreactor test cells simultaneously.
A simple radial symmetric biaxial bending and shear loading geometry was selected to allow even loading on the composite/dentin interface through the backside of bonded restorations with no gaps (Fig. 1a). Loading is applied in the center of the sample by a hemispherical loading rod, while the sample sits on a ring shaped support. The loading rod was guided into the center of the bioreactor using linear bearings that were housed in the bioreactor upper covers (Fig. 1b). To avoid external contamination, the loading rods passed through a flexible rubber barrier where the hole was undersized relative to the rod diameter to ensure a seal was maintained throughout testing. The use of individual bioreactor cells provided a sealed system for individually testing each simulated restoration (Fig. 1b). The bioreactors were fabricated from stainless steel to provide a durable, stiff platform for loading the specimens while also minimizing corrosion and allowing for autoclave sterilization. Bioreactors consisted of five interlocking elements denoted as the upper cover, lower cover, rubber barrier, base and sample stand. The two cover elements and base were manufactured from 316 stainless steel and made up the exterior of the bioreactor. Viton O-rings were used between parts to ensure a sealed system connected with four 316 stainless steel bolts. In order to create an active area for loading, the sample stand (Figs. 1a & 1b) was designed with a 3.5 mm radius rounded edge ring that supported the sample. The sample stand was machined from 17-4 stainless steel heat treated at 482°C for 60 minutes after machining to achieve peak strength for supporting the specimens. Locating pins were integrated into the lower cover and allowed for the sample to be both located and constrained within the bioreactor and centered over the sample stand for symmetric loading. These locating pins were designed to mesh with the bioreactor base, sample stand and sample itself.
Required nutrients and pH buffering were provided via brain-heart infusion (BHI) liquid media. 5% CO2 in air was flowed through the upper fluid inlet/outlet set (Fig. 1b) and controlled with a pressure regulator. BHI media flowed through the lower fluid inlet/outlet set (Fig. 1b). The BHI media flow was designed with the inlet set below the sample and the outlet set above the sample to ensure that liquid covered the entire specimen throughout testing and fresh media continuously flowed past the bottom, or stressed, side of the sample. Liquid flow was controlled using low flow peristaltic pumps (Model FB 70381, Thermo Fisher Scientific Inc., Waltham, MA, USA), with one pump per bioreactor inlet (pushing) and an additional pump on each bioreactor outlet (pulling) to minimize fluids “backing-up” within the chambers. The tube dimensions were 3.175 mm inside diameter and 6.35 mm outside diameter (Fischer Scientific, Pittsburgh, PA, USA).
Cyclic fatigue stress was applied using a computer controlled, servohydraulic fatigue test system (Model 8872, Instron Corporation, Norwood, MA, USA). A ball bearing was used to evenly distribute the load to three loading rods for three separate bioreactors (Fig. 1c). Applied loads were collected for each sample with individual universal fatigue load cells (Model LCF300, FUTEK Advanced Sensor Technology Inc., Irvine, CA, USA). The load distribution system was monitored over a two week period of cyclic loading and the maximum difference between bioreactors never exceeded 5%.
Temperature was maintained throughout testing with the use of a temperature controlled water bath. The bioreactors and inlet fluid tubing were partially immersed in deionized (DI) water and the temperature was controlled using a digital immersion circulator (Polystat, Cole-Parmer, Vernon Hills, IL, USA). Within the bath, the temperature at the bioreactor locations was found to be maintained at 37 ± 0.2 °C using thermocouples. To protect the bath water from mold/bacterial contamination, 5 ml of SterilityAqua-clean (Thermo Fisher Scientific Inc., Waltham, MA, USA) was added per each liter of water. The sealing of the individual bioreactors was verified by pumping water dyed with red food coloring through the entire system for two weeks and observing that no color change occurred in the bath water.
2.2 Sample preparation
Simulated dental restorations were produced in recently extracted human molar teeth (Fig. 1a) that were disinfected by storage in 0.5% chloramine-T solution. The teeth were mounted in dental stone to the cementoenamel junction, and then horizontally sectioned on a diamond saw to remove the cusps. The teeth were carefully examined for cracks, caries and presence of enamel on the flattened tooth surface. If cracks or caries were found, the tooth was discarded. If enough enamel remained such that the entire gap portion of the cavity preparation margin would not be in dentin, the specimen was reduced using 180 grit sandpaper until adequate dentin was uncovered. The mounted specimen was placed in a computer-controlled milling system (CNC specimen former, U. Of Iowa) and subjected to three preparation regimens: 1) The first preparation flattened the sectioned occlusal surface to ensure a uniformly finished and flattened tooth surface; 2) the second prepared the exterior tooth dimensions to a circular 9 mm diameter dimension with two slots opposing each other for placement of the guiding pins on the bioreactor; 3) the final program resulted in 5 mm diameter, 2 mm deep cylindrical cavity preparations. The teeth were then horizontally sectioned to just above the pulp horns, leaving a slab of tooth 3 mm thick and 9 mm diameter with a flat surface of predominantly dentin with some peripheral enamel. The composite used in this study had a resin composed of a 50:50 mixture of bisphenol A glycidyl methacrylate (BisGMA):triethylene glycoldimethacrylate (TEGDMA) monomers with 0.4 wt% of camphorquinone (CQ), 0.8 wt% of 4-dimethylaminobenzoic acid ethyl ether (EDMAB), and 0.05 wt% of 3, 5-di-tertbutyl-4-hydroxytoluene (BHT). The composite was produced by combining the resin with 67 wt% silanated strontium glass (3.0 μm average size, Bisco Inc., Schaumburg, IL, USA) and 5 wt% aerosol-silica filler (OX-50, Degussa) and mixed at 3000 rpm for at least two minutes in a centrifugal speed mixer (DAC 150, Flacktek, South Carolina).
To observe bacterial colonization of marginal gaps, roughly half of the circumference of the restorations were produced with an interfacial gap. The cavity was filled with composite by first applying a dentin bonding agent to the floor and one half of the walls of the cavity. A self-etch, two-step adhesive (Clearfil SE Bond) was used. The self-etching primer was applied for 20 seconds with light scrubbing of the dentin walls and floor and then lightly air dried. The adhesive resin was applied to the cavity and light cured for 10 seconds. In order to keep the adhesive off of the surface not meant to be bonded, a flexible shim made of polyvinylsiloxane impression material was molded to the wall to protect it during adhesive application. After curing the adhesive, the composite was placed into the cavity either with a lubricated metal shim to produce a large gap ∼300 μm, or no shim which allowed the composite to shrink away from the non-bonded wall producing small gaps of approximately 15-30 μm. The composite was cured with a single exposure from the curing light (20 seconds - total radiant exposure ∼12J/cm2; Demi Light, Kerr, Orange, CA, USA). Then the shim, if present, was removed. The sides and bottom of the dentin samples were then coated with a dental adhesive (Optibond FL, Kerr, Orange, CA, USA) in order to seal them from the acidic environment and prevent demineralization of these surfaces. Finally, the surface of the cavity margins were exposed by polishing with coarse grit silicon carbide disks (Sof-lex, 3M ESPE, St. Paul, MN, USA) in a slow-speed handpiece. The specimens were then stored in sterile water before being further sterilized.
2.3 Sample Sterilization
Two sample sterilization techniques were evaluated, one using a sodium hypochlorite (bleach) solution and one using 1% chloramine T. For the bleach procedure, freshly prepared tooth cavities were sterilized by 1) soaking in 50% household bleach solution for 1 h in an ultrasonic water bath, 2) soaking for 10 min in 5% sodium thiosulfate solution in water ultrasonic bath to remove the chlorine, and 3) washing three times in autoclave sterilized deionized (DI) water for 5 minutes in water in an ultrasonic bath. Sterilized teeth were filled with composites and then put into 15 mL autoclaved brain heart infusion (BHI) media and incubated at 37°C, 5% CO2 and 95% relative humidity (BBD 6220 incubator, Thermo, Asheville, NC, USA) to assess sterility. Samples were observed over a >2 week period to ensure the media stayed clear and no biofilm appeared on the surface indicating the samples were sterile.
For the chloramine T procedure, the teeth with prepared cavities were stored for one week in 1% chloramine T at 22°C then rinsed with autoclaved water and stored in sterile water overnight before being filled with composite. After filling, the specimens were soaked for one hour in 70% ethanol, and then washed three times with sterile water before being placed in to BHI media to check for sterilization as described above. It should be noted that other sterilization procedures, such as UV, ethylene oxide gas, prolonged alcohol soaking, were all tried, but caused damage/changes to the tooth and/or composite and were therefore discontinued.
2.4 Biofilm growth procedure
For samples tested with a living biofilm, after the sterilization procedure the samples were co-cultured with Streptococcus mutans using the following procedure. Bacterial cultures were prepared by growing Streptococcus mutans (UA 159 from American Type Culture Collection (ATCC), Manassas, VA) in planktonic form using BHI media. The cultures were monitored by visible light at 600nm to ensure that the bacteria were growing in logarithmic phase. Once they reached a suitable density, 6ml of bacterial broth/well was transferred to culture plates containing the samples. At this time, trypticase soy broth (TSB) with 3% sucrose was used to encourage biofilm formation. Samples were incubated under previously described conditions for 4 days, changing media each day, until a visible biofilm was observed.
2.5 Test Procedure
Two weeks was chosen as the length of test based on typical demineralization studies found in the literature [36,37]. Indeed, results within this study showed that two weeks was long enough to see a robust, measurable demineralization. The cyclic (sine wave) loading schedule was chosen as alternating blocks of two hours cyclic loading at 1.5 Hz and four hours held at the minimum load. A maximum load of ∼113 N was used, which is 25% of the average breaking force (450 N) for samples tested using monotonically increasing quasi-static loading (n=5). The minimum cyclic load was always set as 10% of the maximum load, or 11.3 N. The four hour resting time was chosen to represent a normal time between meals and also should provide ample opportunity for the biofilm to grow and potentially double [39] before being subjected to loading again. While the two hours cyclic loading time is not clinically relevant, it provides a compromise to attain a large number of cycles within the total test period. Considering that a person may chew at about 1.5 Hz [38], and that the actual chewing time per day may be conservatively estimated as about 20 minutes, this would equate to 1800 chews/day or 657,000 chews/year. An intermittent cycling phase of two hours followed by four hours without cycling gives a total of 43,200 cycles per day, or approximately 605,000 cycles in two weeks. Thus, the total number of cycles is equivalent to nearly one year of normal human exposure.
Table 1 shows the four types of experiments that were conducted to assess both the viability of the bioreactor fatigue testing system and synergistic effects of mechanical loading and bacteria (Table 1). Many of the procedures were common among experiments while differences are noted in Table 1 and the following section.
Table 1. Summary of experiments.
| I | sterile BHI media | none |
|
| II | Streptococcus mutans in BHI media | none |
|
| III | Streptococcus mutans in BHI media | cyclic |
|
| IV | Streptococcus mutans in BHI media | cyclic |
|
For type I & III experiments the bleach sterilization procedure was used since it enabled the maintenance of a 100% sterile environment through the course of the two week test, ensuring the system design was adequately sealed from external contaminants. However, it was also found that this procedure deteriorated the dentin. This deterioration was observed as a whitening of the tissue after the test, observed primarily after drying, and a loss of mechanical integrity that caused the dentin to erode under the loading rod during type III experiments (Fig. 2). Thus, for type II & IV experiments the chloramine-T procedure was used. While chloramine-T was found not to degrade the dentin, it also was found not to give 100% sterilization. However, it was observed that this sanitation procedure was adequate to allow S. mutans biofilm growth without excessive competition from other organisms found in the tooth tissue.
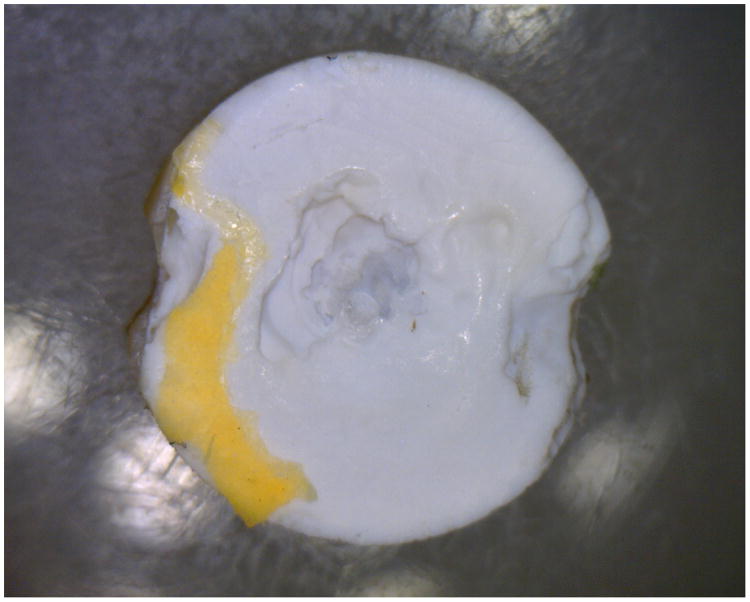
Fig 2.
Image of the discoloration and dentin erosion on the loading surface of the specimen directly under the loading pin that occurred for type III experiments using the bleach sterilization method.
Dehydrated BHI media (Thermo Fisher Scientific Inc., Waltham, MA, USA) was prepared according to manufacturer instructions. A separate 2L Erlenmeyer glass flask was used as the media supply for each bioreactor. Each flask was sealed by a rubber stopper with a glass tube (7mm dia.) penetrating it and reaching the bottom of the flask. When the fluid was pumped out of the flask, air was allowed to enter the flask through a stainless steel syringe needle penetrating the rubber stopper. A 0.2 μm syringe filter (Acrodisc@ Syringe filter 0.2 μm, Pall Corporation, Port Washington, NY USA) was inserted on the needle in order to avoid contamination of the media from the air. The flow of media was maintained at approximately 0.5 ml/min per bioreactor using the peristaltic pumps.
Each 2L of media was autoclaved along with the flask, glass tube, cork, tubing, bioreactors, and loading rods for 45 minutes at 221 °C. All components were then allowed to cool to room temperature under sterile conditions in a BioSafety Cabinet (Baker SterilGARD III, The Baker Company, Sanford, ME, USA) prior to assembling the bioreactor with the sample inside. The media source was replenished approximately every three days by replacing the flasks with freshly prepared media. A 5% CO2-air mixture was flowed through a 0.2 μm syringe filter into each bioreactor at a low flow rate to just maintain visible bubbles at the outlet. The enhanced CO2 helps provide pH buffering for the media.
Media leaving the bioreactors was collected into a waste container and used to check for contamination during the type I & III experiments. Every three days one drop of waste media from the exit tube of each bioreactor was placed onto a BHI agar plate (Anaerobe systems, Morgan Hill, CA, USA) and incubated for 24-48 hours to check for contamination.
After completion of the type I & III experiments, the bioreactors were opened in a biosafety cabinet and samples were put into 10 ml sterile BHI media and again incubated using the previously described conditions to ensure the absence of bacterial activity. After the completion of type II & IV experiments, samples were subjected to a live/dead staining procedure (Life Technologies Live/Dead BacLight Bacterial Viability Kit) following the kit manufacture's protocol and the biofilm was evaluated under confocal fluorescent microscopy to ensure the biofilm was still viable at the end of the experiments. Once sterility or biofilm viability were confirmed the samples were fixed in 10 ml 4%-gluteraldehyde and held at a temperature of 4°C overnight.
For gap analysis the biofilm was carefully removed from the surface with a swab, avoiding the gap area, and the surface was impressed with a dental vinyl polysiloxane impression material (Aquasil Ultra, Dentsply). The impression was later poured in epoxy to make a replica of the surface and the margin for examination in the SEM to determine if debonding or further gap formation occurred as a result of the loading in the bioreactor. The specimens were gram positive stained by applying crystal violet dye (Difco Laboratories, Detroit MI, USA), rinsing, and applying iodine to bind the dye to the bacteria. Samples were then mounted in LR white resin (London Resin Company Ltd, Reading, Berkshire England), sectioned in half on a slow speed diamond saw, and finally examined under the stereomicroscope for the presence of dentin demineralization and the extent of penetration of the stained bacterial biofilm. The sample half with the greatest apparent demineralization from the stereomicroscopic evaluation was further examined with confocal laser scanning microscopy (BioRad/Zeiss Radiance 2100 confocal laser scanning system) using 4×/0.2 objective. A GRE/Ne laser source with excitation band at 543 nm wavelength and a 570 nm long pass filter was used to detect the stained bacteria in the biofilm. Image J (NIH) was used to assess bacterial penetration, which was quantified as a fraction of the total depth of the gap and a student's t-test was used to compare the loaded and non-loaded cases with α≤ 0.05 considered statistically significant.
3 Results and Discussion
Most bioreactor studies are conducted in a well controlled biosafety cabinet environment where potential sources of external contamination (bacteria, fungi, etc.) can be easily controlled. In the present study, that was done for type I and II experiments. In contrast, a significant challenge for the current system was to develop a bioreactor and test protocol sufficiently robust to integrate into a standard servo-hydraulic fatigue testing system found in a typical laboratory environment. Type I and III experiments both served to ensure external contaminants did not enter the bioreactors during the two week test period and that the samples did not degrade in the absence of bacteria. Results of both type I and III experiments using the above procedures typically showed no external contamination of the system during the testing period. However, observations of bleach sterilized samples from type I and III tests indicated structural degradation of the samples (Fig. 2) that motivated the use of chloramine T as an alternative sample sterilizing agent.
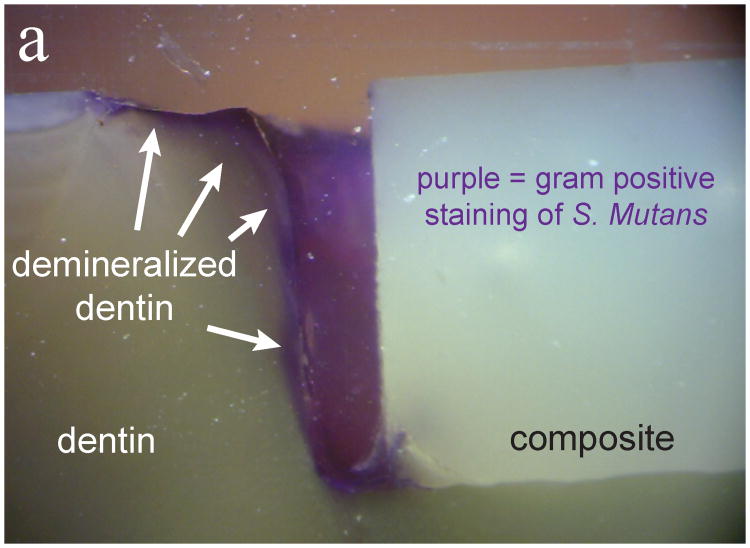
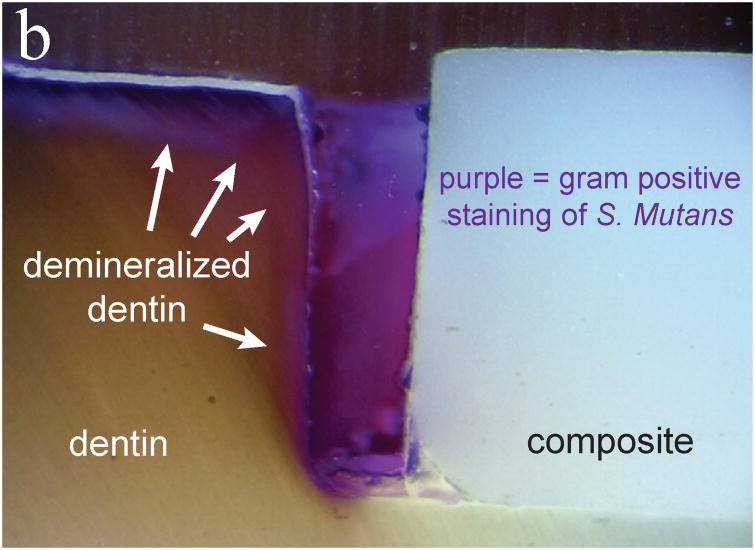
For types II and IV experiments, live/dead staining after each experiment showed that biofilms were viable on all samples. Further gram positive staining and cross sectioning revealed biofilm presence on the surface, in the gaps of the samples, and penetrating to the full depth of the gap for the large gap samples. As Fig. 3 shows, observations of the marginal gaps revealed that for large gap (∼300 μm) samples, there was considerable bacterial penetration and demineralization both with and without loading (Type II and Type IV experiments). Once it was found that bacteria penetrated easily into the large gaps both with and without loading (Fig. 3), experiments were instead focused primarily on the small gap samples.
Fig 3.
Evaluation of marginal gaps of large gap sample. Panel (a) is with loading (type IV) and panel (b) is without loading (type II). Purple gram positive staining shows the bacterial penetration into the full extent of the gaps while darkening of the dentin indicates demineralization (white arrows).
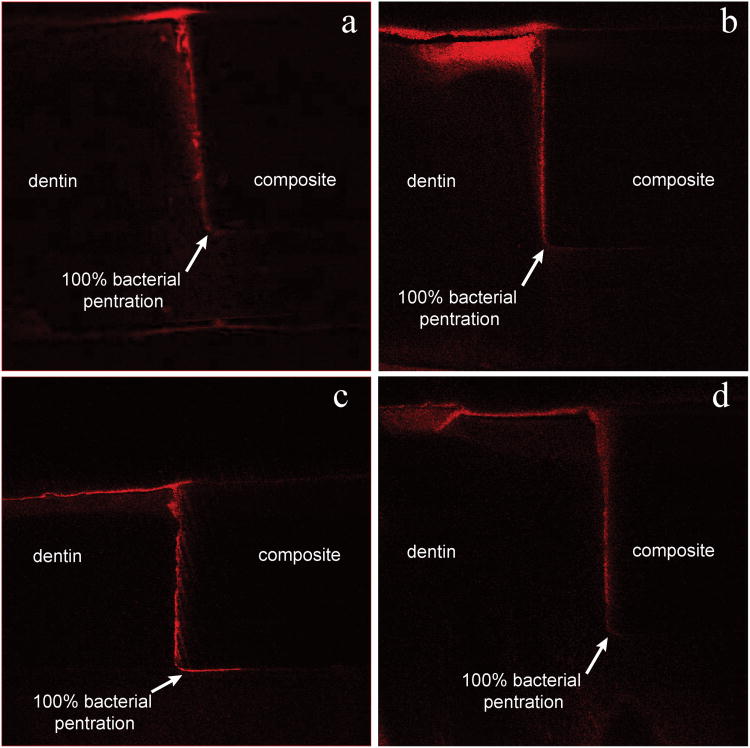
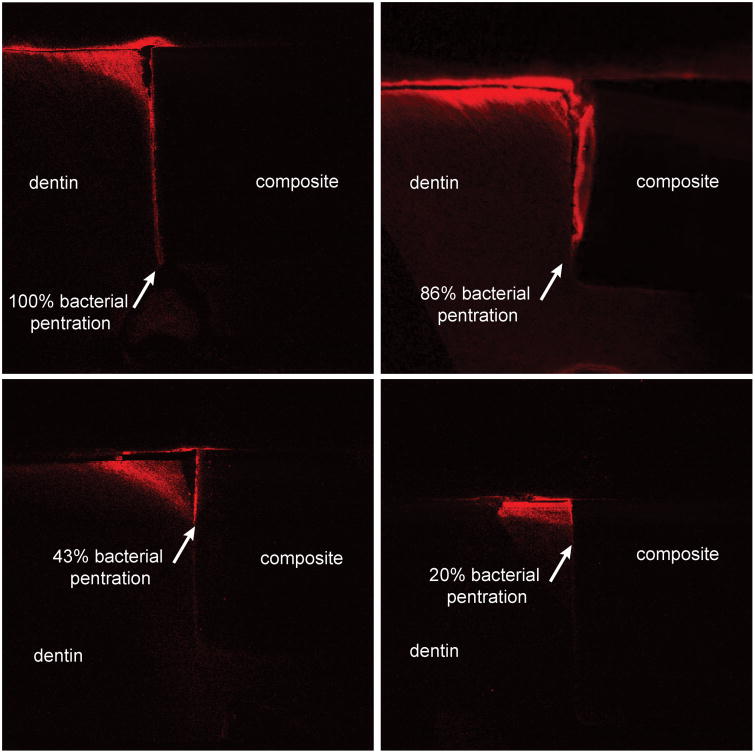
Samples with small gaps showed differences in bacterial penetration depending on whether the sample was cyclically loaded (Fig. 4) or not loaded (Fig. 5). With fatigue loading (Fig. 4) the bacteria penetrated deeply to the floor of the cavity preparation for all six samples. With no loading, the biofilm penetration down the gap was often less severe (Fig. 5). Only two of six samples had full biofilm penetration and the average penetration depth was only 67%. The results for all samples are summarized in Table 2.
Fig 4.
Panels a – d show marginal gaps of four different loaded small gap samples, all panels are configured as in Figs. 3 & 6. Red shows the bacterial penetration, which was fully to the bottom of the restoration for all loaded samples.
Fig 5.
Panels a – d show marginal gaps of four different non-loaded small gap samples, all panels are configured as in Figs. 3 & 6. Red shows the bacterial penetration, which was highly variable for the non-loaded samples.
Table 2. Percentage of biofilm penetration down the gap for loaded and non-loaded samples.
| Sample | Loaded | Non-loaded |
|---|---|---|
| 1 | 100 | 50 |
| 2 | 100 | 100 |
| 3 | 100 | 100 |
| 4 | 100 | 86 |
| 5 | 100 | 43 |
| 6 | 100 | 20 |
| mean (stdv) | 100 (0) | 66.6 (33.5) |
Unlike the consistent results for the cyclically loaded samples, penetration depth for the non-loaded samples was highly variable (20% - 100%). A student's t-test comparing the mean penetration depths for loaded and non-loaded samples showed the difference was statistically significant (p < 0.05). Although there is large scatter in the non-loaded sample data, it can be clearly seen that four out of six of the non-loaded samples had incomplete bacterial penetration. Demineralization within the small confines of the interfacial gap likely results in a saturated solution and an increase in pH above the critical level required for demineralization to occur. In a stagnant environment, demineralization is likely limited or ceases altogether. Thus, cyclic loading is aiding bacterial penetration which may be due to a hydraulic pumping effect whereby the gap closes and opens under the cyclic loading, helping to bring fresh media and bacterial cells into the gap, while possibly removing some of the saturated solution and bacterial waste from the gap.
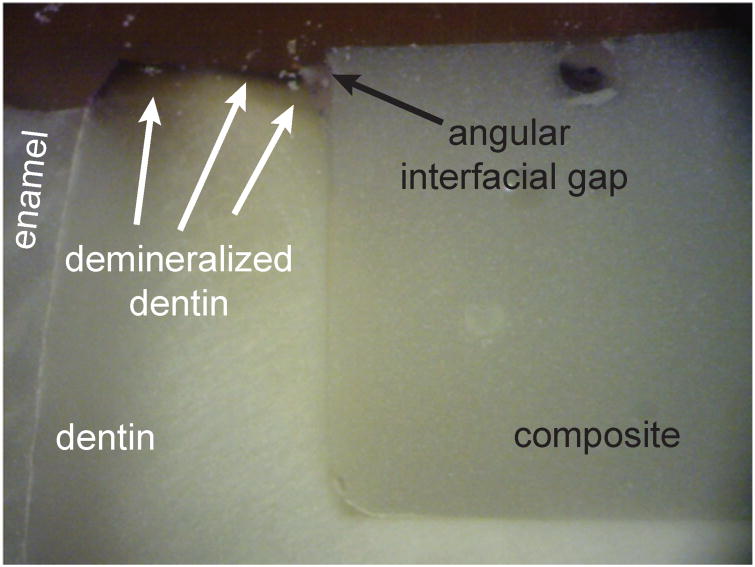
Finally, the well bonded margins were evaluated after loading (type IV) experiments and results revealed superficial angular gap formation and bacterial demineralization to be accelerated along the bonded interface relative to the bulk dentin (Fig. 6). Bacterial exposure in this case leads to a local stress concentration that over time, combined with cyclic loading, may lead to the complete interface debonding, deep bacterial penetration, and secondary caries around and under the restoration.
Fig 6.
A well bonded margin evaluated for a cyclically loaded sample showing preferred demineralization at the bonded composite-dentin interface relative to the bulk dentin.
Based on Fig. 3, it may be concluded that when the gaps are large (∼300 μm wide) bacteria have free access to colonize the gaps and demineralize the dentin along the marginal interface. Accordingly, one may expect the effect of cyclic loading for large gap samples to be minimal. In contrast, for small gap samples (∼15 – 30 μm wide) access of the bacteria deep into the gap appears to be more difficult, especially with no cyclic loading (Fig. 5). Accordingly, it is likely that the synergistic effects of bacterial exposure and loading are most important early in the process of gap formation and secondary caries development. Currently the mechanistic role of cyclic loading is unclear; however, one hypothesis might be that a pumping effect due to cyclic loading allows nutrients and bacteria to flow more easily into the small gaps while possible helping to remove saturated fluid and harmful metabolic waste products. Alternatively, the bacteria may sense the stress on the surfaces which somehow aids the attachment and colonization process. In any case, the bioreactor fatigue test system presented here represents a reasonable methodology for the study of the role of cyclic loading on biofilm colonization, proving that the combination of cyclic loading and bacterial exposure assists secondary caries propagation. Furthermore, it will also be useful for evaluating new restorative materials (e.g., such as those described in [40]) that are intended to slow or prevent secondary tooth decay.
4 Conclusions
Based on an experimental study using a novel bioreactor system and test methodology to study the synergistic effects of cyclic loading and bacterial exposure on simulated tooth fillings in vitro, the following conclusions can be made:
It was found that complete sterilization of the samples was achieved using a sodium hypochlorite based procedure, but significant mechanical degradation of the tooth tissue occurred. In contrast, a chloramine-T based procedure did not achieve perfect sterilization; however, the mechanical integrity of the sample remained intact.
Cyclic loading did not appear to affect biofilm penetration into large gaps (∼ 300 μm wide) since bacteria appear to have easy access. In contrast, non-loaded experiments using small gap (∼ 15 – 30 μm wide) samples demonstrated deep 100% penetration of bacteria into the gaps for only two out of six samples while cyclic loading caused deep 100% bacterial penetration for all samples. Mean penetration depth was found to be 67% vs. 100% for non-loaded versus load samples, respectively.
There is strong in vitro evidence of a synergetic effect of cyclic loading and bacteria exposure that aides bacterial biofilm penetration at the dentin-restoration margin, possibly leading to faster secondary tooth caries development in vivo.
We developed a bioreactor system for in vitro cyclic loading of tooth filling samples
We grew biofilms on tooth filling samples with and without applied cyclic loading
Bacteria penetrated deeper into marginal gaps for the cyclically loaded samples
We report evidence of a synergetic effect of cyclic loading and bacteria exposure
Acknowledgments
This work was supported by NIH/NIDCR GRANT DE021372. The authors thank Jonathon Cummins and Max Breedlove for their assistance developing the first bioreactor prototype. The authors thank Fernanda Gwinner for help with sample preparation and Michael Danilchik for help with the confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lohbauer U, Frankenberger R, Kramer N, Petschelt A. Strength and fatigue performance versus filler fraction of different types of direct dental restoratives. Journal of Biomedical Materials Research. 2006;76B:114–120. doi: 10.1002/jbm.b.30338. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NH, Dunne SM, Gainsford ID. Current materials and techniques for direct restorations in posterior teeth. Part 2: Resin composite systems. International Dental Journal. 1997;47:185–193. doi: 10.1111/j.1875-595x.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 3.Hickel R, Manhart J, Garcia-Godoy F. Clinical results and new developments of direct posterior restorations. American Journal of Dentistry. 2000;13:41–54D. [PubMed] [Google Scholar]
- 4.Combe EC, Burke FJT. Contemporary resin-based composite materials for direct placement restorations: packables, flowables and others. Dental Update. 2000;27:326–332. 334–326. doi: 10.12968/denu.2000.27.7.326. [DOI] [PubMed] [Google Scholar]
- 5.Manhart J, Kunzelmann KH, Chen HY, Hickel R. Mechanical properties of new composite restorative materials. Journal of Biomedical Materials Research. 2000;53:353–361. doi: 10.1002/1097-4636(2000)53:4<353::aid-jbm9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Hickel R, Kaaden C, Paschos E, Buerkle V, Garcia-Godoy F, Manhart J. Longevity of occlusally-stressed restorations in posterior primary teeth. Am J Dent. 2005;18:198–211. [PubMed] [Google Scholar]
- 7.Downer MC, Azli NA, Bedi R, Moles DR, Setchell DJ. How long do routine dental restorations last? A systematic review. Br Dent J. 1999;187:432–439. doi: 10.1038/sj.bdj.4800298a1. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 9.Marks LAM, Weerheijm KL, van Amerongen WE, Groen HJ, Martens LC. Dyract versus Tytin class II restorations in primary molars: 36 months evaluation. Caries Research. 1999;33:387–392. doi: 10.1159/000016538. [DOI] [PubMed] [Google Scholar]
- 10.Mjor IA, Dahl JE, Moorhead JE. Placement and replacement of restorations in primary teeth. Acta Odontol Scand. 2002;60:25–28. doi: 10.1080/000163502753471961. [DOI] [PubMed] [Google Scholar]
- 11.Ostlund J, Moller K, Koch G. Amalgam, composite resin and glass ionomer cement in Class II restorations in primary molars--a three year clinical evaluation. Swed Dent J. 1992;16:81–86. [PubMed] [Google Scholar]
- 12.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children's Amalgam Trial. J Am Dent Assoc. 2007;138:763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 13.Attin T, Opatowski A, Meyer C, Zingg-Meyer B, Buchalla W, Monting JS. Three-year follow up assessment of Class II restorations in primary molars with a polyacid-modified composite resin and a hybrid composite. Am J Dent. 2001;14:148–152. [PubMed] [Google Scholar]
- 14.Loesche WJ. Microbiology of Dental Decay and Periodontal Disease. In: Baron S, editor. Medical Microbiology. The University of Texas Medical Branch at Galveston; Galveston TX: 1996. [PubMed] [Google Scholar]
- 15.Mjör IA, Toffenetti OF. Secondary caries: A literature review with case reports. Quintessence Int. 2000;31:165–179. [PubMed] [Google Scholar]
- 16.Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38:182–191. doi: 10.1159/000077753. [DOI] [PubMed] [Google Scholar]
- 17.Preussker S, Klimm W, Poschmann M, Koch R. Microbial ingrowth around single- and multi-component adhesives studied in vitro. Caries Res. 2003;37:345–351. doi: 10.1159/000072166. [DOI] [PubMed] [Google Scholar]
- 18.Splieth C, Bernhardt O, Heinrich A, Bernhardt H, Meyer G. Anaerobic microflora under Class I and Class II composite and amalgam restorations. Quintessence Int. 2003;34:497–503. [PubMed] [Google Scholar]
- 19.Zivkovic S, Bojovic S, Pavlica D. Bacterial penetration of restored cavities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:353–358. doi: 10.1067/moe.2001.113345. [DOI] [PubMed] [Google Scholar]
- 20.Rezwani-Kaminski T, Kamann W, Gaengler P. Secondary caries susceptibility of teeth with long-term performing composite restorations. J Oral Rehabil. 2002;29:1131–1138. doi: 10.1046/j.1365-2842.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 21.Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43:97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 22.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–473. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal V, Logani A, Jain V, Shah N. Effect of cyclic loading on marginal adaptation and bond strength in direct vs. indirect class II MO composite restorations. Oper Dent. 2008;33:587–592. doi: 10.2341/07-152. [DOI] [PubMed] [Google Scholar]
- 24.Arisu HD, Uctasli MB, Eliguzeloglu E, Ozcan S, Omurlu H. The effect of occlusal loading on the microleakage of class V restorations. Oper Dent. 2008;33:135–141. doi: 10.2341/07-49. [DOI] [PubMed] [Google Scholar]
- 25.Campos PE, Barceleiro Mde O, Sampaio-Filho HR, Martins LR. Evaluation of the cervical integrity during occlusal loading of Class II restorations. Oper Dent. 2008;33:59–64. doi: 10.2341/07-35. [DOI] [PubMed] [Google Scholar]
- 26.Pongprueksa P, Kuphasuk W, Senawongse P. Effect of elastic cavity wall and occlusal loading on microleakage and dentin bond strength. Oper Dent. 2007;32:466–475. doi: 10.2341/06-132. [DOI] [PubMed] [Google Scholar]
- 27.Vandewalle KS, Ferracane JL, Hilton TJ, Erickson RL, Sakaguchi RL. Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dent Mater. 2004;20:96–106. doi: 10.1016/s0109-5641(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 28.Turkun LS, Aktener O, Ates M. Clinical evaluation of different posterior resin composite materials: A 7-year report. Quintessence Int. 2003;34:418–426. [PubMed] [Google Scholar]
- 29.Heintze SD. Systematic reviews: I. The correlation between laboratory tests on marginal quality and bond strength. II. The correlation between marginal quality and clinical outcome. Journal of Adhesive Dentistry. 2007;9:77–106. [PubMed] [Google Scholar]
- 30.Blanc V, Isabal S, Sanchez MC, Llama-Palacios A, Herrera D, Sanz M, Leon R. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J Periodontal Res. 2013 doi: 10.1111/jre.12110. [DOI] [PubMed] [Google Scholar]
- 31.Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, Reilly C, Fok AS, Aparicio C. A reproducible oral microcosm biofilm model for testing dental materials. J Appl Microbiol. 2012;113:1540–1553. doi: 10.1111/j.1365-2672.2012.05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ahmad A, Wiedmann-Al-Ahmad M, Auschill TM, Follo M, Braun G, Hellwig E, Arweiler NB. Effects of commonly used food preservatives on biofilm formation of Streptocaccus mutans in vitro. Arch Oral Biol. 2008;53:765–772. doi: 10.1016/j.archoralbio.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Ledder RG, McBain AJ. An in vitro comparison of dentifrice formulations in three distinct oral microbiotas. Arch Oral Biol. 2012;57:139–147. doi: 10.1016/j.archoralbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Rudney J, Aparicio C, Fok A, Jones RS. Quantifying dental biofilm growth using cross-polarization optical coherence tomography. Lett Appl Microbiol. 2012;54:537–542. doi: 10.1111/j.1472-765X.2012.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayati F, Okada A, Kitasako Y, Tagami J, Matin K. An artificial biofilm induced secondary caries model for in vitro studies. Aust Dent J. 2011;56:40–47. doi: 10.1111/j.1834-7819.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 36.Buren JL, Staley RN, Wefel J, Qian F. Inhibition of enamel demineralization by an enamel sealant, Pro Seal: an in-vitro study. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133:S88–S94. doi: 10.1016/j.ajodo.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Cho A, Suzuki S, Hatakeyama J, Haruyama N, Kulkarni AB. A method for rapid demineralization of teeth and bones. The open dentistry journal. 2010;4:223. doi: 10.2174/1874210601004010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braem M, Lambrechts P, Vanherle G. Clinical relevance of laboratory fatigue studies. J Dent. 1994;22:97–102. doi: 10.1016/0300-5712(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Wong L, Sissons CH. Human dental plaque microcosm biofilms: effect of nutrient variation on calcium phosphate deposition and growth. Arch Oral Biol. 2007;52:280–289. doi: 10.1016/j.archoralbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Khvostenko D, Mitchell JC, Hilton TJ, Ferracane JL, Kruzic JJ. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent Mater. 2013:1139–1148. doi: 10.1016/j.dental.2013.08.207. [DOI] [PMC free article] [PubMed] [Google Scholar]