Abstract
The purpose of this study was to determine whether sex differences in the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol (THC) are due to activational effects of gonadal hormones. Rats were sham-gonadectomized (sham-GDX) or gonadectomized (GDX). GDX females received no hormone replacement (GDX+0), estradiol (GDX+E2), progesterone (GDX+P4), or both (GDX+E2/P4). GDX male rats received no hormone (GDX+0) or testosterone (GDX+T). Two weeks later, antinociceptive potency of THC was determined (pre-chronic test) on the warm water tail withdrawal and paw pressure assays. Vehicle or a sex-specific THC dose (females, 5.7 mg/kg, males, 9.9 mg/kg) was administered twice-daily for 9 days, then the THC dose-effect curves were re-determined (post-chronic test). On the pre-chronic test (both assays), THC was more potent in sham-GDX females than males, and gonadectomy did not alter this sex difference. In GDX females, P4 significantly decreased THC’s antinociceptive potency, whereas E2 had no effect. In GDX males, T did not alter THC’s antinociceptive potency. After chronic THC treatment, THC’s antinociceptive potency was decreased more in sham-GDX females than males, on the tail withdrawal test; this sex difference in tolerance was not altered in GDX or hormone-treated groups. These results suggest that greater antinociceptive tolerance in females, which occurred despite females receiving 40% less THC than males, is not due to activational effects of gonadal hormones.
Keywords: Cannabinoid, Sex Differences, Testosterone, Progesterone, Estradiol
1. Introduction
Sex differences in the behavioral effects of cannabinoid agonists have been reported by several laboratories. For example, drugs such as Δ9-tetrahydrocannabinol (THC), WIN55,212-2 and CP55,940 are more potent or effective in female than male rats on measures of analgesia, anxiolysis, sedation and reward (Tseng and Craft, 2001; Romero et al., 2002; Fattore et al., 2007; Wakley and Craft, 2011; Harte-Hargrove et al., 2012; Craft et al., 2012; 2013). Moreover, a recent study in chronic cannabis users demonstrated greater abuse-related subjective effects in women than in men (Cooper and Haney, 2014). In contrast, male rats and guinea pigs are more sensitive than females to the hyperphagic effects of cannabinoid agonists (Miller et al., 2004; Diaz et al., 2009). Male-female differences in acute behavioral effects of cannabinoids may result from sex differences in pharmacokinetic (Narimatsu et al., 1991; Tseng et al., 2004; Wiley and Burston, 2014), pharmacodynamic (Rodriguez de Fonseca et al., 1994), and hormonal factors (Craft and Leitl, 2008; Wakley and Craft, 2011).
Tolerance to various effects of cannabinoid agonists develops readily upon repeated drug administration, and tolerance development may vary by sex. For example, Wiley (2003) demonstrated that 3 days of twice-daily injection with 10 mg/kg THC led to tolerance development in a locomotor activity test in female but not male mice. In rats, 9 days of twice-daily THC administration led to greater antinociceptive tolerance in females than in males (Wakley et al., 2014b), as well as greater dependence in females, albeit on only one of multiple measures of precipitated withdrawal (Marusich et al., 2014). Furthermore, in the first study the tolerance-inducing dose was adjusted for sex differences in acute potency of THC, such that females received approximately 30% less THC than males did during the chronic treatment period, yet THC potency still decreased more in females than males. Epidemiological evidence indicates that women are more susceptible than men to rapid development of abuse and dependence on a variety of addictive substances, including marijuana (Khan et al., 2013; Lewis et al., 2014), and women are more likely than men to report physical withdrawal symptoms upon cessation of chronic cannabis use (Copersino et al., 2010). Given the significant role of drug tolerance in the development of substance abuse and dependence, females’ greater potential for developing cannabinoid tolerance may contribute to female cannabinoid users’ more rapid trajectory to abuse and dependence.
The purpose of the present study was to determine whether sex differences in the development of antinociceptive tolerance can be attributed to activational effects of gonadal hormones. Gonadal hormones in adult animals have been shown to modulate acute effects of cannabinoid drugs. Female rats in late proestrus showed enhanced THC-induced antinociception compared to females in other estrous stages and compared to males (Wakley and Craft, 2011), suggesting that fluctuating gonadal hormones influence THC sensitivity. In gonadectomized (GDX) rats, estradiol (E2) enhanced THC-induced antinociception in females (Craft and Leitl, 2008; Wakley et al., 2014a), while testosterone (T) decreased the sedative effects of THC in males (Craft and Leitl, 2008). Kellert and colleagues (2009) found that E2 blunted cannabinoid-induced hyperphagia in ovariectomized (OVX) female guinea pigs, possibly explaining the lesser cannabinoid-induced hyperphagia seen in gonadally intact females compared to males. Given E2’s role in sex differences in behavioral effects of acutely administered cannabinoids, it was hypothesized that sex differences in tolerance to THC would also be E2-dependent. Very little is known about T or progesterone (P4) modulation of even acute cannabinoid effects, so it is possible that these gonadal hormones also influence the development of tolerance to THC.
To determine whether sex differences in the development of antinociceptive tolerance to THC are due to activational effects of gonadal steroid hormones, rats were GDX and Silastic capsules containing E2 or nothing (blanks) were implanted in females, and blank or T-containing capsules were implanted in males. P4 (or oil vehicle) was administered by injection every 5 days. Rats were tested for THC-induced antinociception, then given vehicle or THC (using sex-specific ED80 doses) twice-daily for 9 days to induce tolerance, and then THC effects were reexamined to determine the extent of tolerance development in each hormone group.
2. Methods
2.1 Subjects
Adult female and male Sprague-Dawley rats were used (60–90 days old, bred in-house from rats purchased from Harlan Laboratories, Livermore, CA). Ad libitum access to food and water was provided except during testing. The vivarium was maintained at 21±1°C on a 12:12 h light:dark cycle with lights on at 0700 h. After surgery, each rat was housed with another rat of the same sex, in the same hormone condition. Rats used in this study were treated in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2 Surgery and Hormone Administration
Chlordiazepoxide (3 mg/kg; Sigma Aldrich, St. Louis, MO) and pre-operative morphine (0.5 mg/kg; Sigma Aldrich, St. Louis, MO) were injected s.c.; 15 min later, rats were anesthetized with ketamine plus xylazine i.p. (90 mg/kg ketamine + 10 mg/kg xylazine in females, 100 mg/kg ketamine + 10 mg/kg xylazine in males; Patterson Veterinary Supply Inc., Kansas City, MO). All rats were either sham-GDX or GDX as described previously (Stoffel et al., 2003; Craft and Leitl, 2008). Following gonadectomy or sham-gonadectomy, constant-release Silastic® capsules were implanted s.c. between the shoulder blades (for females, one 1-mm blank or E2-filled capsule; for males, two 10-mm blank capsules or one 10-mm T-filled capsule/100 g body weight); we have shown previously that these E2 and T treatment regimens produce reproductive behavior and physiology that are similar to that seen in gonadally intact females (in proestrus to estrus) and gonadally intact males, respectively (Stoffel et al., 2003). Post-operative morphine (2.0 mg/kg) was administered before rats awoke from anesthesia. Behavioral testing began 14 days after surgery.
P4 (Steraloids, Newport, RI) was dissolved in safflower oil, which served as the vehicle. Vehicle and P4 were administered in a 0.1-mL volume. Starting on the 4th day after surgery, at 0700 h, either safflower oil or P4 (500 μg) was administered s.c. to each rat every 5 days. Females received either oil or P4 and males received only oil. The various hormone treatment groups are shown in Table 1.
Table 1.
Treatment groups.
| Capsule Implant | Hormone Injectiona | |
|---|---|---|
| FEMALES | ||
| Sham-GDX | Blank | Oil |
| GDX + 0 | Blank | Oil |
| GDX + E2 | Estradiol | Oil |
| GDX + P4 | Blank | Progesterone |
| GDX + E2/P4 | Estradiol | Progesterone |
| MALES | ||
| Sham-GDX | Blank | Oil |
| GDX + 0 | Blank | Oil |
| GDX + T | Testosterone | Oil |
oil or P4 was injected s.c. once every 5 days starting on the 4th day after the surgery day; THC dose-effect curves were obtained before and after chronic exposure to THC, starting approximately 4 hr after oil or P4 injection.
2.3 Apparatus
Tail withdrawal antinociception was assessed using a 2.5-L water bath (Precision Scientifics Inc., Winchester, VA) maintained at 50±0.5°C. Paw pressure antinociception was assessed using an analgesy-meter (Ugo-Basile, Varese, Italy). The pressure on the paw began at 30 g and increased at a constant rate of 48 g/s to a maximum of 606 g. Catalepsy was measured using a bar test: a 1.5-cm diameter horizontal bar for females was set at 12 cm above the table surface, and for males, the bar was set at 15 cm above the table surface.
2.4 Drug
THC (National Institute on Drug Abuse, Bethesda, MD) was dissolved in 1:1:18 ethanol:cremophor:saline solution, which served as the vehicle. Vehicle or THC was administered i.p. in a volume of 1 mL/kg, except doses >10 mg/kg. Due to solubility limitations, these larger doses were administered in larger volumes of a 10 mg/mL THC solution. Previous work from our lab has shown that these larger volumes of vehicle (with ethanol doses up to approximately 0.35 g/kg) do not alter nociception on the tail withdrawal or paw pressure tests (Wakley et al., 2014b).
2.5 Determination of estrous stage
Beginning on post-surgery day 7, a daily vaginal cell sample was obtained from sham-GDX females via lavage between 0700–0730 h; GDX females and males were handled daily to ensure similar amounts of handling among rats in the various groups. Vaginal cell samples were collected from all female rats immediately after behavioral testing on the pre-chronic and post-chronic test days. Classification of vaginal cytology for each stage has been described previously (see Bradshaw et al., 2006; Freeman, 1988; Wakley and Craft, 2011).
2.6 Behavioral procedure
The timeline for the experiment is illustrated in Figure 1. On post-surgery day 14, pre-chronic testing began at 1100 h (4 h after oil/P4 injection). The testing procedure used was identical to that previously described for gonadally intact rats (Wakley et al., 2014b). First, baselines were obtained consecutively on the warm water tail withdrawal and paw pressure tests 3 times, approximately 5 min apart. For the tail withdrawal assay, the distal 5 cm of the tail was submerged in the warm water bath and latency to withdraw the tail was measured to the nearest 0.01 s with a cutoff of 12 s. For the paw pressure test, latency to withdraw or attempt to withdraw the hindpaw was measured to the nearest 0.1 s and a cutoff of 12 s (606 g) was used. After baseline measures were obtained, THC dose-effect curves were obtained via cumulative dosing, using ¼-log-unit dose intervals and starting with a dose of 1.0 mg/kg (i.e., doses of 1.0, 0.8, 1.4, 2.4, 4.4, 8.0 mg/kg were given, resulting in total cumulative doses of 1.0, 1.8, 3.2, 5.6, 10, 18 mg/kg). Fifteen min after the first injection, rats were tested on tail withdrawal then paw pressure tests; immediately thereafter the next highest dose of THC was injected, and 15 min later rats were tested on both nociceptive assays again, and so on. Once a rat reached cutoff on both nociceptive tests, catalepsy was assessed and no more THC was given. For the catalepsy test, the rat’s forepaws were placed on the bar and latency to remove both paws from the bar or jump onto the bar was recorded to the nearest 0.01 s (cutoff 12 s). Rats were placed on a chronic injection regimen the next morning (post-surgery day 15).
Figure 1.
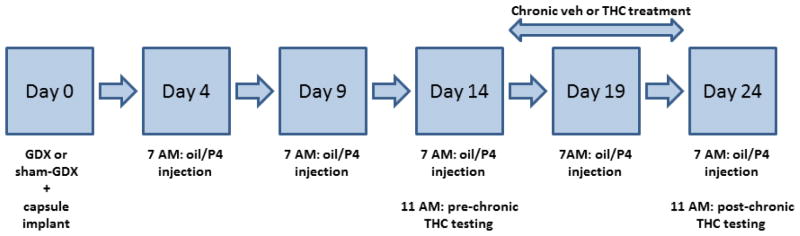
Experimental timeline.
To induce tolerance, vehicle or the sex-specific ED80 for THC was administered i.p. twice daily (at approximately 0700 h and 1600 h) for 9 days. ED80 values were estimated from the THC dose-effect curves obtained from the first 6 sham-GDX rats/sex tested: 5.7 mg/kg in females and 9.9 mg/kg in males. At 0700 h on post-surgery day 24 (post-chronic test day), an oil or P4 injection was given and the THC dose-effect curve was re-determined starting 4 h later (same as on the pre-chronic test day). The THC cumulative dosing range on the post-chronic test day was 1.8–32.0 mg/kg in the chronic vehicle-treated groups and 18–180 mg/kg in the chronic THC-treated groups; behavioral testing was conducted as described for the pre-chronic test day. Following the catalepsy test, rats were euthanized and the uterus (from GDX females) and seminal vesicles (from GDX males) were harvested, fixed in 10% formalin, and later trimmed and weighed. These organs are highly sensitive to circulating E2 (uterus) and T (seminal vesicles), so that organ weight can be used to confirm that gonadectomies were complete (in GDX+0 and GDX+P4 groups) and that E2- and T-filled Silastic capsules were releasing hormone as expected (Stoffel et al., 2003).
2.7 Serum Hormone Levels and Hormone-Sensitive Organs
To confirm that E2 and T administration by Silastic capsule implantation provided relatively stable exposure to these hormones during the weeks-long experiment, a separate group of rats underwent gonadectomy and capsule implantation. Rats were euthanized 2, 6, 8 or 10 weeks after gonadectomy + capsule implantation; trunk blood was harvested for later analysis of E2 (females) or T (males) in serum, and the uterus (females) or seminal vesicles (males) were harvested, fixed in 10% formalin, and later trimmed and weighed.
2.8 Radioimmunoassay for E2 and T
Trunk blood samples were centrifuged for 20 min at 2000 rpm at 4°C; serum was removed and stored at −80°C until analysis. Hormone levels were determined via double antibody radioimmunoassay kits (estradiol (Cat # KE2D1) and testosterone (Cat # TKTT1): Siemens Healthcare Diagnostics, Los Angeles, CA, USA). Manufacturer’s instructions were followed except in the E2 assay, samples and standards were extracted twice using methyl tert butyl ether (done in duplicate); after the ether evaporated, the antibody was added to the extraction tubes, which were incubated for 24 h at 4°C, followed by addition of the tracer and then overnight incubation.
2.9 Data analysis
Baseline nociceptive latencies for each rat on the tail withdrawal and paw pressure tests were calculated as the mean of the three pre-injection trials. Because baseline responding changed from pre- to post-chronic testing and differed among groups in some cases, individual response latencies following drug were converted to % Maximum Possible Effect (% MPE) on each day: (drug latency – baseline latency)/(cutoff latency – baseline latency) x 100, using the baseline latency on the same day on which drug was tested. Negative % MPE values were set to 0. On the pre-chronic test day, THC potency (ED50 value) was estimated for each treatment group by log-linear regression and dose-effect curves were compared between groups by ANOVA, using PharmToolsPro (version 1.27); these values and statistical comparisons are shown in Table 2. On the post-chronic test day, THC-induced antinociception did not reach 50% MPE in most rats that had been treated with THC chronically, and in most cases the slope of the post-chronic dose-effect curve was shallower than the slope of the pre-chronic dose-effect curve; thus, potency could not be estimated accurately or compared between pre- and post-chronic tests. Therefore, pre- vs. post-chronic dose-effect curves (% MPE values) were compared by ANOVA using 5 doses of the dose-effect curve: 1.8–18 mg/kg for all rats at the pre-chronic test and for vehicle-treated rats at the post-chronic test, and 18–180 mg/kg for all THC-treated rats at the post-chronic test. Baseline latencies to respond and catalepsy at pre- to post-chronic tests were also compared among treatment groups using ANOVA.
Table 2.
Sex differences in acute antinociceptive potency of THC, and gonadal hormone modulation of acute THC potency (pre-chronic test day).
| Sex/Hormone Group (n) | Mean ED50 (95% C.L), in mg/kg | |
|---|---|---|
| Tail Withdrawal | Paw Pressure | |
| Sham-GDX Females (24) | 3.63 (3.04, 4.40) | 3.47 (2.90, 4.18) |
| Sham-GDX Males (24) | 8.30 (6.65, 10.97)* | 6.73 (5.30, 8.83)* |
| GDX + 0 Females (24) | 3.05 (2.64, 3.52) | 3.10 (2.47, 3.87) |
| GDX + P4 Females (21) | 4.43 (3.72, 5.30)† | 6.99 (5.32, 9.93)† |
| GDX + E2 Females (23) | 3.00 (2.53, 3.53) | 4.62 (3.31, 7.51) |
| GDX + E2/P4 Females (24) | 2.75 (2.34, 3.20) | 5.37 (4.22, 7.48)† |
| GDX + 0 Males (23) | 8.88 (6.96, 12.33) | 8.22 (6.13, 12.32) |
| GDX + T Males (24) | 7.89 (6.73, 9.48) | 5.99 (4.90, 7.36) |
sex difference: significantly different from sham-GDX females, p<0.05
hormone effect: significantly different from GDX+0 females, p<0.05
For each dependent variable, first it was determined whether there were sex differences, by comparing sham-GDX females to sham-GDX males, with factors of Sex (female or male), Chronic Treatment (vehicle or THC) and Time (pre-chronic or post-chronic, repeated); for comparison of dose-effect curves, the additional variable of Dose (5 levels, repeated) was included in the ANOVA. Second, to determine whether ovarian hormones influenced dependent measures in females, variables were compared among GDX female groups, with factors of E2 (2 levels), P4 (2 levels), Chronic Treatment (vehicle, THC), and Time (pre-chronic, post-chronic, repeated); for comparison of dose-effect curves, the additional variable of Dose (5 levels, repeated) was included. Third, to determine whether testosterone influenced dependent measures in males, variables were compared between GDX male groups, with factors of Testosterone (2 levels), Chronic Treatment (vehicle, THC), and Time (pre-chronic, post-chronic, repeated); the additional variable of Dose (5 levels, repeated) was included when comparing dose-effect curves. Group differences were considered significant at p≤0.05.
To control for differences in organ weight that were due to differences in body weight, organ weights (g) were divided by the rat’s body weight in kg, before analysis. Organ weights and serum hormone levels were compared over time by ANOVA, with the factor of Week (2, 6, 8, 10).
3. Results
Based on hormone-sensitive organ weights and vaginal cytology, data from five rats were dropped before analysis. Three rats in the GDX+P4 group (1 chronic vehicle-treated, 2 chronic THC-treated) had uterine weights that were similar to those of gonadally intact females in proestrus (see Stoffel et al., 2003) and more than 2 standard deviations above the rest of the GDX+P4 group, indicating that ovariectomy was not complete. An additional female in the GDX+P4 group (chronic vehicle-treated) had a uterine weight that was intermediate to those of no-hormone vs. E2-treated females; data from that rat were removed when vaginal cytology samples revealed that the rat was in proestrus on both pre- and post-chronic test days. One male rat in the GDX+0 group (chronic THC-treated) had seminal vesicles that weighed the same as those of T-treated males (more than 2 standard deviations above the rest of the group), indicating that castration was incomplete. Data from that rat were also dropped before analysis.
3.1 Nociceptive Baselines
On both nociceptive tests, latency to respond was significantly shorter in sham-GDX females than sham-GDX males (Tail Withdrawal Test: F(1,44)=5.27, p=0.026; Paw Pressure Test: F(1,44)=18.02, p<0.001) (data not shown). Sex differences in baseline latencies averaged less than 0.5 sec. For example, tail withdrawal latency to respond on the pre-chronic test day was 5.59 ± 0.13 vs. 6.04 ± 0.15 sec in females vs. males, respectively; paw pressure latency to respond on the pre-chronic test day was 4.99 ± 0.15 vs. 5.42 ± 0.13 sec in females vs. males, respectively. Baseline tail withdrawal latencies were longer on the post-chronic test day than on the pre-chronic test day, but only in chronic vehicle-treated males (Sex x Chronic Treatment x Time: F(1,44)=4.28, p=0.044) (data not shown). On the paw pressure test as well, baseline latencies were longer on the post- than on the pre-chronic test day, only in males (Sex x Time: F(1,44)=5.73, p=0.021) (data not shown). These changes in baseline latency from pre- to post-chronic test days averaged no more than 0.5 sec.
Among female GDX groups, baseline tail withdrawal latencies did not differ, and no significant change in latencies occurred pre- to post-chronic (data not shown). On the paw pressure test, E2-treated females’ latencies averaged 0.35 sec shorter than females without E2 (F(1,83)=10.58, p=0.002) (data not shown). Baseline paw pressure latencies did not change significantly from pre- to post-chronic test days in any GDX female group. Baseline latencies in GDX females were very similar to those in sham-GDX females. For example, GDX+0 females had pre-chronic latencies of 5.69 ± 0.15 and 5.08 ± 0.14 sec on the tail withdrawal and paw pressure tests, respectively.
Between male GDX groups, baseline tail withdrawal latencies averaged 0.4 sec longer in chronic vehicle-treated rats than in chronic THC-treated rats (F(1,43)=8.49, p=0.006), but there was no significant effect of T, and no significant change from pre- to post-chronic test days (data not shown). Baseline paw pressure response latencies did not differ between male GDX groups, and there was no significant change from pre- to post-chronic tests. Baseline latencies in GDX males were very similar to those in sham-GDX males. For example, GDX+0 males had pre-chronic latencies of 5.91 ± 0.15 and 5.57 ± 0.13 sec on the tail withdrawal and paw pressure tests, respectively.
3.2 Sex Differences in and Hormone Modulation of Acute THC Potency (Pre-Chronic Test Day)
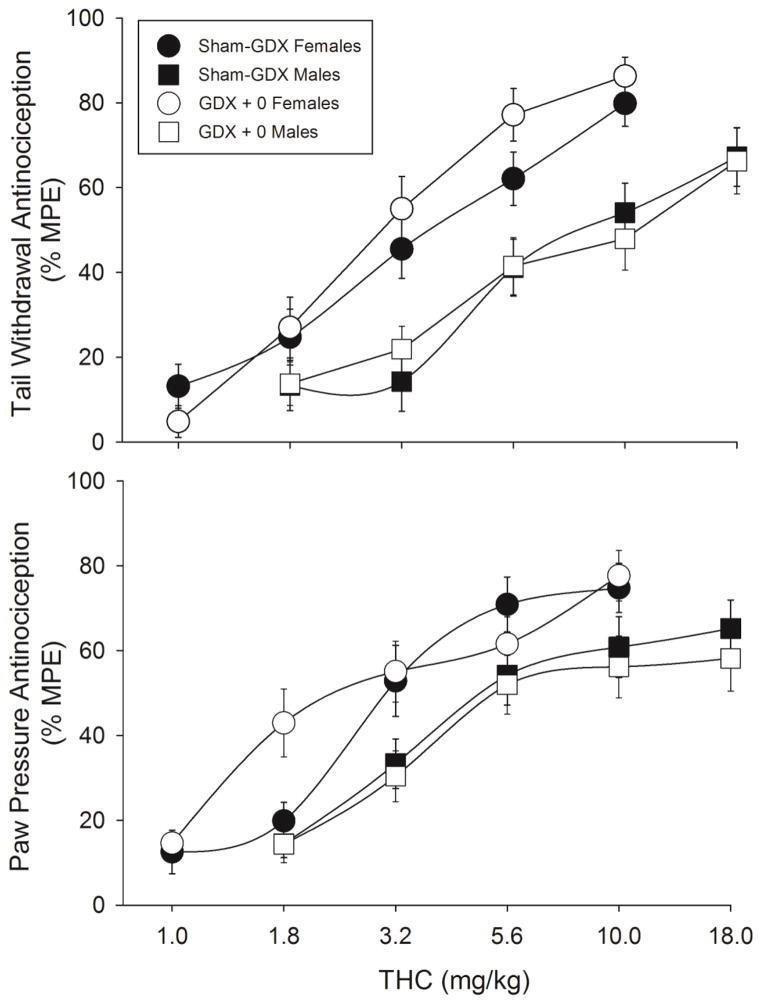
Figures 2 and 3 show THC dose-effect curves on the pre-chronic test day, the first time that THC was administered, on the tail withdrawal and paw pressure tests. ED50 values derived from these dose-effect curves are shown in Table 2.
Figure 2.
Sex differences in acute THC-induced antinociception on the 50°C warm water tail withdrawal test (top panel) and the paw pressure test (bottom panel). Rats were either sham-gonadectomized (sham-GDX) or gonadectomized (GDX+0) two weeks before testing. Each point is the mean ± 1 SEM percent maximum possible effect (%MPE) of 23–24 rats. For clarity of data presentation, the 1.0 mg/kg dose is not shown for male groups (all points <20% MPE).
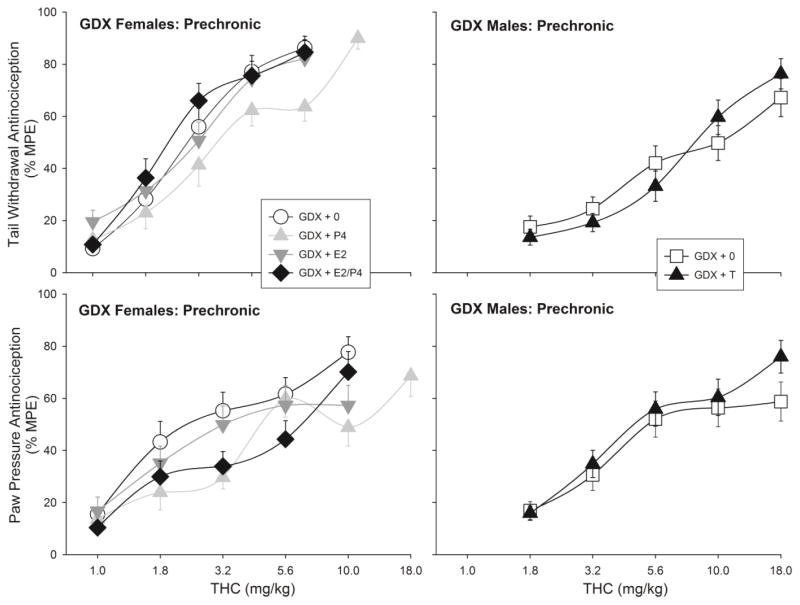
Figure 3.
Gonadal hormone modulation of acute THC-induced antinociception on the 50°C warm water tail withdrawal test (top panel) and the paw pressure test (bottom panel). Gonadectomized females (left panels) were treated with no hormone (GDX+0), estradiol (GDX+E2), progesterone (GDX+P4), or both ovarian hormones (GDX+E2/P4). Gonadectomized males (right panels) were treated with no hormone (GDX+0) or testosterone (GDX+T). Each point is the mean ± 1 SEM percent maximum possible effect (%MPE) of 21–24 rats. The 1.0 mg/kg dose is not shown for male groups (all points <20% MPE).
Figure 2 shows that on the pre-chronic test day, THC was more potent in females than males on both the tail withdrawal and paw pressure tests; ED50 values were significantly higher in sham-GDX males than sham-GDX females (Table 2). Gonadectomy did not affect THC potency in either sex: there was no significant difference in ED50 values between sham-GDX vs. GDX+0 females, or between sham-GDX and GDX+0 males, on either test (Fig. 2, Table 2).
To illustrate the influence of gonadal hormones on acute THC potency, Figure 3 shows THC’s antinociceptive effects on the pre-chronic test day in GDX groups only. On the tail withdrawal test, THC was significantly less potent in P4-treated females than in GDX+0 females; however, neither E2 nor E2+P4 combined significantly altered THC potency (Fig. 3, top left panel; Table 2). On the paw pressure test, P4 also significantly reduced THC potency: ED50 values were higher in both GDX+P4 and GDX+E2/P4 females than in GDX+0 females, whereas females treated with E2 alone did not differ from no-hormone controls (Fig. 3, bottom left panel; Table 2). THC potency did not differ significantly between GDX+0 and GDX+T males on either test (Fig. 3, right panels; Table 2).
3.3 Sex Differences in and Hormone Modulation of Antinociceptive Tolerance to THC
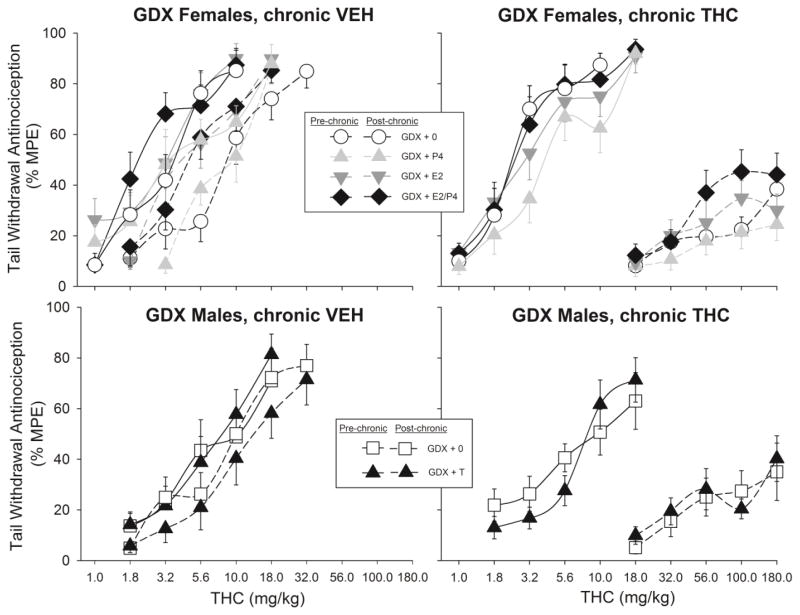
Figures 4–6 show THC dose-effect curves on the pre- vs. post-chronic test days, for the tail withdrawal and paw pressure tests. As expected, THC dose-effect curves shifted farther to the right (and slopes became shallower) from pre- to post-chronic tests in rats that had received twice-daily THC injections (right panels) compared to those that had received twice-daily vehicle injections (left panels).
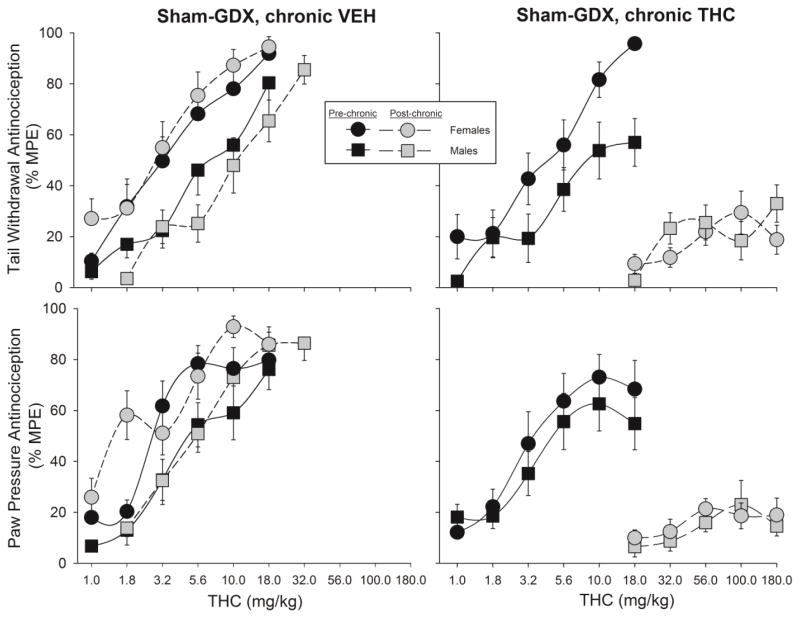
Figure 4.
Sex differences in development of antinociceptive tolerance to THC on the 50°C warm water tail withdrawal test (top panels) and the paw pressure test (bottom panels). Rats were sham-gonadectomized (sham-GDX); two weeks later, the first THC dose-effect curve was obtained (Pre-chronic), then either vehicle (left panels) or THC (right panels) was administered twice-daily for 9 days, and the THC dose-effect curve was re-determined (Post-chronic). Each point is the mean ± 1 SEM percent maximum possible effect (%MPE) of 12 rats. For clarity of data presentation, the 1.0 mg/kg dose is not shown for some groups (all points <20% MPE).
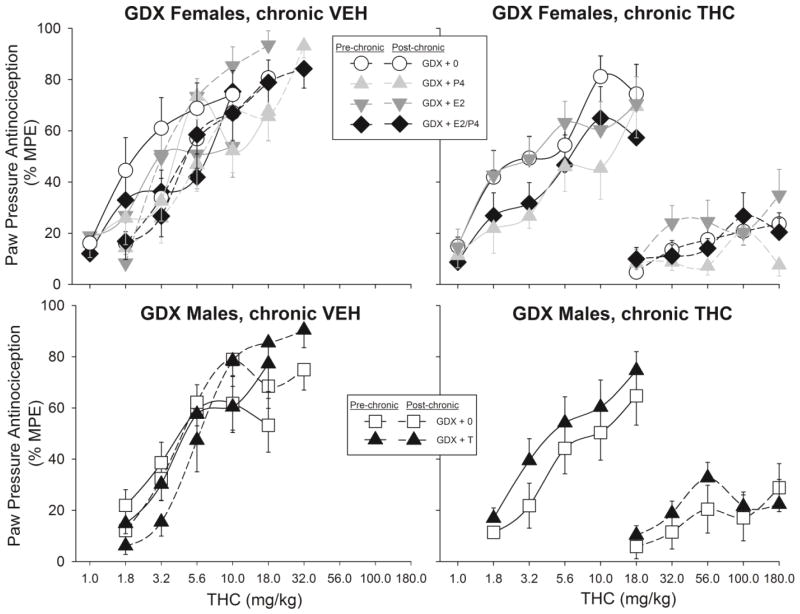
Figure 6.
Antinociceptive tolerance to THC on the paw pressure test in gonadectomized females (top panels) and males (bottom panels). Details same as in Fig. 5.
Figure 4 shows the THC dose-effect curves in sham-GDX females vs. males only. On the tail withdrawal test (top panels), change in THC effect was significantly greater in sham-GDX females than sham-GDX males (Sex x Chronic Treatment x Dose: F(4,176)=2.56, p=0.04; Sex x Dose x Time: F(4,176)=3.38, p=0.011); subsequent analysis revealed a sex difference in tolerance development only in the chronic THC-treated groups (Sex x Dose x Time: F(4,88)=4.94, p=0.001). On the paw pressure test (Fig. 4, bottom panels), change in THC effect after chronic THC treatment did not vary significantly between females and males.
Figure 5 shows pre- and post-chronic THC dose-effect curves on the tail withdrawal test, in GDX females (top panels) and GDX males (bottom panels) that received no hormone vs. those that received hormones. Among GDX females, E2 increased THC-induced antinociception (that is, including all E2-treated females at both time points: F(1,83)=12.80, p=0.001), and P4 moderated the E2 effect (Estradiol x Progesterone: F(1,83)=4.23, p=0.043). This hormone interaction also depended on chronic treatment group and time (Estradiol x Progesterone X Chronic Treatment x Time: F(1,83)=5.24, p=0.025); subsequent analysis revealed that the E2/P4 interaction was only significant in chronic THC-treated females (Estradiol x Progesterone x Time: F(1,42)=6.68, p=0.013). Further analyses within the pre- or post-chronic time points revealed non-significant E2/P4 interactions (p=0.07–0.14). As shown in Fig. 5 (top right panel), at both pre- and post-chronic tests, females treated with both E2 and P4 tended to be more sensitive to THC than females receiving either hormone alone. Because this same pattern was observed at both pre- and post-chronic tests, it cannot be concluded that E2 or P4 altered the development of tolerance to THC.
Figure 5.
Antinociceptive tolerance to THC on the 50°C warm water tail withdrawal test in gonadectomized females (top panels) and males (bottom panels). Females were treated with no hormone (GDX+0), estradiol (GDX+E2), progesterone (GDX+P4), or both ovarian hormones (GDX+E2/P4). Males were treated with no hormone (GDX+0) or testosterone (GDX+T). Two weeks later, the first THC dose-effect curve was obtained (Pre-chronic), then either vehicle (left panels) or THC (right panels) was administered twice-daily for 9 days, and the THC dose-effect curve was re-determined (Post-chronic). Each point is the mean ± 1 SEM percent maximum possible effect (%MPE) of 10–12 rats. For clarity of data presentation, lower doses are not plotted for some groups (all points <20% MPE).
Figure 5 (bottom panels) shows that in GDX male rats, T did not affect tolerance development on the tail withdrawal test.
Figure 6 shows pre- and post-chronic THC dose-effect curves on the paw pressure test, in GDX females (top panels) and GDX males (bottom panels) that received no hormone vs. those that received hormones. In GDX females, E2 and P4 again interacted to affect THC-induced antinociception (Estradiol x Progesterone x Time: F(1,83)=5.36, p=0.023; Fig. 6 top panels); however, as opposed to the tail withdrawal test on which E2/P4-treated females showed the greatest THC effect, on the paw pressure test P4 tended to decrease THC effect in E2-treated females (as well as in females that did not receive E2). Subsequent analyses within pre- and post-chronic tests revealed that the E2/P4 interaction was significant at the pre-chronic test only (E2 x P4 x Dose: F(4,348)=3.12, p=0.015). Neither E2 nor P4 appeared to alter tolerance development in GDX females, although the post-chronic curves were relatively flat so differential tolerance among groups could not be accurately quantified.
Figure 6 (bottom panels) shows that in GDX males, T did not affect tolerance development on the paw pressure test.
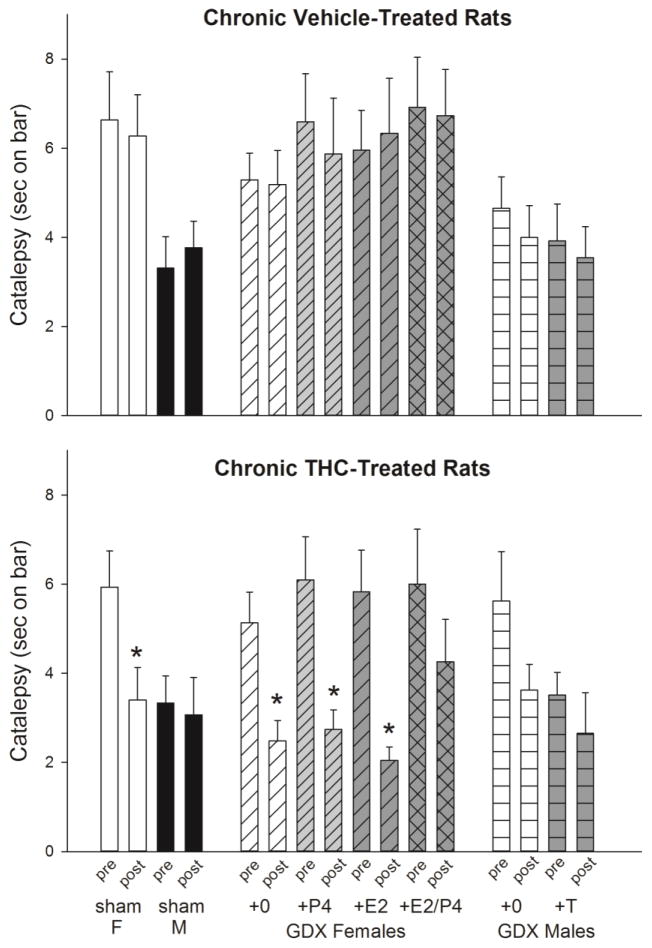
3.4 Sex Differences in and Hormone Modulation of THC-Induced Catalepsy
Immediately following antinociceptive testing on the pre- and post-chronic test days, catalepsy was assessed in each rat. Because catalepsy was examined after rats reached cutoff on the nociceptive tests, most but not all rats were tested after the same THC dose. Table 3 shows that catalepsy was typically tested after 18 mg/kg on the pre-chronic test day (91% of rats), and after 32 mg/kg (88% of chronic vehicle-treated rats) or 180 mg/kg (100% of chronic THC-treated rats) on the post-chronic test day. Figure 7 shows that on the pre-chronic test day (first bar in each sex/hormone group), THC produced greater catalepsy in sham-GDX females than sham-GDX males (Sex: F(1,46)=14.64, p<0.001). Gonadectomy increased THC-induced catalepsy in males but slightly decreased it in females (Sex x GDX: F(1,91)=7.13, p=0.009). In GDX females, neither E2 nor P4 significantly altered THC-induced catalepsy. In GDX males, T decreased THC-induced catalepsy non-significantly (F(1,45)=3.40, p=0.07).
Table 3.
Number of rats in each treatment group that were tested for catalepsy after each THC dose. After a rat reached cutoff on both nociceptive tests (or after the maximal dose, 180 mg/kg, was given on the post-chronic test), catalepsy was tested.
| Treatment Group | Pre-Chronic THC dose (mg/kg) | Post-Chronic THC (mg/kg)dose | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3.2 | 5.6 | 10 | 18 | 5.6 | 10 | 18 | 32 | 180 | |
| Sham-GDX Females | |||||||||
| Vehicle-treated | 2 | 10 | 1 | 2 | 1 | 8 | |||
| THC-treated | 1 | 1 | 10 | 12 | |||||
| Sham-GDX Males | |||||||||
| Vehicle-treated | 12 | 12 | |||||||
| THC-treated | 12 | 12 | |||||||
| GDX + 0 Females | |||||||||
| Vehicle-treated | 1 | 1 | 10 | 1 | 11 | ||||
| THC-treated | 1 | 2 | 9 | 12 | |||||
| GDX + P4 Females | |||||||||
| Vehicle-treated | 11 | 11 | |||||||
| THC-treated | 10 | 10 | |||||||
| GDX + E2 Females | |||||||||
| Vehicle-treated | 1 | 10 | 1 | 2 | 8 | ||||
| THC-treated | 12 | 12 | |||||||
| GDX + E2/P4 Fem. | |||||||||
| Vehicle-treated | 1 | 11 | 1 | 1 | 10 | ||||
| THC-treated | 2 | 1 | 9 | 12 | |||||
| GDX + 0 Males | |||||||||
| Vehicle-treated | 1 | 11 | 1 | 11 | |||||
| THC-treated | 11 | 11 | |||||||
| GDX + T Males | |||||||||
| Vehicle-treated | 1 | 11 | 12 | ||||||
| THC-treated | 12 | 12 | |||||||
Figure 7.
Sex differences in, and gonadal hormone modulation of THC-induced catalepsy. Sham-gonadectomized females (sham F) and males (sham M), and gonadectomized (GDX) rats treated with no hormone (GDX+0), estradiol (+E2), progesterone (+P4), both ovarian hormones (+E2/P4), or testosterone (+T) were tested for catalepsy after completion of antinociceptive testing on the pre-chronic test day (pre) and the post-chronic test day (post). Each bar is the mean ± 1 SEM of 10–12 rats. *significant decrease in catalepsy from pre- to post-chronic tests.
Tolerance developed to THC-induced catalepsy from pre- to post-chronic test days in some groups but not others (Fig. 7, compare first and second bar within each group). As expected, THC-induced catalepsy did not change significantly in chronic vehicle-treated, sham-GDX females or males (Fig. 7, top panel). In contrast, THC-induced catalepsy significantly decreased from pre- to post-chronic tests in chronic THC-treated, sham-GDX females, but not in sham-GDX males (Sex x Time: F(1,44)=3.94, p=0.05) (Fig. 7, bottom panel). In GDX female groups, chronic THC treatment but not chronic vehicle treatment decreased THC-induced catalepsy (Chronic Treatment x Time: F(1,83)=15.21, p<0.001), with no significant influence of E2 or P4 on tolerance development (E2 x P4 x Chronic Treatment x Time: F(1,83)=0.94, NS). In GDX males, decreases in THC-induced catalepsy were significant overall (Time: F(1,43)=5.35, p=0.026); tolerance appeared to occur primarily in chronic THC-treated, GDX+0 males, yet this group difference was not significant (T x Chronic Treatment x Time: F(1,43)=0.25, NS) (Fig. 7, bottom panel).
3.5 Estrous stage in females
Table 4 shows the percent of females in each group that were in each estrous stage on the pre- and post-chronic test days. As expected, sham-GDX females were distributed among all stages, whereas GDX rats that did not receive E2 were nearly all in diestrus, and E2-treated rats were all in proestrus to estrus. There was little difference in estrous stage distribution in any treatment group on the pre- vs. post-chronic test days. However, chronic THC-treated, sham-GDX females spent somewhat less time in proestrus to estrus than chronic vehicle-treated, sham-GDX females, when the entire period of chronic treatment was examined: during the 8-day period before chronic injections began, percent of days in proestrus to estrus averaged 29 ± 7 vs. 29 ± 5% in chronic vehicle- vs. chronic THC-assigned rats, whereas during the 10-day period of chronic injection, percent of days in proestrus to estrus averaged 40 ± 8% in rats that were treated with vehicle daily, vs. 23 ± 5% in rats that were treated with THC daily (Chronic Treatment x Time: F(1,22)=1.42, NS).
Table 4.
Percent of females in each estrous stage (Pro=proestrus; Pro/Est=intermediate between proestrus and estrus; Est=estrus; Diest=diestrus day 1 or day 2) immediately after the pre-chronic and post-chronic tests.
| Treatment Group (n) | Pre-chronic Test | Post-chronic Test | ||||||
|---|---|---|---|---|---|---|---|---|
| Pro | Pro/Est | Est | Diest | Pro | Pro/Est | Est | Diest | |
| Sham-GDX | ||||||||
| Vehicle (12) | 25 | 16.7 | 16.7 | 41.6 | 8.3 | 16.7 | 25 | 50 |
| THC (12) | 16.7 | 25 | 0 | 58.3 | 16.7 | 16.7 | 25 | 41.6 |
| GDX + 0 | ||||||||
| Vehicle (12) | 100 | 100 | ||||||
| THC (12) | 100 | 100 | ||||||
| GDX + P4 | ||||||||
| Vehicle (10) | 10 | 90 | 100 | |||||
| THC (10) | 100 | 100 | ||||||
| GDX + E2 | ||||||||
| Vehicle (11) | 18.2 | 81.8 | 18.2 | 81.8 | ||||
| THC (12) | 8.3 | 91.7 | 8.3 | 25 | 66.7 | |||
| GDX + E2/P4 | ||||||||
| Vehicle (12) | 25 | 75 | 16.7 | 83.3 | ||||
| THC (12) | 8.3 | 91.7 | 8.3 | 8.3 | 83.3 | |||
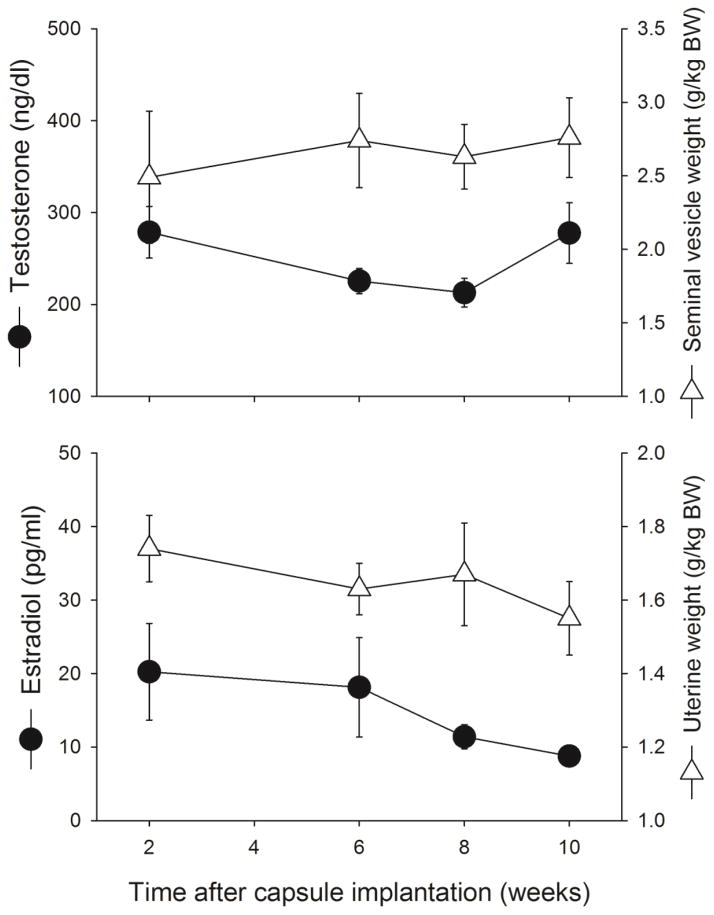
3.6 Serum hormone levels
Figure 8 shows that T and E2 replacement by Silastic capsule yielded relatively stable serum hormone levels and hormone-sensitive reproductive organ weights for up to 10 weeks after capsule implantation in GDX rats. From 2 to 10 weeks after capsule implantation, T levels changed slightly but not significantly (Week: F(3,16)=2.51, p=0.096), and seminal vesicle weights remained stable (Week: F(3,17)=0.23, NS) (Fig. 8, top panel). E2 levels declined slightly but not significantly over time (Week: F(3,17)=1.52, NS), and uterine weights remained stable (Week: F(3,18)=0.91, NS) (Fig. 8, bottom panel).
Figure 8.
Serum levels of testosterone and seminal vesicle weight in males (top panel) and serum levels of estradiol and uterine weight in females (bottom panel) at various time points after gonadectomy + hormone capsule implantation. Each point is the mean ± 1 SEM of 4–6 rats.
4. Discussion
The present study replicated two previously published findings: (1) greater acute antinociceptive potency of THC in female compared to male rats; (2) greater development of antinociceptive tolerance to THC in females compared to males. The primary novel finding of the present study is that sex differences in the development of antinociceptive tolerance to THC are not explained by activational effects of gonadal hormones. After 9 days of twice-daily THC treatment, despite receiving approximately 40% less THC than sham-GDX males, sham-GDX females were more tolerant than males to THC’s antinociceptive effect on the tail withdrawal test. Gonadectomy did not eliminate this sex difference: GDX females without hormone replacement showed as much tolerance development as sham-GDX females, and GDX males without hormone replacement showed antinociceptive tolerance development very similar to that observed in sham-GDX males. Neither E2 nor P4 significantly affected tolerance development in GDX females, and T did not significantly affect tolerance development in GDX males.
In the present study rats developed more tolerance overall than those in our previous study (Wakley et al., 2014b). THC efficacy, as indicated by shallower slopes on the post-chronic compared to the pre-chronic dose-effect curves, as well as THC potency decreased after chronic THC treatment. This dramatic decrease in THC effect is similar to what was observed in the first study of antinociceptive tolerance to THC that included both female and male rats, in which Harlan Long-Evans were used, albeit with higher dose-THC administration given during the chronic treatment period (Wiley et al., 2007). The sex-specific tolerance induction protocols used in the present study and the Wakley et al. (2014b) study were nearly identical, and data were collected by the same experimenter (A.A.W.). The primary differences between the two studies were rat vendor (Taconic vs. Harlan), laboratory location (the Craft laboratory moved to a different building with a different vivarium just before the present study began), and the fact that rats in the present study underwent anesthesia plus at least a small incision (sham-GDX) two weeks prior to the start of THC testing. Each of these variables could contribute to discrepant tolerance across studies. For example, it has been shown that drug effects can vary between Sprague-Dawley rats from different vendors (e.g. Oliff et al., 1996).
Regardless of the source of the between-study variability in tolerance development, sex differences in antinociceptive potency of acute THC were still observed in the present study, and these could be accurately quantified. At the pre-chronic test, THC was approximately twice as potent in sham-GDX females than in sham-GDX males on both nociceptive tests. These sex differences were not eliminated by gonadectomy: THC potency did not differ between GDX+0 and sham-GDX females, or between GDX+0 and sham-GDX males, on either test. Gonadectomy effects in females may depend on the estrous stage of sham-GDX rats (Becker et al., 2005; Greenspan et al., 2007). Proestrus-to-estrus is the period during which Taconic Sprague-Dawley female rats were most sensitive to THC and most different from males (Craft and Leitl, 2008; Wakley and Craft, 2011), so assuming that Harlan Sprague-Dawley females show similar estrous cycle variability in THC sensitivity, the likelihood of observing an effect of gonadectomy would be greatest if all sham-GDX females were in proestrus-to-estrus on the pre-chronic test day, instead of only 21% (see Table 4). Given the estrous stage-specific enhancement of THC-induced antinociception that we have previously observed, E2 or E2+P4 was expected to alter acute THC potency; however, this was not observed (see Table 2). T also did not significantly alter THC’s acute antinociceptive potency in GDX males. The lack of T effect agrees with a previous study (Craft and Leitl, 2008), but the lack of E2 effect does not: E2 increased the antinociceptive effects of a single dose of THC on the tail withdrawal and paw pressure tests, when THC was administered systemically (Craft and Leitl, 2008) or i.c.v. (Wakley et al., 2014a). If the peak increase in response to THC in females occurs at a different point in the estrous cycle in Harlan vs. Taconic females, the use of a constant-release E2 replacement approach instead of a cyclic E2 injection regimen in GDX females may have resulted in the lack of E2 enhancement of THC effect in the present study. It should be noted that when all dose-effect curves were included in the analysis (pre- and post-chronic), E2 did significantly increase THC effect on the tail withdrawal test, which may suggest that the E2 effect is small in this population and may only be evident when sample size is very large. The only other study of ovarian hormone modulation of cannabinoid antinociception, conducted in female mice, showed that gonadectomy increased antinociception produced by WIN55,212-2, and this increase was reversed by E2 given in a single large dose approximately 4 h before testing (Kalbasi Anaraki et al., 2008). Thus, E2 modulation of cannabinoid antinociception may depend on species, strain, and other variables, and should be investigated further.
In contrast to the lack of consistent E2 effect in the present study, acute THC potency (but not tolerance development) was significantly decreased by P4 on both nociceptive tests. A previous study also reported that P4 decreased antinociception produced by a moderate (but not low) dose of WIN55,212-2 in GDX female mice (Kalbasi Anaraki et al., 2008). In rats, P4 decreased i.c.v. THC-induced antinociception slightly but not significantly (Wakley et al., 2014a). In the latter study, P4 was given 4 h before THC, and P4-induced blunting of THC’s effect was primarily observed 15–30 min rather than 120–180 min post-THC injection (unpublished data, A. Wakley), such that overall the P4 effect was not significant. In the present study, cumulative THC dose-effect curves were obtained in 60–90 min, which may be why the P4 effect was significant. One additional finding that suggests a P4-cannabinoid interaction is that progesterone receptor blockade has been reported to enhance THC’s effects: mifepristone, a progesterone receptor antagonist, increased THC-induced hypothermia and catalepsy (Pryce et al., 2003). The mechanism underlying P4-cannabinoid interactions is not known. Interestingly, the steroid hormone precursor pregnenolone has been shown to reduce many effects of THC, including antinociception (Vallée et al., 2014), and pregnenolone can be metabolized to P4 (Robel et al., 1995), so perhaps pregnenolone’s effects are actually mediated by P4.
One mechanism that likely contributes to sex differences in both acute THC-induced antinociception as well as THC tolerance development is females’ greater production of the major active metabolite, 11-OH-THC. For example, two hours after THC injection, brain 11-OH-THC levels were significantly higher in female than male rats, and blocking the metabolism of THC eliminated sex differences in THC’s acute antinociceptive effect (Tseng et al., 2004). Furthermore, Wiley and Burston (2014) reported that after chronic THC administration, 11-OH-THC levels in blood increased in female rats but dropped in males; greater production of 11-OH-THC with repeated THC administration would be expected to cause greater down-regulation and/or greater desensitization of CB1 receptors, thereby resulting in greater tolerance. However, adult females do not show necessarily greater decreases than males in CB1 receptor density or desensitization after chronic THC treatment, in the brain regions examined thus far, including the periaqueductal gray (Burston et al., 2010).
In the present study, sex differences in THC-induced catalepsy were also observed. At the pre-chronic test, catalepsy was greater in sham-GDX females than males; additionally, tolerance developed in sham-GDX females but not in sham-GDX males (see Fig. 7). Because catalepsy was tested only after the dose-effect curves for antinociception were obtained, the catalepsy score represents drug effect after a single dose, the highest one tested in each rat. A majority of rats were tested after 18 mg/kg THC at the pre-chronic test, and after 32 mg/kg (chronic vehicle-treated rats) or 180 mg/kg (chronic THC-treated rats) at the post-chronic test; those rats that were tested at lower doses than these were nearly all females, yet females still showed greater catalepsy than males. Mean catalepsy scores for males were only in the 3–4 sec range, so it is possible that a floor effect made tolerance difficult to observe; however, we have shown previously that baseline catalepsy scores are in the 1–2 sec range in vehicle-injected males and females (Tseng and Craft, 2001; Craft and Leitl, 2008; Wakley et al., 2014b), suggesting that decreases in THC effect still could have been observed from pre- to post-chronic tests. Sex differences in acute THC-induced catalepsy similar to those observed in the present study have been reported previously (Tseng and Craft, 2001; Craft et al., 2012). Furthermore, greater tolerance development to THC-induced catalepsy in females than males was also apparent in a previous developmental study, although sex differences in tolerance development were not explicitly tested for (Wiley et al., 2007).
In the present study, gonadectomy tended to decrease THC-induced catalepsy in females and increase it in males; furthermore, ovarian hormones slightly increased catalepsy in females and T tended to decrease catalepsy in males, yet none of these hormone effects were statistically significant. Similar hormone modulation of THC’s motoric effects was reported in a previous study (Craft and Leitl, 2008), suggesting that activational effects of gonadal steroid hormones do account for sex differences in THC’s acute motoric effects (and possibly sex differences in the development of tolerance to THC’s motoric effects). A more highly powered study, for example, one that includes multiple doses of THC instead of just one, will be necessary to confirm gonadal hormone modulation of THC’s motoric effects.
The estrous cycle in most female rats is 4–5 days long, with females in proestrus to estrus approximately 40–45% of each 4–5 day cycle (Feder, 1981; Freeman, 1988; Haim et al., 2003). In the present study, during the week before chronic injections began, the average percent time in proestrus to estrus averaged only 29% in sham-GDX females, which is approximately 10–15% lower than expected. This result suggests that rats were somewhat stressed from handling/lavage alone (they were not handled or habituated to daily vaginal lavage until these samples were taken). In support of this hypothesis, during the subsequent chronic treatment phase, average time spent in proestrus to estrus (40%) reached the normal range in chronic vehicle-treated females. In contrast, average time spent in proestrus to estrus remained below normal (23%) in chronic THC-treated females during the chronic treatment phase. Although these group differences were not statistically significant, the differential trajectories in the two groups suggest that THC suppressed estrous cycling. Chronic THC administration at relatively high doses has been shown to significantly disrupt estrous cycling in female rats (Marusich et al., 2014), as well as menstrual cycling in female monkeys (Smith et al., 1983) and humans (Mendelson et al., 1986). The lesser effect in the present study likely reflects the lower chronic THC dose used (5.7 mg/kg THC twice-daily) relative to doses used in previous studies (25–30 mg/kg THC once- or twice-daily: Marusich et al., 2014; O’Connell et al., 1987).
5. Conclusion
The present study suggests that greater antinociceptive tolerance development in females compared to males, which occurred despite the fact that females were given approximately 40% less THC than males were during the tolerance induction phase, is not due to activational effects of gonadal hormones. Thus, sex differences may be due to organizational effects of gonadal steroid hormones, or to direct effects of the sex chromosomes themselves (Becker et al., 2005). Given the role that drug tolerance plays in the development of substance abuse and dependence, greater THC tolerance development in females may explain the more rapid transition from first use to problematic use of marijuana observed in women compared to men (Khan et al., 2013; Hernandez-Avila et al., 2004; Lewis et al., 2014).
Highlights.
On the pre-chronic test, THC was more potent in sham-GDX females than males.
In GDX females, progesterone decreased THC’s antinociceptive potency.
In GDX males, testosterone did not alter THC’s antinociceptive potency.
Tolerance to THC was greater in sham-GDX females than males, but this sex difference was not hormone-mediated.
Acknowledgments
This research was supported by the U.S. National Institute on Drug Abuse [Grant DA016644].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw H, Rimmerman N, Krey J, Walker J. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–58. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characteristics of cannabis smokers and experience of cannabis withdrawal. Am J Drug Alcohol Abuse. 2010;36:311–319. doi: 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft R, Leitl M. Gonadal hormone modulation of the behavioral effects of Delta(9)-tetrahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kandasamy R, Davis SM. Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain. 2013;154:1709–17. doi: 10.1016/j.pain.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Laggart JD. Sex differences in CB1 vs. CB2 receptor-selective antagonism of antinociception produced by delta-9-THC and CP55,490 in the rat. J Pharmacol Exp Ther. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–40. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: Sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HH. Estrous cyclicity in mammals. In: Adler NT, editor. Neuroendocrinology of Reproduction. New York: Plenum Press; 1981. pp. 279–348. [Google Scholar]
- Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 1893–1928. [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26:1013–22. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res. 2012;231:48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kalbasi Anaraki D, Sianati S, Sadeghi M, Ghasemi M, Paydar MJ, Mehr SE, Dehpour AR. Modulation by female sex hormones of the cannabinoid-induced catalpepsy and analgesia in ovariectomized mice. Eur J Pharmacol. 2008;586:189–96. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: Results from the National Epidemiologic Survery of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130:101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Hoffman LA, Nixon SJ. Sex differences in drug use among polysubstance users. Drug Alcohol Depend. 2014;145:127–133. doi: 10.1016/j.drugalcdep.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend. 2014;137:20–8. doi: 10.1016/j.drugalcdep.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Ellingboe J, Skupny AS, Lex BW, Griffin M. Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther. 1986;237:862–6. [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–6. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative-metabolism of delta-9-tetrahydrocannabinol in the rat. Biochem Pharmacol. 1991;41:1187–94. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. Washington DC: The National Academies Press; 2011. [Google Scholar]
- O’Connell ME, Morrill GA, Fujimoto GI, Kostellow AB. Factors affecting the response of the female rat reproductive system to cannabinoids. Toxicol Appl Pharmacol. 1987;88:411–7. doi: 10.1016/0041-008x(87)90215-8. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Marek P, Miyazaki B, Weber E. The neuroprotective efficacy of MK-801 in focal cerberal ischemia varies with rat strain and vendor. Brain Res. 1996;731:208–12. doi: 10.1016/0006-8993(96)00582-3. [DOI] [PubMed] [Google Scholar]
- Pryce G, Giovannoni G, Baker D. Mifepristone or inhibition of 11β-hydroxylase activity potentiates the sedating effects of the cannabinoid receptor-1 agonist Δ(9)-tetrahydrocannabinol in mice. Neurosci Lett. 2003;341:164–6. doi: 10.1016/s0304-3940(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Robel P, Young J, Corpechot C, Mayo W, Perche F, Haug M, Simon H, Baulieu EE. Biosynthesis and assay of neurosteroids in rats and mice: functional characteristics. J Steroid Biochem Molec Biol. 1995;53:355–360. doi: 10.1016/0960-0760(95)00074-a. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos J, Martin M, Fernandez-Ruiz J. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–70. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Romero E, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, de Miguel R, Ramos J, Viveros M. Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res. 2002;136:85–92. doi: 10.1016/s0165-3806(02)00306-1. [DOI] [PubMed] [Google Scholar]
- Smith CG, Almirez RG, Berenberg J, Asch RH. Tolerance develops to the disruptive effects of delta 9-tetrahydrocannabinol on primate menstrual cycle. Science. 1983;219:1453–5. doi: 10.1126/science.6298938. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Craft R. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–7. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng A, Harding J, Craft R. Pharmacokinetic factors in sex differences in Delta(9)-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Vallée M, Viviello S, Bellocchio L, Hébert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–8. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Craft RM. Antinociception and sedation following intracerebroventricular administration of Δ9-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216:200–6. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wakley AA, McBride AA, Vaughn LK, Craft RM. Cyclic ovarian hormone modulation of supraspinal Δ9-tetrahydrocannabinol-induced antinociception and cannabinoid receptor binding in the female rat. Pharmacol Biochem Behav. 2014a;124C:269–77. doi: 10.1016/j.pbb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alc Depend. 2014b;143:22–8. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. Sex-dependent effects of Delta(9)-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett. 2003;352:77–80. doi: 10.1016/j.neulet.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ. Sex differences in Δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett. 2014;576:51–5. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O’Connell MM, Tokarz ME, Wright MJ. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]