Abstract
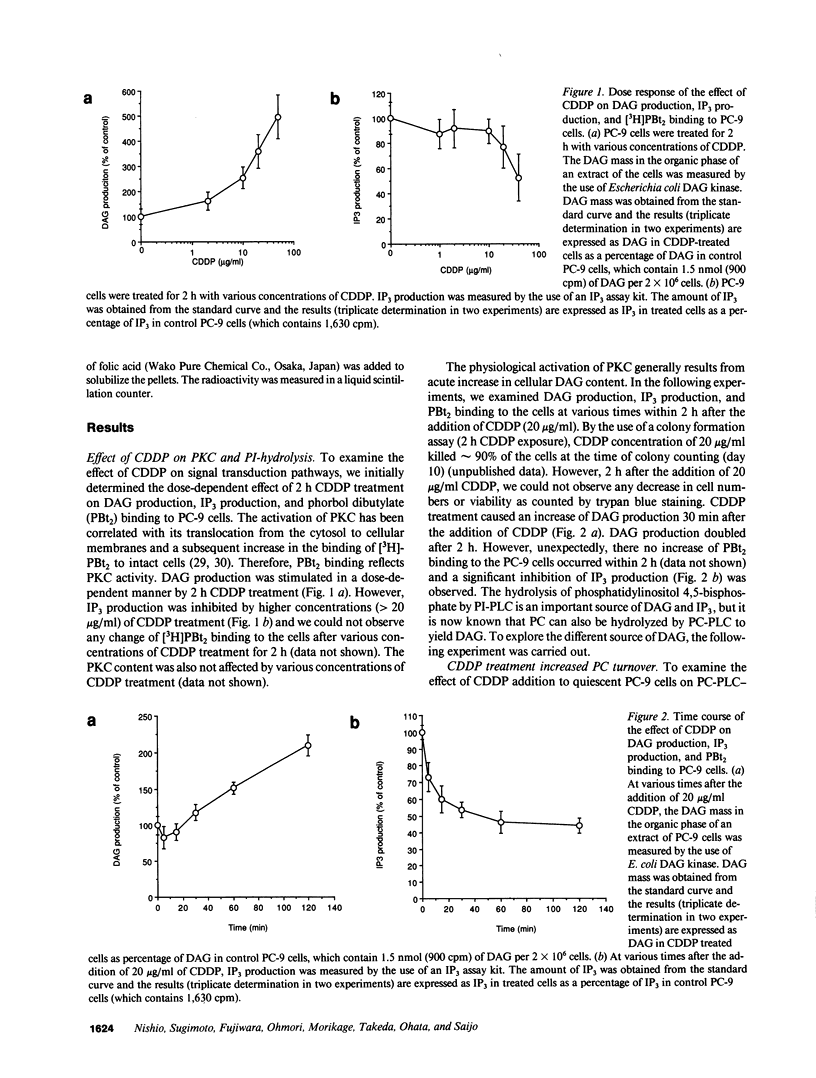
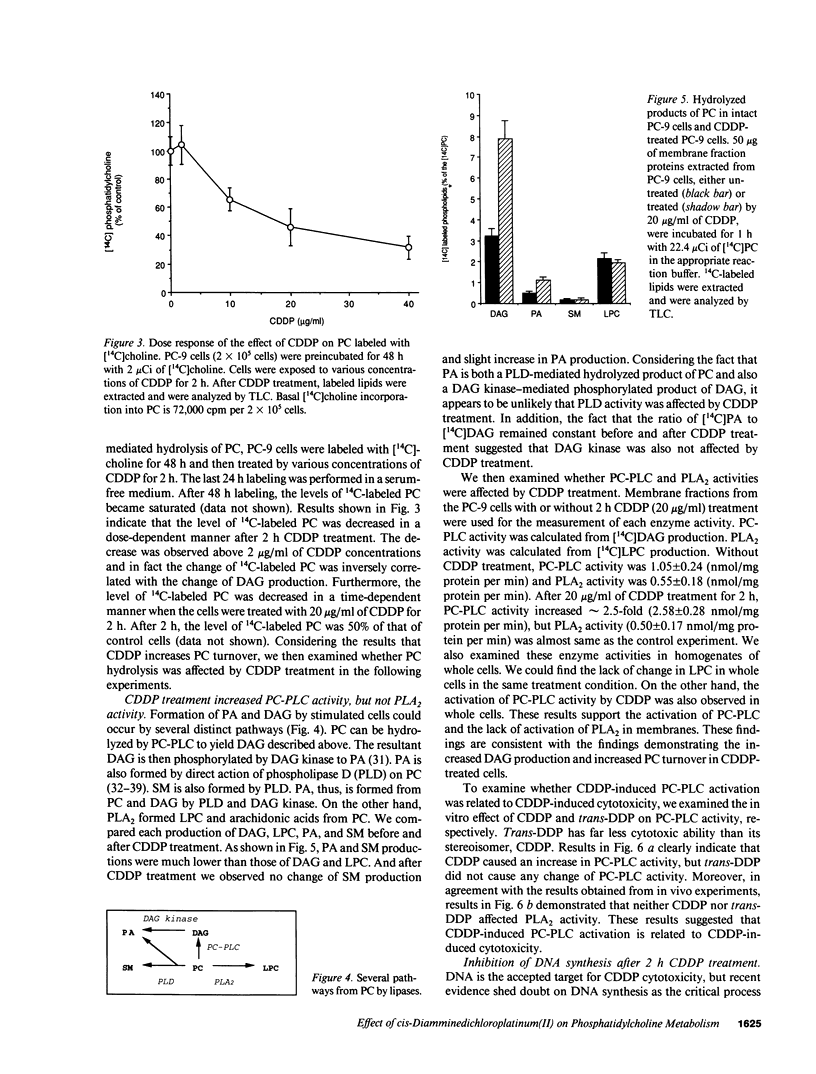
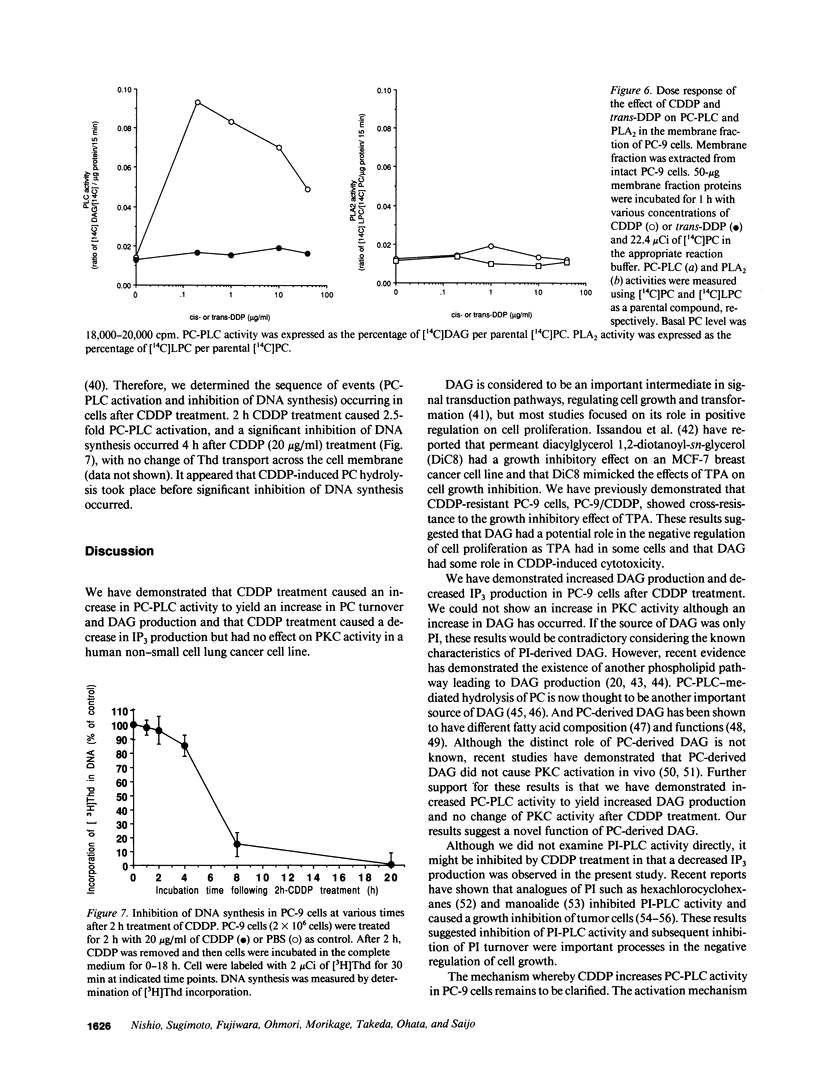
We have investigated the effect of cis-diamminedichloroplatinum(II) (CDDP) on signal transduction pathways. CDDP treatment did not cause any change in the binding of [3H]-phorbol dibutyrate to PC-9 (human lung adenocarcinoma cell line) cells, a measure of protein kinase C activation. However, 2-h CDDP treatment (20 micrograms/ml) caused approximately 200% increase in 1,2-sn-diacylglycerol (DAG) production and approximately 50% decrease in inositol 1,4,5-triphosphate production. To explore the different source of DAG, we analyzed phospholipids labeled with [14C]choline by TLC and revealed that [14C]choline-labeled phosphatidylcholine (PC) was decreased to 50% by CDDP treatment. This suggested that PC turnover was increased by CDDP-treatment. PC-specific phospholipase C (PC-PLC) activity was increased to 2.5-fold (2.58 +/- 0.28 nmol/mg protein per min) by 2 h CDDP (20 micrograms/ml) treatment compared with control (1.05 +/- 0.24 nmol/mg protein per min). Treatment of CDDP also stimulated PC-PLC in the crude membrane extract from PC-9 cells. CDDP had no effect on the activities of phospholipase A2 and D. Trans-DDP, which has far less cytotoxicity than its stereoisomer, CDDP, did not cause any change in PC-PLC activity. A significant inhibition of DNA synthesis (less than 80%) occurred 4 h after 2 h CDDP (20 micrograms/ml) treatment. These results demonstrated that CDDP-induced PC-PLC activation was an early event in CDDP-induced cytotoxicity and suggested that the effects of CDDP on signal transduction pathways had an important role in CDDP-induced cytotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- Araki S., Kawahara Y., Kariya K., Sunako M., Fukuzaki H., Takai Y. Stimulation of phospholipase C-mediated hydrolysis of phosphoinositides by endothelin in cultured rabbit aortic smooth muscle cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1072–1079. doi: 10.1016/0006-291x(89)92218-3. [DOI] [PubMed] [Google Scholar]
- Araki S., Kawahara Y., Kariya K., Sunako M., Fukuzaki H., Takai Y. Stimulation of phospholipase C-mediated hydrolysis of phosphoinositides by endothelin in cultured rabbit aortic smooth muscle cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1072–1079. doi: 10.1016/0006-291x(89)92218-3. [DOI] [PubMed] [Google Scholar]
- Augert G., Blackmore P. F., Exton J. H. Changes in the concentration and fatty acid composition of phosphoinositides induced by hormones in hepatocytes. J Biol Chem. 1989 Feb 15;264(5):2574–2580. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Basu A., Teicher B. A., Lazo J. S. Involvement of protein kinase C in phorbol ester-induced sensitization of HeLa cells to cis-diamminedichloroplatinum(II). J Biol Chem. 1990 May 25;265(15):8451–8457. [PubMed] [Google Scholar]
- Bennett C. F., Mong S., Wu H. L., Clark M. A., Wheeler L., Crooke S. T. Inhibition of phosphoinositide-specific phospholipase C by manoalide. Mol Pharmacol. 1987 Nov;32(5):587–593. [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Ca2+-mobilizing hormones elicit phosphatidylethanol accumulation via phospholipase D activation. FEBS Lett. 1987 Dec 10;225(1-2):201–204. doi: 10.1016/0014-5793(87)81157-2. [DOI] [PubMed] [Google Scholar]
- Brock T. A., Rittenhouse S. E., Powers C. W., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Phorbol ester and 1-oleoyl-2-acetylglycerol inhibit angiotensin activation of phospholipase C in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 15;260(26):14158–14162. [PubMed] [Google Scholar]
- Cao Y. Z., Reddy C. C., Mastro A. M. Evidence for protein kinase C independent activation of phospholipase D by phorbol esters in lymphocytes. Biochem Biophys Res Commun. 1990 Sep 28;171(3):955–962. doi: 10.1016/0006-291x(90)90777-k. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Hydrolysis of phosphatidylcholine by phospholipase D is a common response to mitogens which stimulate inositol lipid hydrolysis in Swiss 3T3 fibroblasts. Biochim Biophys Acta. 1991 Apr 17;1092(2):265–272. doi: 10.1016/0167-4889(91)90166-u. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Wykle R. L. A novel mechanism of diglyceride formation. 12-O-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986 Jul 15;261(20):9128–9132. [PubMed] [Google Scholar]
- Diaz-Laviada I., Larrodera P., Nieto J. L., Cornet M. E., Diaz-Meco M. T., Sanchez M. J., Guddal P. H., Johansen T., Haro A., Moscat J. Mechanism of inhibition of adenylate cyclase by phospholipase C-catalyzed hydrolysis of phosphatidylcholine. Involvement of a pertussis toxin-sensitive G protein and protein kinase C. J Biol Chem. 1991 Jan 15;266(2):1170–1176. [PubMed] [Google Scholar]
- Diaz-Meco M. T., Larrodera P., Lopez-Barahona M., Cornet M. E., Barreno P. G., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is activated by muscarinic agonists. Biochem J. 1989 Oct 1;263(1):115–120. doi: 10.1042/bj2630115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. W., Niedel J. E. Cytosolic calcium regulates phorbol diester binding affinity in intact phagocytes. J Biol Chem. 1986 Mar 25;261(9):4097–4100. [PubMed] [Google Scholar]
- Duronio V., Nip L., Pelech S. L. Interleukin 3 stimulates phosphatidylcholine turnover in a mast/megakaryocyte cell line. Biochem Biophys Res Commun. 1989 Oct 31;164(2):804–808. doi: 10.1016/0006-291x(89)91530-1. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fujiwara Y., Sugimoto Y., Kasahara K., Bungo M., Yamakido M., Tew K. D., Saijo N. Determinants of drug response in a cisplatin-resistant human lung cancer cell line. Jpn J Cancer Res. 1990 May;81(5):527–535. doi: 10.1111/j.1349-7006.1990.tb02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go M., Yokoyama M., Akita H., Fukuzaki H. Phorbol ester modulates serotonin-stimulated phosphoinositide breakdown in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 May 31;153(1):51–58. doi: 10.1016/s0006-291x(88)81188-4. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Effects of 12-O-tetradecanoylphorbol 13-acetate on glycerolipid metabolism in cultured myoblasts. Biochim Biophys Acta. 1982 May 13;711(2):272–280. doi: 10.1016/0005-2760(82)90036-4. [DOI] [PubMed] [Google Scholar]
- Hofmann J., Doppler W., Jakob A., Maly K., Posch L., Uberall F., Grunicke H. H. Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer. 1988 Sep 15;42(3):382–388. doi: 10.1002/ijc.2910420313. [DOI] [PubMed] [Google Scholar]
- Hofmann J., Ueberall F., Posch L., Maly K., Herrmann D. B., Grunicke H. Synergistic enhancement of the antiproliferative activity of cis-diamminedichloroplatinum(II) by the ether lipid analogue BM41440, an inhibitor of protein kinase C. Lipids. 1989 Apr;24(4):312–317. doi: 10.1007/BF02535169. [DOI] [PubMed] [Google Scholar]
- Huang C. F., Cabot M. C. Phorbol diesters stimulate the accumulation of phosphatidate, phosphatidylethanol, and diacylglycerol in three cell types. Evidence for the indirect formation of phosphatidylcholine-derived diacylglycerol by a phospholipase D pathway and direct formation of diacylglycerol by a phospholipase C pathway. J Biol Chem. 1990 Sep 5;265(25):14858–14863. [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Isonishi S., Andrews P. A., Howell S. B. Increased sensitivity to cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1990 Mar 5;265(7):3623–3627. [PubMed] [Google Scholar]
- Issandou M., Bayard F., Darbon J. M. Inhibition of MCF-7 cell growth by 12-O-tetradecanoylphorbol-13-acetate and 1,2-dioctanoyl-sn-glycerol: distinct effects on protein kinase C activity. Cancer Res. 1988 Dec 1;48(23):6943–6950. [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- Liscovitch M. Phosphatidylethanol biosynthesis in ethanol-exposed NG108-15 neuroblastoma X glioma hybrid cells. Evidence for activation of a phospholipase D phosphatidyl transferase activity by protein kinase C. J Biol Chem. 1989 Jan 25;264(3):1450–1456. [PubMed] [Google Scholar]
- Martin T. W., Feldman D. R., Michaelis K. C. Phosphatidylcholine hydrolysis stimulated by phorbol myristate acetate is mediated principally by phospholipase D in endothelial cells. Biochim Biophys Acta. 1990 Jul 12;1053(2-3):162–172. doi: 10.1016/0167-4889(90)90009-3. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Minnicozzi M., Anthes J. C., Siegel M. I., Billah M. M., Egan R. W. Activation of phospholipase D in normodense human eosinophils. Biochem Biophys Res Commun. 1990 Jul 31;170(2):540–547. doi: 10.1016/0006-291x(90)92125-j. [DOI] [PubMed] [Google Scholar]
- Muir J. G., Murray A. W. Bombesin and phorbol ester stimulate phosphatidylcholine hydrolysis by phospholipase C: evidence for a role of protein kinase C. J Cell Physiol. 1987 Mar;130(3):382–391. doi: 10.1002/jcp.1041300311. [DOI] [PubMed] [Google Scholar]
- Mullmann T. J., Siegel M. I., Egan R. W., Billah M. M. Phorbol-12-myristate-13-acetate activation of phospholipase D in human neutrophils leads to the production of phosphatides and diglycerides. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1197–1202. doi: 10.1016/0006-291x(90)90520-w. [DOI] [PubMed] [Google Scholar]
- Nishio K., Sugimoto Y., Nakagawa K., Niimi S., Fujiwara Y., Bungo M., Kasahara K., Fujiki H., Saijo N. Cross-resistance to tumour promoters in human cancer cell lines resistant to adriamycin or cisplatin. Br J Cancer. 1990 Sep;62(3):415–419. doi: 10.1038/bjc.1990.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Activation of phospholipase D by chemotactic peptide in HL-60 granulocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):355–364. doi: 10.1016/0006-291x(88)90528-1. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Parries G. S., Hokin-Neaverson M. Inhibition of phosphatidylinositol synthase and other membrane-associated enzymes by stereoisomers of hexachlorocyclohexane. J Biol Chem. 1985 Mar 10;260(5):2687–2693. [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Pfeffer L. M., Strulovici B., Saltiel A. R. Interferon-alpha selectively activates the beta isoform of protein kinase C through phosphatidylcholine hydrolysis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6537–6541. doi: 10.1073/pnas.87.17.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Sorenson C. M., Barry M. A., Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990 May 2;82(9):749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- Trilivas I., Brown J. H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem. 1989 Feb 25;264(6):3102–3107. [PubMed] [Google Scholar]
- Tritton T. R., Hickman J. A. How to kill cancer cells: membranes and cell signaling as targets in cancer chemotherapy. Cancer Cells. 1990 Apr;2(4):95–105. [PubMed] [Google Scholar]
- Workman P. Antitumor ether lipids: endocytosis as a determinant of cellular sensitivity. Cancer Cells. 1991 Aug;3(8):315–317. [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]
- Yamatani T., Yamaguchi A., Nakamura A., Morishita T., Kadowaki S., Fujita T., Chiba T. Activation of PKC inhibits NaF-induced inositol phospholipid turnover in rat insulinoma cells. Am J Physiol. 1990 Jul;259(1 Pt 1):E73–E79. doi: 10.1152/ajpendo.1990.259.1.E73. [DOI] [PubMed] [Google Scholar]


