Abstract
Background
Ketoconazole is a well-known CYP-17-targeted systemic treatment for castration-resistant prostate cancer (CRPC). However, most of the published data has been in the pre-chemotherapy setting; its efficacy in the post-chemotherapy setting has not been as widely described. Chemotherapy-naïve patients treated with attenuated doses of ketoconazole (200-300 mg three times daily) had prostate specific antigen (PSA) response rate (greater than 50% decline) of 21% to 62%. We hypothesized that low-dose ketoconazole would likewise possess efficacy and tolerability in the CRPC post-chemotherapy state.
Methods
Men with CRPC and performance status (PS) 0-3, adequate organ function and who had received prior docetaxel were treated with low-dose ketoconazole (200 mg PO three times daily) and hydrocortisone (20 mg PO qAM and 10 mg PO qPM) until disease progression. Primary endpoint was PSA response rate (greater than 50% reduction from baseline) where a PSA response rate of 25% was to be considered promising for further study (versus a null rate of less than 5%); 25 patients were required. Secondary endpoints included PSA response greater than 30% from baseline, progression-free survival (PFS), duration of stable disease, and evaluation of adverse events (AEs).
Results
Thirty patients were accrued with median age of 72 years (range 55-86) and median pre-treatment PSA of 73 ng/ml (range 7-11,420). Twenty-nine patients were evaluable for response and toxicity. PSA response (>50% reduction) was seen in 48% of patients; PSA response (>30% reduction) was seen in 59%. Median PFS was 138 days; median duration of stable disease was 123 days. Twelve patients experienced grade 3 or 4 AEs. Of the 17 grade 3 AEs, only 3 were attributed to treatment. None of the 2 grade 4 AEs was considered related to treatment.
Conclusions
In docetaxel pre-treated CRPC patients, low-dose ketoconazole and hydrocortisone is a well-tolerated, relatively inexpensive and clinically active treatment option. PSA response to low-dose ketoconazole appears historically comparable to that of abiraterone in this patient context. A prospective, randomized study of available post-chemotherapy options is warranted to assess comparative efficacy.
Introduction
In 2014, the National Cancer Institute's SEER database estimates that 233,000 men in the United States will be diagnosed with prostate cancer and 29,480 patients will die from this disease. While the majority of patients will have early stage prostate cancer, a proportion of these patients will progress and develop metastasis and a small fraction of men will be diagnosed with metastatic disease at the outset. Androgen deprivation therapy (ADT) using luteinizing hormone releasing hormone (LHRH) agonists (with or without androgen receptor [AR] antagonists) is considered standard first-line treatment in patients with locally advanced or metastatic disease. Although all metastatic prostate cancer patients will eventually progress despite ADT to a ‘castration-resistant’ state, the disease remains androgen receptor-driven and hence still potentially responsive to modulation of hormonal pathways.
Traditional hormonal manipulation in the castrate-resistant context, including ketoconazole, megestrol and AR antagonists (bicalutamide, nilutamide and flutamide) have been often used in CRPC, however none had been shown to prolong survival until recently with newer agents such as abiraterone and enzalutamide. Ketoconazole, an oral antifungal agent, was first described to have activity in prostate cancer over 30 years ago and has long been used off-label in the treatment of CRPC prior to docetaxel. It blocks androgen synthesis by inhibiting the C17,20-desmolase enzyme, a key microsomal cytochrome P450-dependent enzyme, necessary for adrenal androgen and testosterone biosynthesis. It is also suspected to have direct cytotoxic effects on prostate cancer cells by inhibiting DNA synthesis in-vitro and to possibly induce G0/G1 cell cycle arrest in other cancers.[1-3] High-dose ketoconazole therapy with 400 mg three-times daily and replacement hydrocortisone has become the standard dose schedule. However, toxicities (some due to off-target effects) including fatigue, abdominal pain, hepatic dysfunction, nausea, diarrhea, and mucositis have often limited its long-term use.[4] Low-dose ketoconazole at 200 mg three times daily and intermediate-dose ketoconazole at 300 mg three times daily have been explored in the pre-chemotherapy setting with comparable response rates (28% to 55%) to high-dose ketoconazole and better tolerability.[5-7]
Cytotoxic chemotherapy was considered relatively ineffective in CRPC until two landmark clinical trials established a survival benefit for docetaxel in this setting.[8, 9] Docetaxel subsequently received FDA approval in 2004 and subsequently became a standard of care in metastatic CRPC treatment. Despite progression on ADT, castration-resistant disease continues to rely on androgen pathways within the tumor, testes, and adrenal glands for growth, reaffirming the key role of the AR and AR signaling in the progression of prostate cancer throughout the course of the disease. CYP17 plays a crucial role within the androgen biosynthesis pathway and is the target of androgen synthesis inhibitors such as ketoconazole and abiraterone. Abiraterone received FDA approval in 2011 after showing a survival benefit in docetaxel pre-treated CRPC patients with expanded approval in 2013 for its use prior to docetaxel.[10, 11] Similar to other AR antagonists, enzalutamide binds to AR, but is unique in its ability to also inhibit AR nuclear translocation and interaction with DNA. Enzalutamide received FDA approval in 2013 after showing a survival benefit in docetaxel pre-treated CRPC patients.[12]
Most of the published data for ketoconazole (with few exceptions[13, 14]) has been in an era when patients benefiting from ketoconazole had not yet received any prior chemotherapy. This study was conducted to prospectively assess the response rate to low-dose ketoconazole after CRPC patients had received at least one prior chemotherapy regimen.
Methods
Patients
Inclusion criteria
Eligible patients must have had biopsy-proven prostate cancer deemed to be castrate-resistant despite androgen deprivation and have evidence of either radiographic progression or rising PSA (two consecutive rises at least two weeks apart). Other major eligibility criteria included age at least 18 years old and Zubrod performance status of 0-3 with adequate end-organ and marrow function.
They must have received at least one prior docetaxel-based regimen more than 3 weeks prior to initiation of study. Patients must have been surgically or medically castrated with a total serum testosterone level less than 50 ng/dL. If the method of castration was LHRH agonists (leuprolide or goserelin), then the patient must have been willing to continue the use of LHRH agonists. If the patient had been treated with non-steroidal anti-androgens (flutamide, bicalutamide or nilutamide) or other hormonal treatment (megestrol acetate or steroids), the patient must have stopped these agents at least 28 days prior to enrollment for flutamide, megestrol or steroids and at least 42 days prior to enrollment for bicalutamide or nilutamide; patients must have demonstrated progression of disease since these agents were suspended.
Exclusion criteria
Patients could not have had any prior treatment with ketoconazole for prostate cancer or have had any other active malignancies in the past 3 years except non-melanoma skin cancer. They were ineligible if they could not take oral medications or had a known hypersensitivity to ketoconazole. Patients were not allowed to take certain medications, including terbinafine, astemizole, triazolam, statins (except pravastatin and fluvastatin) and acid suppressive agents (antacids, H2 blockers, proton pump inhibitors) while on trial given the known interactions with ketoconazole.
Study Design
This was an open-label, single-institution investigator initiated phase II study sponsored by the University of California Davis Comprehensive Cancer Center Support Grant. CRPC patients received ketoconazole, administered orally at a dose of 200 mg three times a day, and hydrocortisone, administered orally at 20 mg in the morning and 10 mg in the evening. Each treatment cycle was 28 days and treatment was continued until disease progression, unacceptable toxicity despite dose reduction, death, or upon the patient or physician's discretion.
Adverse events that resolved promptly with supportive care required no dosing adjustment. For grade 3 or higher AE related to ketoconazole that did not resolve to grade 2 or less despite maximum supportive care for 72 hours, ketoconazole was held until resolution of AE to grade 2 or less and then continued at one dose level reduction. The ketoconazole dose would be reduced from an initial 200 mg three times/day to 200 mg twice/day and if necessary to 100 mg three times/day; no additional reductions were permitted after this last dose level reduction and patients would subsequently be removed from the study if still intolerant. Patients with grade 3 AE that did not resolve to grade 2 at the lowest reduced dose level were removed from study.
Assessments
Physical examinations and blood tests were completed as close as possible to the study enrollment date (within 28 days) and then at four-week intervals thereafter. For patients with measurable disease, imaging studies were done every 8 weeks. Serum PSA was measured at time of study enrollment and every 4 weeks. PSA progression was defined as an increase in PSA by at least 50% over the nadir or baseline (pretreatment) value, whichever was lower. The lowest PSA value at any timepoint was recorded as best response.
Adverse events were classified according to the National Cancer Institute CTCAE 3.0 Common Terminology Criteria (CTC). Serious AE were fatal or life-threatening, resulted in prolonged or substantial disability, required hospitalization. Patients were followed for 8-12 weeks after removal from study or until death, whichever occurred first. Patients removed from study for unacceptable AE were followed until resolution or stabilization of the adverse event.
Statistical Considerations
The primary endpoint was PSA response defined as greater than 50% reduction from baseline. The lowest PSA achieved at any point was recorded. Secondary endpoints included PSA response (greater than 30% from baseline), progression-free survival (PFS) defined as time from registration until disease progression employing RECIST radiographic criteria for progression (i.e., PCWG2 criteria were not used for this study), duration of stable disease defined as time from start of treatment until disease progression, and safety. Imaging studies (CT and/or bone scans) were performed after every 8 weeks in patients with measurable or evaluable disease. To estimate the planned sample size, a single-arm Simon single-stage phase II minimax design was employed. A sample size of 25 patients had a power of at least 90% to detect PSA response in greater than 25% of patients versus the null hypothesis of a PSA response in less than 5% of patients. To account for dropouts, a target of 30 patients was recruited for this study.
Results
Patient Characteristics
Between November 2009 and December 2013, we enrolled 30 patients with CRPC who had failed at least one line of docetaxel-based chemotherapy. The date of data cutoff was December 2013. Table 1 summarizes the baseline patient characteristics of all 30 patients, of which 29 patients were evaluable for treatment response and AE at time of data cutoff. The median age of the patients was 72 years with a range from 55-86 years. All patients had Zubrod PS of 0-1 except for one (PS 2). Baseline pre-study PSA was 73 ng/ml with a wide range 7-11,420 ng/ml. Median values of hemoglobin were 12.8 g/dL, alkaline phosphatase of 96 U/L and creatinine of 0.87 mg/dl.
Table I. Patient Characteristics.
| Characteristic | Total (n = 30) |
|---|---|
| Median age, range (years) | 72, 55 – 86 |
| Zubrod Performance Status | |
| 0 | 16 |
| 1 | 13 |
| 2 | 1 |
| Median pre-treatment PSA, range (ng/ml) | 73, 7 – 11,420 |
| Median hemoglobin, range (g/dL) | 12.8, 9.7 – 15.5 |
| Median alkaline phosphatase, range (U/L) | 96, 36 – 271 |
| Median creatinine, range (mg/dL) | 0.87, 0.60 – 1.83 |
| Prior therapy (# patients) | |
| LHRH antagonist | 30 |
| AR antagonist | 30 |
| Docetaxel | 30 |
| Cabazitaxel | 1 |
| Vinorelbine | 1 |
| Abiraterone | 4 |
| Orteronel | 2 |
| Enzalutamide | 1 |
| Sipuleucel-T | 4 |
| Atrasentan | 1 |
| Silutuximab | 1 |
| Cabozantanib | 1 |
Two patients had received systemic chemotherapy in addition to docetaxel (cabazitaxel or vinorelbine) prior to study entry. Six patients had received prior androgen synthesis inhibitors, four with abiraterone and two with orteronel. One patient had previously received enzalutamide. Four patients had received immunotherapy with sipuleucel-T. Five patients had received prior biologic therapy including one patient with cabozantinib, one patient with siltuximab, and three patients with atrasentan.
Outcomes/Efficacy
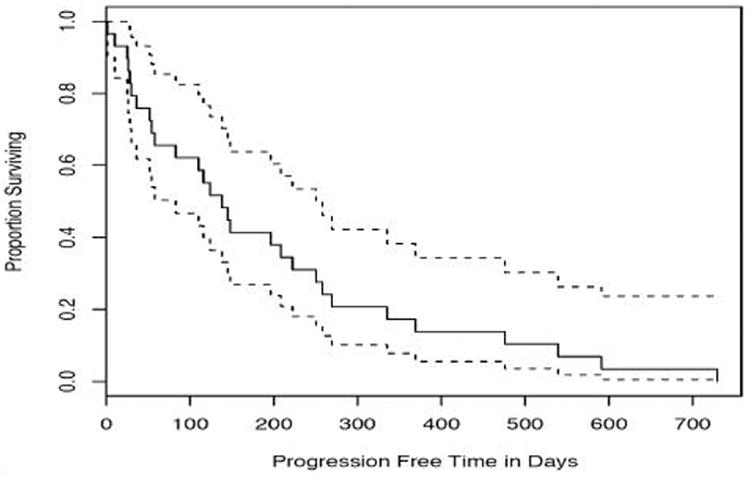
A waterfall plot of the best PSA response (>50% decline from baseline) among the 29 evaluable patients (Figure 1) showed that 14 (48%) patients experienced PSA response. A total of 17 (59%) patients experienced at least 30% decline in their PSA (Table 2). Responses were durable with a median PFS of 138 days (4.6 months) while duration of stable disease was 123 days (Table 2). Figure 2 shows the Kaplan-Meier curve for PFS with 95% confidence intervals.
Figure 1. Waterfall Plot of PSA Response.
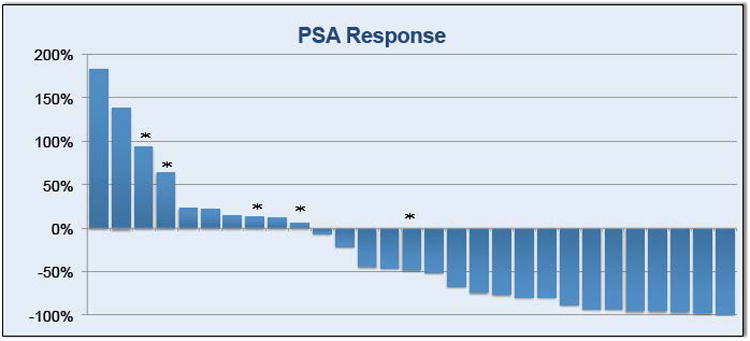
*indicates receipt of prior CYP inhibitor therapy (either abiraterone or orteronel)
Table 2. Efficacy.
| Efficacy | Total (n = 29) |
|---|---|
| PSA response rate > 50% (# patients,%) | 14, 48% |
| PSA response rate > 30% (# patients,%) | 17, 59% |
| Median progression free survival (days) | 138 (95% CI: 80, 260) |
| Median duration of stable disease (days) | 123 (range: 2-728) |
Figure 2. Kaplan-Meier Curve for Progression Free Survival.
Adverse Events
The incidence of grade 3 or 4 AE is shown in table 3. The most frequently reported grade 3 or 4 AE was cardiotoxicity (4 patients), followed equally by hepatoxicity, neurotoxicity, electrolyte disturbance, malaise/fatigue, and pain. Thirteen unique patients experienced at least one AE; an AE led to discontinuation of low-dose ketoconazole in one patient. No deaths occurred while the patient was receiving the study drug. The three Grade 3 toxicities considered possibly or probably related to treatment included hyperglycemia, elevated aspartate aminotransferase, and fatigue. No grade 4 toxicities were attributed to treatment.
Table 3. All Grade 3 or Grade 4 Adverse Events (CTCAE v3.0).
| Event | Grade 3 (# events) | Grade 4 (# events) |
|---|---|---|
| Cardiac general | Angina (1) | Myocardial infarct (1) |
| Congestive heart failure (1) | - | |
| Myocarditis (1) | - | |
| Cardiac arrhythmia | Atrial fibrillation (1) | - |
| Constitutional symptoms | Weight gain (2) | - |
| Fatigue (1) | - | |
| Metabolic | Transaminitis (2) | Hyperkalemia (1) |
| Hyponatremia (1) | - | |
| Hyperglycemia (1) | - | |
| Musculoskeletal | Fracture (1) | - |
| Neurology | Syncope (1) | - |
| Mood alteration (1) | - | |
| Psychosis (1) | - | |
| Pain | Pain (2) | - |
Discussion
In this single-arm phase II trial, the efficacy of low-dose ketoconazole was studied in men with CRPC who had failed ADT and had undergone at least one line of docetaxel-based chemotherapy. The characteristics of patients in this trial were typical of those commonly seen in the clinical oncology practice. Most patients had bone metastases and high serum PSA. We found that PSA response rates, PFS, and duration of response all suggest reasonable efficacy for low-dose ketoconazole in this chemotherapy-pretreated setting. Low-dose ketoconazole demonstrated PSA response (>50% from baseline) of 49%, which appears at least comparable to that seen in the only phase III randomized study of high-dose ketoconazole (response rate of 32% in pre-docetaxel CRPC).[15] The PSA response rate in this study also appears similar to those described in aforementioned phase III studies of newer hormonal therapies such as abiraterone (29%) and enzalutamide (54%) in the post-docetaxel setting.[10, 12] However, median PFS of 4.6 mos (138 days) was somewhat less than that observed with abiraterone (5.6 mos) or enzalutamide (8.3 mos), although our study was limited by its use of RECIST-based PFS criteria. Furthermore, in a recent retrospective study, men with docetaxel pre-treated CRPC who then received either abiraterone or ketoconazole were analyzed.[16] In that study, outcomes such as PSA response and PFS (either biochemical or radiological) appeared to favor patients treated with abiraterone, although overall survival was not statistically different between the groups.
While mature overall survival (OS) data are not yet available at time of data cutoff, a PSA response greater than 30% from baseline was seen in 59% of patients. Prior reports suggest that a PSA decline of this magnitude may potentially be associated with subsequent OS benefit, despite some controversy surrounding the use of PSA as an intermediate marker of disease response and outcome.[17, 18] Further complicating comparisons of treatment benefit, especially OS, is the ‘cross-contamination’ of the placebo arm with subsequent lines of active therapy. This was seen in a phase III study of the novel CYP17 inhibitor orteronel compared to placebo, which showed a significant PFS but no OS benefit.[19] One likely reason was that many in the placebo arm were treated upon progression with abiraterone, an agent similar to orteronel. It is highly likely that any future randomized trial of low-dose ketoconazole versus placebo might also witness the same confounding effect.
Given the relative abundance of treatment options in CRPC, one important question is the optimal sequence of therapy. There is data suggesting that docetaxel's anti-tumor effect comes in part from its ability to suppress AR expression and that cross-resistance is seen when docetaxel is used after abiraterone.[20] With abiraterone, a recent trial has shown decreased PSA response and median PFS when used after enzalutamide, but not in every patient.[21] When sequential therapies targeting the AR pathway show decreased efficacy, this suggests some degree of acquired resistance despite different mechanisms of action and highlights the current gaps in our understanding. In a large retrospective study on the effect of prior ketoconazole on subsequent docetaxel therapy, there was no apparent difference in survival between men receiving prior ketoconazole or not.[22] Identifying those patients most likely to benefit from androgen synthesis inhibitors or AR antagonists would be one way that subsequent trials can help define the optimal sequence of therapies in the CRPC setting.
In the current and future healthcare landscape, the cost per quality adjusted life year (QALY) may become as important as OS, PFS, and response rate when evaluating a therapy's overall efficacy. From this perspective, a generic drug like ketoconazole has the potential to compare favorably to newer and more expensive agents. A recent formal study of abiraterone vs placebo calculated the cost at $94,000/QALY.[23] It must be noted that ketoconazole is a generic medication and while the QALY for ketoconazole has not to our knowledge been formally studied, the cost for a one-month supply of low-dose ketoconazole (200 mg tab, three times daily) is $193, based on acquisition costs at this institution as of this writing.
Encouragingly, low-dose ketoconazole was well-tolerated in almost all patients with the vast majority of AEs being grade 1 or 2 and only one patient had to withdraw due to treatment intolerance. None of the grade 4 AE was thought related to the study treatment and no patients died while on treatment.
This study had several limitations. It is a single-institution trial with a modest sample size. Furthermore, RECIST criteria (and not PCWG2) were used, and confirmation of PSA response was not required. Another limitation was the lack of a double-blind design that required us instead to rely on external trials as a comparator. While low-dose ketoconazole appears to have activity in this setting, it is also important to recognize that part of this response might be attributable to concurrent hydrocortisone, which is needed to replace decreased cortisol production while on ketoconazole. Replacement doses of hydrocortisone were shown in a large phase III trial to result in a PSA response in 16% of patients.[24]
To our knowledge, this is the first prospective study of the efficacy of low-dose ketoconazole in the post-docetaxel setting. Our findings provide evidence that low-dose ketoconazole has continued activity after docetaxel that may be comparable to high-dose ketoconazole, abiraterone, and enzalutamide. It appears to be a well-tolerated, relatively inexpensive and clinically active treatment option. A prospective randomized study is ultimately needed to assess the comparative efficacy, survival benefit, and cost effectiveness of low-dose ketoconazole against the newer AR-pathway inhibitors in this setting.
Acknowledgments
This study was supported by the VA Career Development Award-2 (PI: Pan), VA Merit (PI: Pan; Grant # 1I01BX001784) and the NCI Cancer Center Support Grant (PI: de Vere White). Statistical Support was provided by the Biostatistics Shared Resource through the UC Davis Comprehensive Cancer Center Support Grant, P30CA093373-06.
Footnotes
Presented in part at the 10th Annual Meeting of the American Society of Clinical Oncology Genitourinary Symposium, January 2014, San Francisco, CA
Clinical Trials Registration: ClinicalTrials.gov Identifiers NCT00895310
References
- 1.Chen RJ, et al. Ketoconazole induces G0/G1 arrest in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Pharmacol. 2000;169(2):132–41. doi: 10.1006/taap.2000.9062. [DOI] [PubMed] [Google Scholar]
- 2.Eichenberger T, et al. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141(1):190–1. doi: 10.1016/s0022-5347(17)40639-2. [DOI] [PubMed] [Google Scholar]
- 3.Rochlitz CF, et al. Cytotoxicity of ketoconazole in malignant cell lines. Cancer Chemother Pharmacol. 1988;21(4):319–22. doi: 10.1007/BF00264198. [DOI] [PubMed] [Google Scholar]
- 4.Keizman D, et al. Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: clinical factors associated with PSA response and disease progression. Prostate. 2012;72(4):461–7. doi: 10.1002/pros.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris KA, et al. Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol. 2002;168(2):542–5. [PubMed] [Google Scholar]
- 6.Nakabayashi M, et al. Response to low-dose ketoconazole and subsequent dose escalation to high-dose ketoconazole in patients with androgen-independent prostate cancer. Cancer. 2006;107(5):975–81. doi: 10.1002/cncr.22085. [DOI] [PubMed] [Google Scholar]
- 7.Ngo LS, et al. Efficacy of low-dose ketoconazole in hormone refractory prostate cancer patients at the National Cancer Centre and The Cancer Institute, Singapore. Ann Acad Med Singapore. 2007;36(10):811–4. [PubMed] [Google Scholar]
- 8.Petrylak DP, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 13.Galsky MD, et al. Ketoconazole retains activity in patients with docetaxel-refractory prostate cancer. Ann Oncol. 2009;20(5):965–6. doi: 10.1093/annonc/mdp199. [DOI] [PubMed] [Google Scholar]
- 14.Nakabayashi M, et al. Activity of ketoconazole after taxane-based chemotherapy in castration-resistant prostate cancer. BJU Int. 2010;105(10):1392–6. doi: 10.1111/j.1464-410X.2009.08971.x. [DOI] [PubMed] [Google Scholar]
- 15.Small EJ, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22(6):1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Peer A, et al. Comparison of abiraterone acetate versus ketoconazole in patients with metastatic castration resistant prostate cancer refractory to docetaxel. Prostate. 2014;74(4):433–40. doi: 10.1002/pros.22765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong AJ, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25(25):3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 18.Hussain M, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27(15):2450–6. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapoor A. What's new in prostate cancer research? Highlights of GU-ASCO 2014. Can Urol Assoc J. 2014;8(3-4 Suppl 2):S8–S12. doi: 10.5489/cuaj.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezynski J, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23(11):2943–7. doi: 10.1093/annonc/mds119. [DOI] [PubMed] [Google Scholar]
- 21.Loriot Y, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24(7):1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal R, et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance) Cancer. 2013;119(20):3636–43. doi: 10.1002/cncr.28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong L, et al. Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis. PLoS One. 2013;8(5):e64275. doi: 10.1371/journal.pone.0064275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small EJ, et al. Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. J Clin Oncol. 2000;18(7):1440–50. doi: 10.1200/JCO.2000.18.7.1440. [DOI] [PubMed] [Google Scholar]


