Abstract
Aims
Within a parent study examining ovarian hormone effects on smoking cessation in women, we conducted an exploratory short-term trial of varenicline versus transdermal nicotine patch.
Design
Double-blind double-dummy randomized trial.
Setting
Single-site outpatient research clinic in the United States.
Participants
Female smokers, ages 18-45 and averaging ≥10 cigarettes per day for at least 6 months (N=140).
Interventions
Participants were randomized to receive a four-week course of (a) varenicline tablets and placebo patches (n=67), or (b) placebo tablets and nicotine patches (n=73). Two brief cessation counseling sessions were provided for all participants.
Measurements
The outcome of primary clinical interest was two-week end-of-treatment abstinence. Secondary outcomes included one- and four-week end-of treatment abstinence and abstinence at a post-treatment follow-up visit occurring four weeks after treatment conclusion. Breath carbon monoxide (≤10 parts per million) was used to confirm biochemically self-reported abstinence.
Findings
Two-week end-of-treatment abstinence was achieved by 37.3% (25/67) of varenicline participants and by 17.8% (13/73) of nicotine patch participants (odds ratio [OR] (95% confidence interval [CI]) 2.7 (1.3-6.0), p=0.011). One-week (44.8% vs 20.6%, OR 3.1 (1.5-6.6), p=0.003) and four-week (22.4% vs 9.6%, OR 2.7 (1.0-7.2), p=0.043) end-of-treatment abstinence similarly favored varenicline, though post-treatment follow-up Russell Standard abstinence was not significantly different between groups (23.9% vs 13.7%, OR 2.0 (0.8-4.7), p=0.126).
Conclusion
In an exploratory four-week head-to-head trial in female smokers, varenicline, compared with nicotine patch, more than doubled the odds of end-of-treatment abstinence, although this diminished somewhat at post-treatment follow-up.
Keywords: Nicotine, Tobacco, Pharmacotherapy, Varenicline, Nicotine Patch, Women, Gender, Randomized Clinical Trial, NRT
Introduction
Despite significant advances in prevention and treatment, cigarette smoking remains the leading cause of preventable death in the United States (US) and the world (1,2). Pharmacotherapies have been among the most important advances in smoking cessation treatment, but the lack of head-to-head trials limits the ability to compare efficacy across medications, leading researchers and clinicians to rely on indirect comparisons across studies with heterogeneous methodologies.
Varenicline (VAR) and transdermal nicotine patch (TNP) are both established US Food and Drug Administration (FDA) approved smoking cessation pharmacotherapies. However, no head-to-head trials comparing these medications have employed double-blind methods, and only two open-label efficacy comparison trials have been published. One (n=746) yielded superior end-of-treatment outcomes with VAR versus TNP (Odds Ratio [OR] 1.70) (3) and the other (n=272) yielded statistically equivalent outcomes between treatment groups (4). A number of effectiveness comparisons have been conducted, with VAR generally yielding superior abstinence outcomes when compared to single NRT (e.g., patch, gum, lozenge, inhaler; OR ranging 1.8-2.0), but more modest advantage when compared to combined NRT (OR ranging 1.1-1.3) (5-9). A recent network meta-analysis concluded that VAR is superior to single NRT (OR=1.5) but not combined NRT (OR 1.1) (10).
In light of the aforementioned prior findings, and given the need for smoking-related research focused on females (11), we sought, within a parent study evaluating ovarian hormone effects on smoking cessation (12), to compare the short-term efficacy of VAR versus TNP in female smokers. We incorporated a double-blind, double-dummy design for enhanced methodological rigor, and hypothesized that women receiving VAR would have higher rates of abstinence than those receiving TNP.
Methods
Design
Treatment-seeking female smokers were randomized, in 1:1 parallel group allocation, to receive a double-blind, double-dummy, four-week course of (a) varenicline (VAR) tablets and placebo patches, or (b) placebo tablets and nicotine patches (TNP). Post-treatment follow-up occurred four weeks after treatment conclusion. Smoking self-report (cigarettes per day) was logged by participants and confirmed via Timeline Follow-Back procedures (TLFB) (13) at all visits. Breath carbon monoxide (CO; <10 parts per million) was used for biochemical verification of abstinence. These procedures were conducted as part of a parent study, in which plasma progesterone and estradiol levels were obtained at baseline and weekly throughout the cessation pharmacotherapy trial, to examine ovarian hormone influences on abstinence (12). All clinical procedures were conducted in a research clinic at the Medical University of South Carolina (MUSC) in Charleston, South Carolina, USA, and approved by the MUSC Institutional Review Board.
Participants
To enroll in the study, women were required to (a) be 18 to 45 years old, (b) smoke at least an average of 10 cigarettes per day for at least 6 months, with desire to quit, (c) be post-menarcheal and premenopausal and have regular menstrual cycles between 25 and 35 days (for investigation of ovarian hormone influence on smoking outcomes), (d) not take hormonal contraceptives, (e) lack current comorbid substance use disorders, (f) have no acutely unstable psychiatric or medical illness, and (g) lack history of adverse reaction to VAR or TNP. Recruitment occurred primarily through community media and clinical referrals. If an initial telephone screen suggested potential eligibility, individuals were scheduled for an informed consent and baseline assessment visit. After a complete description of the study, written participant consent was obtained before initiation of any assessments or procedures. Participants were compensated for time and effort involved in study participation.
Study Procedures
Screening
All procedures were conducted in the university research clinic. At the screening visit, comprehensive psychiatric and substance use diagnostic assessment (14,15), physical examination, and laboratory testing (complete blood count, comprehensive metabolic panel, urine pregnancy test, and urine drug test) were performed. Participants completed general demographic and smoking history questionnaires, as well as the Fagerström Test for Nicotine Dependence (FTND) and the Questionnaire on Smoking Urges (QSU) (16,17).
Medication Trial
Eligible participants were enrolled in the study and returned one to two weeks following the screening visit for double-blind randomization to receive either VAR or TNP. The university investigational pharmacy conducted 1:1 allocation block randomization (block size = 6) and medication dispensing. All research team members were blind to the randomization sequence and individual participant assignments. At the participant level, medication was received at weekly dispensation visits, with standard packaging bearing no indication of active versus placebo assignment. The target quit date (TQD) was set for one week following randomization, and the treatment phase lasted for a total of four weeks beyond the TQD. Participants were seen in clinic weekly during the four-week medication trial and returned for post-treatment follow-up four weeks after treatment conclusion. At all visits, the study physician or physician assistant provided medication management.
Interventions
Medication
Enrolled participants were randomized to double-blind treatment assignment (VAR versus TNP). Double-dummy procedures were used, so that VAR participants received placebo patches, and TNP participants received placebo tablets. VAR/placebo tablets were titrated during the week prior to the TQD (0.5 mg daily on days -7 through -5 and 0.5 mg twice daily on days -4 through - 1), with goal dosing (1 mg twice daily) thereafter. Nicotine/placebo patches were started at 21 mg daily on the TQD. Medication dose reduction or discontinuation was available if indicated in the context of medication management. VRN and matched placebo tablets were supplied by Pfizer, Inc. Nicotine patches (Nicoderm CQ brand) were purchased from GlaxoSmithKline, and matched placebo patches (designed to appear identical to active Nicoderm CQ patches) were purchased from Rejuvenation Labs, Inc.
Cessation Counseling
All participants received two formal cessation counseling sessions, each lasting about 30 minutes. The first, conducted prior to medication initiation, included sharing and reviewing the National Cancer Institute brochure Clearing the Air, as well as planning for the upcoming target quit date. The second session, conducted on the TQD, focused on strategies for relapse prevention. The remainder of cessation counseling during active treatment was informal and brief, occurring in the context of medication management. At the conclusion of active treatment, participants were offered referral for ongoing cessation counseling (e.g., state quitline).
Outcomes
Efficacy
In order to maximize power and gain clear understanding of potential efficacy difference at the end of active treatment, the efficacy outcome of primary clinical interest was CO-verified self-reported two-week end-of-treatment abstinence. Secondary measures of interest included one- and four-week end-of-treatment abstinence, as well as one-week and continuous abstinence at the post-treatment follow-up visit. Per the initial study protocol, times to smoking lapse and relapse were analyzed, with lapse defined as the first self-reported smoking day following the TQD and smoking relapse defined as the first of three or more consecutive days of self-reported smoking following the TQD.
Safety/Tolerability
A thorough safety evaluation was conducted at each clinic visit: (a) physician or physician assistant evaluation of adverse events via open-ended interview and comprehensive, structured review of systems, (b) suicide ideation and risk assessment (18), (c) urine pregnancy testing, and (d) vital signs measurement.
Statistical Analyses
Sample Size Determination
The study sample size was determined via power analysis focused on the parent study's goal of assessing ovarian hormone influence on smoking cessation (12). Power for detection of VAR versus TNP efficacy outcomes was not formally assessed, though the sample size attained was sufficiently powered to detect a doubling of abstinence rates (i.e., 20% for TNP and 40% for VAR).
Analysis of Smoking Outcomes
Abstinence from smoking was assessed by self-report and weekly CO measurements. An intent-to-treat (ITT) approach (including all randomized participants) was used, with those lost to follow-up or missing study visits coded as having continued to smoke. A Wilcoxon rank sum test was used to evaluate continuous baseline demographic and clinical measures between groups while the normal Pearson chi-square test was used to assess the relationship for categorical and ordinal variables. The efficacy of VAR versus TNP was analyzed over four weeks of treatment and at the post-treatment follow-up visit. Time naïve logistic regression models were used to assess the primary end point of two-week end-of-treatment abstinence. Similar models were used to evaluate secondary end points, including one- and four-week endof-treatment abstinence as well as one-week abstinence at the post-treatment follow-up visit. An additional method of measuring efficacy outcomes in smoking cessation trials is the Russell Standard (RS) (19). RS abstinence is operationally defined as reporting no more than five (5) cigarettes smoked between the TQD and the post-treatment follow-up visit, with a ≤10 ppm CO reading at the follow-up visit. This method allows for periodically missing self-report or weekly visit data without the assumption of relapse. This method is more rigorous in definition than one-week abstinence at the post-treatment follow-up, yet less strict than traditional continuous abstinence measures. Additionally, per the study protocol's intent, times to first lapse and relapse were analyzed using a Cox proportional hazards regression model with the baseline time set as the TQD. Data were determined to conform to the assumption of proportional hazards.
Covariate adjusted models were adjusted for baseline smoking characteristics and demographic data shown in Table 1. Covariate adjusted analysis were performed to account for any baseline smoking characteristics that could possibly be related to the smoking efficacy outcomes of interest (20). Given missing baseline covariate data on some participants, adjusted analysis was completed on multiply imputed data sets (m=50) using the MCMC algorithm (SAS PROC MI) to avoid biased estimates (21,22). Missing covariate data were assumed to be arbitrarily missing at random. Pooled estimates of the odds ratios (OR) and associated 95% confidence intervals (CI) are presented (PROC MIANALYZE).
Table 1.
Demographics and baseline smoking characteristics overall and by treatment assignment.
| Characteristics | Treatment Assignment |
||
|---|---|---|---|
| Overall n=140 | Nicotine Patch n=73 | Varenicline n=67 | |
| Demographics | |||
| Age; years (SD) | 31.9 (7.7) | 33.0 (7.4) | 30.7 (7.9) |
| % Caucasian (n) | 78.6 (110) | 78.1 (57) | 79.1 (53) |
| Education | |||
| % High School or Less | 30.0 (42) | 31.5 (23) | 28.4 (19) |
| % Some College | 39.3 (55) | 39.7 (29) | 38.8 (26) |
| % College or Beyond | 30.7 (43) | 28.8 (21) | 32.8 (22) |
| Marital Status! | |||
| % Never Married | 38.1 (53) | 31.9 (23) | 44.8 (30) |
| % Married | 35.3 (49) | 33.3 (24) | 37.3 (25) |
| % Separated/Divorced | 26.6 (37) | 34.7 (25) | 17.9 (12) |
| Smoking Characteristics | |||
| Cigarettes per day (SD)* | 17.0 (7.1) | 17.0 (6.5) | 16.9 (7.7) |
| Years Smoking (SD)† | 13.5 (7.8) | 14.1 (7.7) | 13.0 (7.9) |
| Age became regular smoker (SD) | 17.3 (4.0) | 17.7 (4.3) | 17.0 (3.6) |
| ≥24-Hour Quit Attempts (SD)Δ | 3.1 (3.4) | 3.1 (3.6) | 3.1 (3.3) |
| Last quit attempt; years (SD) | 2.4 (3.9) | 2.2 (3.2) | 2.7 (4.5) |
| % Living with a smoker (n) | 52.1 (73) | 60.3 (44) | 46.3 (31) |
| Carbon Monoxide (CO), ppm (SD) | 12.3 (7.6) | 12.8 (7.6) | 11.7 (7.6) |
| Fagerström Test for Nicotine Dependence (FTND) (SD)‡ | 5.0 (2.3) | 5.2 (2.3) | 4.8 (2.3) |
| Questionnaire of Smoking Urges (QSU) (SD)# | 4.0 (1.4) | 4.1 (1.4) | 4.0 (1.3) |
| Factor 1 | 5.0 (1.6) | 5.0 (1.7) | 5.0 (1.6) |
| Factor 2 | 3.1 (1.4) | 3.1 (1.5) | 3.0 (1.3) |
Continuous data are shown as means and associated standard deviations (SD) and categorical data are shown as percent (n). There was no statistical evidence of imbalances in the distributions of demographic variables or smoking characteristics between treatment groups (all p>0.05).
Data on marital status missing for 1 participant from the TNP group.
Data on cigarettes per day missing for 3 participants (2 VAR, 1 TNP).
Data on years of regular smoking missing for 2 participants (1 VAR, 1 TNP).
Data on years since last quit attempt missing for 17 participants (6 VAR, 11 TNP).
Data on FTND missing for 10 participants (3 VAR, 7 TNP).
Data on QSU missing for 11 participants (4 VAR, & TNP).
A sensitivity analysis of the effect of missing response data on the parameter estimates was performed comparing the stated ITT analysis with a completers analysis approach that analyzed the data “as-is” with no imputation for missing smoking data. Study participation rates at various time points and overall adverse event rates were compared between treatment groups using Pearson's Chi-Square test statistic. All statistical analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, N.C.).
Results
Participant Characteristics
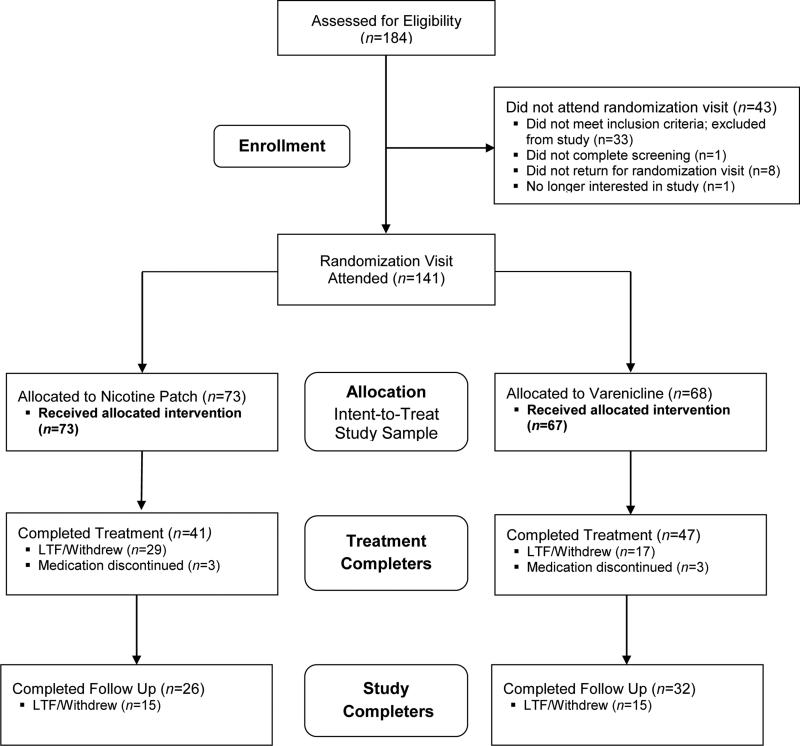
Of the 184 individuals screened, 33 failed to meet study inclusion criteria. Of the remaining 151 participants, 141 were randomized to receive either VAR (n=68) or TNP (n=73) (Figure 1). One participant randomized to VAR failed to return for medication (ITT analysis data: VAR, n=67 and TNP, n=73). The TQD visit was attended by 83% (116/140) of randomized participants (VAR, 78% (52/67) and TNP 84% (61/73), (p=0.375)). The end-of-treatment visit was attended by 63% (88/140) of randomized study participants (completers analysis data: VAR, n=47 (70%) and TNP, n=41 (56%); p=0.089). The post-treatment follow-up visit was attended by 41% (58/140) of randomized participants (Follow up analysis data: VAR, n=32 (48%) and TNP, n=26 (36%); p=0.110).
Figure 1.
CONSORT diagram of participant flow through study procedures. LTF=Lost to Follow-up
Baseline demographic and clinical characteristics of the participants by treatment assignment are presented in Table 1. The sample was predominately Caucasian (79%) and had some education beyond high school (62%). Participants, on average, had been smoking for nearly 14 years and averaged 17 cigarettes per day in the 90 days prior to study entry. There were no between group differences in any measured baseline demographic or clinical variables.
Efficacy
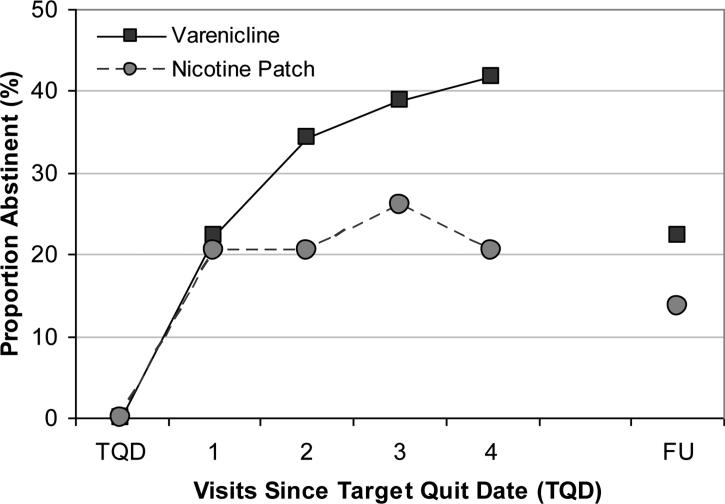
Rates of abstinence over time are presented in Figure 2 (ITT sample, with non-abstinence assumed for all missed visits). VAR participants, compared to TNP participants, had greater than twice the odds of abstinence during the final two weeks of treatment (unadjusted and adjusted; Table 2). Assessment of secondary endpoints of treatment efficacy yielded similar magnitudes of estimates. During the final week of treatment, VAR participants had greater than three times the odds of abstinence as compared to TNP participants and similar odds of abstinence were observed over the final four weeks of active study treatment. During the week preceding the post-treatment follow-up visit, 22.4% of VAR participants were abstinent, compared to only 13.7% of TNP participants. RS abstinence measured at the post-treatment follow-up visit showed similar abstinence proportions; 23.9% (16/67) of VAR participants were RS abstinent, compared to only 13.7% (10/73) of TNP participants. Lastly, the two treatment groups did not differ on time to first lapse (HR=0.86 (0.60-1.23); p=0.411) or time to first relapse (HR=0.81 (0.45-1.44); p=0.470).
Figure 2.
Proportions of participants abstinent at each weekly visit (since the prior weekly visit; intent-to-treat with non-abstinence assumed at each missed visit).
Table 2.
Outcome Measures.
| Measure and Group | N | % (95% CI) | Intent-to-Treat Unadjusted OR (95% CI) | Adjusted‡ OR (95% CI) |
|---|---|---|---|---|
| Primary Outcome Measure | ||||
| Two-week end-of-treatment abstinence | 2.74 (1.26-5.98)* | 2.77 (1.17-6.59)* | ||
| Varenicline | 25 | 37.3 (26.7-49.3) | ||
| Transdermal Nicotine Patch | 13 | 17.8 (10.7-28.1) | ||
| Difference in proportion (95% CI) | 20.0 (4.9-34.0) | |||
| Secondary Outcome Measures | ||||
| One-week end-of-treatment abstinence | 3.14 (1.49-6.60)† | 3.12 (1.38-7.07)† | ||
| Varenicline | 30 | 44.8 (33.5-56.7) | ||
| Transdermal Nicotine Patch | 15 | 20.6 (12.9-31.2) | ||
| Difference in proportion | 24.2 (9.1-39.3) | |||
| Four-week end-of-treatment abstinence | 2.72 (1.03-7.16)* | 3.25 (1.11-9.58)* | ||
| Varenicline | 15 | 22.4 (14.1-33.7) | ||
| Transdermal Nicotine Patch | 7 | 9.6 (4.7-18.5) | ||
| Difference in proportion | 12.8 (0.7-24.9) | |||
| One-week abstinence at post-treatment follow-up | 1.82 (0.75-4.38) | 1.93 (0.68-5.48) | ||
| Varenicline | 15 | 22.4 (14.1-33.7) | ||
| Transdermal Nicotine Patch | 10 | 13.7 (7.6-23.4) | ||
| Difference in proportion | 8.7 (−4.0-21.4) | |||
| Russell Standard abstinence at follow-up | 1.98 (0.83-4.73) | 1.94 (0.75-5.04) | ||
| Varenicline | 16 | 23.9 (14.3-35.9) | ||
| Transdermal Nicotine Patch | 10 | 13.7 (7.6-23.4) | ||
| Difference in proportion | 10.2 (−2.7-23.1) |
Self reported two-, one-, and four-week end-of-treatment abstinence, one-week post-treatment, and Russell Standard follow-up abstinence, all confirmed by weekly breath carbon monoxide (CO) < 10 ppm. In the intent-to-treat (ITT) logistic regression analysis, missing data was assumed to be non-abstinent. ITT analysis sample size n=140 (VAR n=67, TNP n=73). OR=Odds Ratio.
p<0.05
p<0.01
Covariate adjusted models were adjusted for all baseline smoking characteristics and demographic shown in Table 1.
Sensitivity Analysis
Logistic regression analysis of the completer data revealed a similar magnitude and direction of the effect as in the ITT analysis, favoring VAR over TNP for two-week end-of-treatment abstinence (OR = 2.5 (1.0-5.9)). The secondary endpoints of abstinence results using the per-protocol analysis also showed similar effect directions and magnitudes as in the ITT analysis (one-week end-of-treatment abstinence OR = 3.1 (1.3-7.3); four-week end-of-treatment abstinence OR = 2.3 (0.8-6.3); one-week post-treatment follow-up abstinence OR = 1.4 (0.5-4.0)).
Post-Hoc Power Analysis
Comparison of VAR versus TNP efficacy was an exploratory endeavor within a parent study formally powered on another outcome. While significant between-group differences were noted in end-of-treatment abstinence outcomes, the study follow-up outcomes were statistically insignificant. Thus, it was deemed appropriate to conduct a post-hoc analysis to determine the study's power to detect differences in abstinence at the follow-up visit. Given the observed differences between treatment groups at that visit (22.4% versus 13.7%) and the sample size attained, the post-hoc power to determine such a difference (1 – beta) is 0.30 (with alpha=0.05). The study therefore had a 30% probability of detecting this observed difference at the post-treatment follow-up visit. Thus, the sample size was not adequate to detect an effect of this magnitude, if it exists.
Safety/Tolerability
Adverse events are summarized in Table 3. The most commonly reported adverse events included gastrointestinal discomfort, vivid dreams, and nausea. Two FDA-defined serious adverse events (in this case, defined as such due to the need for emergent medical intervention) occurred (both in the same TNP group participant): skin irritation (generalized rash not near the patch site) and fever, both of which resolved with medical intervention prior to study completion and were deemed unlikely to be related to study medication (23). Adverse events in both TNP and VAR groups were generally mild with no action required. Discontinuation of medication was necessary for 6 participants (3 from each medication arm), who experienced a total of 13 adverse events (many concurrently) that led to the clinical decision to discontinue. A small percentage of events were considered “definitely related” (2% in the TNP group, and 0 in the VAR group). No participants reported suicide ideation or clinically significant suicide risk during study participation. Vital signs did not change significantly over the course of treatment in either group.
Table 3.
Summary of adverse events.
| Adverse Event | Varenicline (n=67) | Nicotine Patch (n=73) | Total (N=140) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Gastrointestinal | 19 | 28.4% | 22 | 30.1% | 41 | 29.3% |
| Vivid Dreams | 18 | 26.9% | 23 | 31.5% | 41 | 29.3% |
| Nausea | 21 | 31.3% | 8 | 11.0% | 29 | 20.7% |
| Insomnia | 13 | 19.4% | 13 | 17.8% | 26 | 18.6% |
| Irritability | 8 | 11.9% | 16 | 21.9% | 24 | 17.1% |
| Headache | 13 | 17.9% | 11 | 15.1% | 23 | 16.4% |
| Respiratory | 7 | 10.4% | 13 | 17.8% | 20 | 14.3% |
| Skin Irritation at Patch Site | 2 | 3.0% | 13 | 17.8% | 15 | 10.7% |
| Drowsiness/Fatigue | 7 | 10.4% | 7 | 9.6% | 14 | 10.0% |
| Anxiety/Depression | 4 | 6.0% | 7 | 9.6% | 11 | 7.9% |
| Dizziness/Lightheadedness | 3 | 4.5% | 8 | 11.0% | 11 | 7.9% |
| Mouth Dryness | 7 | 10.4% | 4 | 5.5% | 11 | 7.9% |
| Musculoskeletal | 5 | 7.5% | 5 | 6.8% | 10 | 7.1% |
| Skin Irritation other than Patch Site | 2 | 3.0% | 7 | 9.6% | 9 | 6.4% |
| Restlessness/Jitteriness | 3 | 4.5% | 5 | 6.8% | 8 | 5.7% |
| Urinary/Genital | 4 | 6.0% | 4 | 5.5% | 8 | 5.7% |
| Increased Heart Rate | 1 | 1.5% | 3 | 4.1% | 4 | 2.9% |
| Fever | 0 | 0% | 1 | 1.4% | 1 | 0.7% |
Discussion
Evaluation of medication efficacy is strengthened by direct trials, using rigorous methods. Although the current study was not a full-course clinical trial, and was an opportunistic exploration within a parent study of ovarian hormone influences on abstinence, we believe this is the first double-blind randomized trial of VAR versus TNP for smoking cessation. VAR, compared to TNP, more than doubled the odds of abstinence at the end of four-week treatment among female smokers, though abstinence outcomes were not statistically significant at the post-treatment follow-up visit. These findings suggest that VAR may be preferred over TNP, at least in the first four weeks of treatment, for women wishing to quit smoking.
The magnitude of the between-group abstinence difference was greater in this trial than in prior open-label trials of VAR versus TNP that included both male and female participants (3,4). However, the absolute abstinence rates at the four-week mark were lower in both groups than in the aforementioned studies. While it may be tempting to infer gender differences from these discrepancies, comparisons at this stage are indirect, and future gender × treatment trials, with appropriate power to detect interaction effects, are needed. Prior work does suggest that men are more responsive to NRT than women (24). In contrast, the initial VAR efficacy trials did not demonstrate gender differences in treatment response (25,26), and a recent VAR versus combined NRT effectiveness study did not yield gender differences in response to either treatment (5). These discrepancies suggest that more systematic study of the influence of gender on cessation outcome is warranted.
While this study's double-blind approach is a significant strength, findings should also be interpreted in light of several limitations. The parent study was designed and powered to detect ovarian hormone, rather than VAR versus TNP treatment, effects on smoking cessation. The trial may have thus been underpowered for formal VAR versus TNP efficacy comparison. Additionally, treatment lasted only four weeks, notably shorter than the standard course for either medication (twelve weeks for VAR and eight weeks for TNP). This compromised the potential to detect longer-term treatment response and limited comparison with other studies employing longer courses of treatment. Additionally, participant dropout, while not significantly different between groups, likely affected the magnitude of ITT response to both treatments.
The present findings provide a significant, novel contribution to the literature on comparative efficacy of smoking cessation pharmacotherapies, with particular relevance for female smokers, who may bear a disproportionate disease burden from cigarette smoking (27). More double-blind head-to-head trials of smoking cessation pharmacotherapies, designed and powered to prospectively assess gender differences and to examine gender specific variation in treatment response to different cessation interventions, are needed to inform clinical practice.
Acknowledgments
The authors wish to thank the study participants and the outstanding clinical research team, including Jessica Hinton, Christine Horne, S. Ashley McCullough, and Erin Klintworth.
Funding sources: NIH grants P50DA16511 Component 4 (KMG & MES), K23DA020482 (MJC), and UL1RR029882 & UL1TR000062 (South Carolina Clinical & Translational Research Institute). Varenicline and matched placebo were supplied by Pfizer, Inc. NIH and Pfizer, Inc., had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Dr. Gray has received research funding from Merck, Inc., and Supernus Pharmaceuticals and Dr. Hartwell has received research funding from Pfizer, Inc., for other projects.
Footnotes
Declarations of Interest The other authors report no potential conflicts of interest.
Clinical Trials Registration clinicaltrials.gov identifier #NCT00664755
References
- 1.Centers for Disease Control and Prevention Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2.World Health Organization . WHO Report on the Global Tobacco Epidemic, 2011. World Health Organization; Geneva: 2011. [Google Scholar]
- 3.Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: Results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: Parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis. 2012;16:268–272. doi: 10.5588/ijtld.11.0183. [DOI] [PubMed] [Google Scholar]
- 5.Brose LS, West R, Stapleton JA. Comparison of the effectiveness of varenicline and combination nicotine replacement therapy for smoking cessation in clinical practice. Mayo Clin Proc. 2013;88:226–233. doi: 10.1016/j.mayocp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Hsueh S-C, Hsueh K-C, Chou M-Y, Tu M-S. A comparison of the effectiveness of varenicline and transdermal nicotine patch in outpatients following a standardized smoking cessation program in southern Taiwan. Eval Health Prof. 2012;0:1–11. doi: 10.1177/0163278712466868. doi: 10.1177/0163278712466868. [DOI] [PubMed] [Google Scholar]
- 7.Kotz D, Brown J, West R. Effectiveness of varenicline versus nicotine replacement therapy for smoking cessation with minimal professional support: Evidence from an English population study. Psychopharmacology. 2014;231:37–42. doi: 10.1007/s00213-013-3202-x. [DOI] [PubMed] [Google Scholar]
- 8.Kralikova E, Kmetova A, Stepankova L, Zvolska K, Davis R, West R. Fifty-two-week continuous abstinence rates of smokers being treated with varenicline versus nicotine replacement therapy. Addiction. 2013;108:1497–502. doi: 10.1111/add.12219. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: A pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 10.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: An overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amos A, Greaves L, Nichter M, Bloch M. Women and tobacco: A call for including gender in tobacco control research, policy and practice. Tob Control. 2012;21:236–243. doi: 10.1136/tobaccocontrol-2011-050280. [DOI] [PubMed] [Google Scholar]
- 12.Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM. Increasing progesterone levels are associated with smoking abstinence in free cycling women smokers receiving brief pharmacotherapy. Nicotine Tob Res. doi: 10.1093/ntr/ntu262. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Brit J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 14.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders, Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2004. [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 19911991:861119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 18.Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, et al. Columbia-Suicide Severity Rating Scale (C-SSRS) 2007 [Google Scholar]
- 19.West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 21.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 23.What is a Serious Adverse Event? Silver Spring; U.S. Food and Drug Administration; MD: [2 December 2014]. [updated 10 January 2014]. Available from: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm. [Google Scholar]
- 24.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 25.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Mucha L, Stephenson J, Morandi N, Dirani R. Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gender Med. 2006;3:279–291. doi: 10.1016/s1550-8579(06)80216-0. [DOI] [PubMed] [Google Scholar]