Abstract
Multipotent stem cells with neural crest-like properties have been identified in the dermis of human skin. These neural crest stem cell (NCSC)-like cells display self-renewal capacity and differentiate into neural crest derivatives, including epidermal pigment-producing melanocytes. NCSC-like cells share many properties with aggressive melanoma cells, such as high migratory capabilities and expression of the neural crest markers. However, little is known about which intrinsic or extrinsic signals determine the proliferation or differentiation of these neural crest-like stem cells. Here, we show that in NCSC-like cells, Notch signaling is highly activated, similar to melanoma cells. Inhibition of Notch signaling reduced proliferation of NCSC-like cells, induced cell death, and down-regulated non-canonical Wnt5a, suggesting that the Notch pathway contributes to the maintenance and motility of these stem cells. In three-dimensional skin reconstructs, canonical Wnt signaling promoted the differentiation of NCSC-like cells into melanocytes. This differentiation was triggered by the endogenous Notch inhibitor Numb, which is up-regulated in the stem cells by Wnt7a derived from UV-irradiated keratinocytes. Together, these data reveal a crosstalk between the two conserved developmental pathways in postnatal human skin, and highlight the role of the skin microenvironment in specifying the fate of stem cells.
Introduction
The dermis of human skin contains progenitor cells capable of differentiating into neural crest lineages, including melanocytes, neuronal cells, and smooth muscle cells. These neural crest stem cell-like cells (NCSC-like cells) share many features with melanoma cells, such as high migratory and invasive capabilities, and expression of the neural crest markers p75 NGF receptor (CD271) and nestin (Joannides et al., 2004; Li et al., 2010; Toma et al., 2005). Foreskin-derived NCSC-like cells are able to differentiate into melanocytes when maintained in 3D skin reconstructs, which reflect the physiological architecture of human skin (Li et al., 2010). These findings suggest that NCSC-like cells serve as a continuous reservoir for epidermal pigment-producing melanocytes, which contribute to the life-long pigmentation in skin areas devoid of hair or with few hair follicles. The shared features between NCSC-like cells and melanoma cells indicate that melanoma cells are more similar to progenitor cells than to differentiated melanocytes. Thus, understanding pathways that are responsible for normal stem cell self-renewal and differentiation will help us to better define tumor-specific changes and to discover novel therapeutic approaches.
It is largely unknown which intrinsic and extrinsic signals determine the proliferation or differentiation of NCSC-like cells. Previous studies implied two conserved developmental pathways—Wnt and Notch—to be involved in melanocytic differentiation. The Wnt pathway has a variety of roles in neural crest development, including fate determination of the melanocyte lineage. During development, the activation of Wnt-β-catenin signaling in neural crest stem cells promotes melanocyte formation and disturbs neuronal/glial differentiation (Dorsky et al., 1998). In contrast, the ablation of β-catenin in the neural crest causes loss of melanocytes (Hari et al., 2002). Thus, we hypothesized that canonical Wnt signals might determine the melanocyte fate of NCSC-like cells in human skin.
The Notch pathway participates in diverse processes during normal development and in tissue homeostasis. Dysregulation of the Notch pathway is involved in the pathogenesis of many cancers including melanoma (Balint et al., 2005; Bedogni et al., 2008; Hansson et al., 2004; Pinnix et al., 2009; Ranganathan et al., 2011; Weng and Aster, 2004). Ablation of Notch signaling results in progressive premature hair graying in mouse hair follicles, suggesting that the Notch pathway plays an essential role in the maintenance of melanocyte stem cells, as well as the maintenance and proliferation of neural crest-derived progenitors in mice (Moriyama et al., 2006; Nikopoulos et al., 2007; Okamura and Saga, 2008). Based on those previous studies and our recent findings that Notch activation dedifferentiates mature melanocytes into NCSC-like cells (Zabierowski et al., 2011), we hypothesized that the Notch pathway plays a critical role in regulating the phenotype of human NCSC-like cells.
Here, we show that interactions of the Wnt and Notch signaling pathways play essential roles in the self-renewal and differentiation of NCSC-like cells in human skin. The Notch pathway is highly activated in NCSC-like cells, and inhibition of that pathway impairs proliferation and induces cell death of NCSC-like cells. Wnt7a, produced by keratinocytes, not only promotes melanocytic differentiation of NCSC-like cells, but also suppresses the Notch pathway by up-regulating the Notch inhibitor Numb in a canonical Wnt pathway-dependent manner. Our 3D skin reconstruct model validates that the skin-derived Wnt-β-catenin signaling pathway plays an essential role in melanocytic differentiation of NCSC-like cells. The findings of this study reveal a crosstalk between the two conserved developmental pathways in somatic stem cells within the human skin environment, which may help us to discover effective strategies to treat melanoma.
Results
Notch activation is required for the sustained maintenance of NCSC-like cells
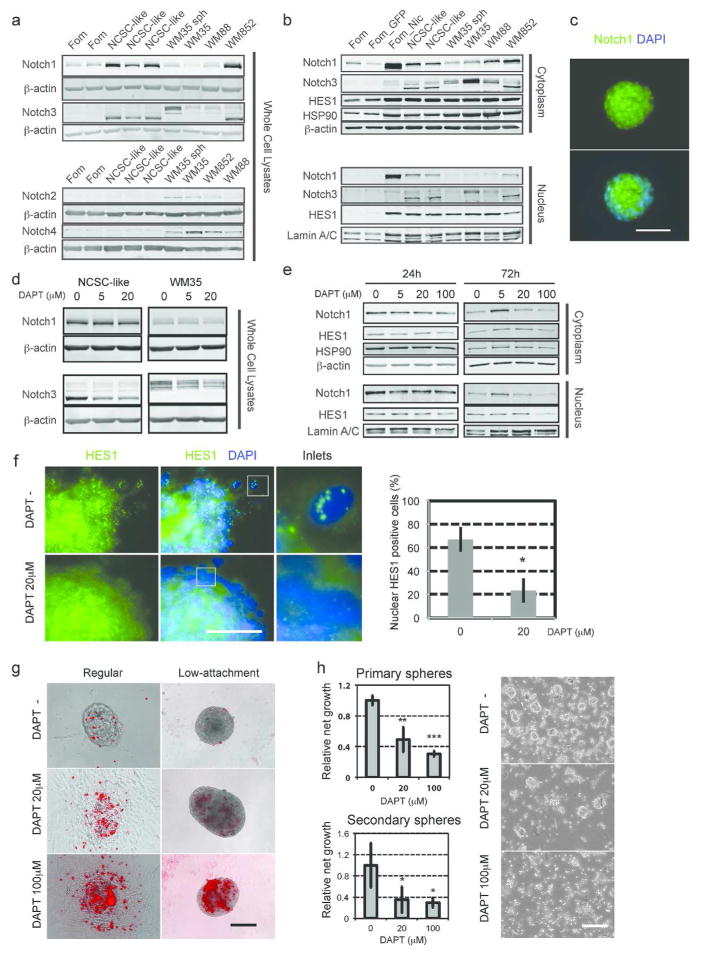
Our previous study showed that forced expression of activated Notch1 dedifferentiates melanocytes to multipotent NCSC-like cells (Zabierowski et al., 2011), suggesting a role for Notch signaling in the maintenance of a neural crest-like state. NCSC-like cells expressed high levels of Notch1 and Notch3 as truncated proteins, while all 3 melanoma cell lines expressed all 4 Notch receptors (Fig. 1a). Truncated Notch1 and Notch3 were also translocated to the nuclei of NCSC-like cells (Fig. 1b, c). Of Notch target genes, the hairy and enhancer of split (HES) family was highly expressed by NCSC-like cells (Supplementary Fig. 1). Hairy/enhancer-of-split related with YRPW motif (HEY) 1 (HEY1) and HEY2 were barely expressed in NCSC-like cells compared with the 3 melanoma cell lines (Supplementary Fig. 1), suggesting that the HEY family is specifically up-regulated in malignant cells. The Notch ligand delta-like 1 gene (DLL1) was predominantly expressed by NCSC-like cells, while another Notch ligand, Jagged 1 (JAG1), was expressed predominantly by melanocytes and melanomas (Supplementary Fig. 1), indicating that the Notch pathway is activated differently between NCSC-like cells and melanoma cells. We next sought to determine whether the Notch pathway contributes to the self-renewal ability of NCSC-like cells. DAPT, a γ-secretase inhibitor, didn’t alter the total Notch1 expression, however, it mildly decreased nuclear Notch1 in NCSC-like cells after 72 h (Fig. 1d, e, Supplementary Fig. 2a). Reduction of Notch1 nuclear translocation was also observed in the NCSC-like cells treated with RO4929097, another γ-secretase inhibitor (Supplementary Fig. 2b). Notch NCSC-like cells and DAPT decreased total Notch3 expression in both bands of differently sized Notch3 (Fig. 1d), suggesting that Notch3 is directly regulated by the Notch pathway as previously reported (Zuurbier et al., 2010). Treatment with 20 μM DAPT or 5 μM RO4929097 significantly decreased the expression of Notch target molecules HES1 and HEY1 mRNA (Fig. 1e, Supplementary Fig. 2c, d), and reduced the punctate nuclear HES1 protein expression (Fig. 1f), suggesting that 20~100 μM DAPT is effective at blocking the Notch pathway in NCSC-like cells. Staining with the cell viability indicator ethidium homodimer-1 (EthD-1) revealed that DAPT and RO4929097 induced robust cell death in NCSC-like cells (Fig. 1g, Supplementary Fig. 2e). The induction of cell death was more prominent when NCSC-like cells were cultured on a low-attachment surface (Fig. 1g). Notably, DAPT induced the adhesion of NCSC-like cells on conventional culture plates. The adherent cell population was still viable, suggesting that Notch inhibition particularly decreases survival of the sphere-forming stem cells. Next, we performed serial sphere forming assays to assess the impact of Notch inhibition in the self-renewal of NCSC-like cells. The primary sphere forming capacity was impaired by DAPT in a dose-dependent manner (Fig. 1h). Furthermore, the number of secondary spheres was significantly reduced by treatment with DAPT and RO4929097 (Fig. 1h, Supplementary Fig. 2f). Together, these results showed that the activation of Notch signaling contributes to the maintenance of NCSC-like cells in vitro. Our previous study showed that NCSC-like cells are highly migratory in a collagen matrix in a similar manner to melanoma cells (Li et al., 2010). The expression of a non-canonical Wnt ligand Wnt5a is up-regulated in metastatic melanoma and has been implicated in motility in melanoma cells (Weeraratna et al., 2002). Wnt5a has also been shown to be expressed in embryonic and cancer associated fibroblasts, in addition to being a target of the Notch pathway in dermal papilla cells and endothelial progenitor cells (Hu et al., 2010; Koyanagi et al., 2007; Pourreyron et al., 2012; Sato et al., 2010). Among those tested, Wnt5a was highly expressed in NCSC-like cells (Supplementary Fig. 2c). Inhibition of Notch signaling down-regulated WNT5A, suggesting that the Notch pathway also regulates the expression of Wnt5a in NCSC-like cells (Supplementary Fig. 2c, d).
Figure 1. The Notch pathway is highly expressed in NCSC-like cells and is critical for their self-renewal.
(a) Immunoblot analysis with Notch1~4 antibodies showing that Notch1 is highly expressed in NCSC-like cells isolated from human dermis. Fom: Foreskin-derived melanocytes, NCSC-like: NCSC-like cells, WM35, WM88, WM852: human melanoma cell lines, WM35 sph: sphere-cultured WM35 melanoma cells. Blotting for β-actin serves as a loading control. (b) Activated Notch1 and the Notch target HES1 are detected in nuclear fractions of NCSC-like cells. Fom_GFP: control vector transduced melanocytes, Fom_Nic: active Notch1 transduced melanocytes (Pinnix et al., 2009). Blotting for β-actin, HSP90 and Lamin A/C serves as loading controls. (c) Immunofluorescence showing that Notch1 is localized mainly in nuclei of NCSC-like cells. Scale bar = 50 μm. (d) Immunoblot analysis showing that treatment with the γ-secretase inhibitor DAPT does not alter total expression of Notch1, but decreases total expression of Notch3 in NCSC-like cells and WM35 melanoma cells. Blotting for β-actin serves as loading controls. (e) Immunoblot analysis showing that 72 h treatment with the γ-secretase inhibitor DAPT decreases nuclear Notch1 expression in NCSC-like cells. Blotting for HSP90 and Lamin A/C serves as loading controls. (f) Left: Immunoblot analysis showing that 72 h treatment with the γ-secretase inhibitor DAPT decreases nuclear HES1 expression in NCSC-like cells. Immunofluorescence showing that the characteristic punctate staining pattern of HES1 disappears in NCSC-like cells treated with DAPT for 72 h. Scale bar = 50 μm. Right: Quantification of the nuclear HES1 positive cells. Data represent means ± SD, n = 5. *p ≤ 0.01. (g) Representative images of NCSC-like cells from control DMSO-treated and from DAPT (20 μM or 100 μM) treated cells. DAPT induces the adhesion of NCSC-like cells on the regular plastic surface and induces cell death, indicated by EthD-1 staining (red) on the low-attachment surface. Scale bar = 100 μm. (h) Left: Histogram depicting the relative growth of NCSC-like cells generated by each population. Data represent means ± SD, n = 5 for primary spheres, n=7 for secondary spheres. *p ≤ 0.01, **p ≤ 0.001, ***p ≤ 0.0001. Right: DAPT impairs the self-renewal ability of NCSC-like cells. Representative images were taken at day 13 after secondary sphere formation from single cell suspension of primary spheres. Scale bar = 100 μm.
The canonical Wnt pathway promotes melanocyte differentiation of NCSC-like cells
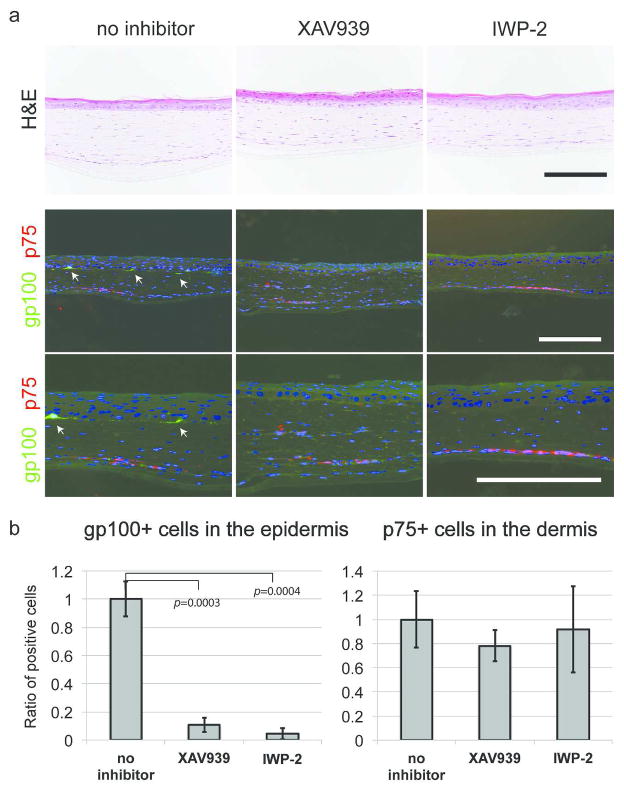
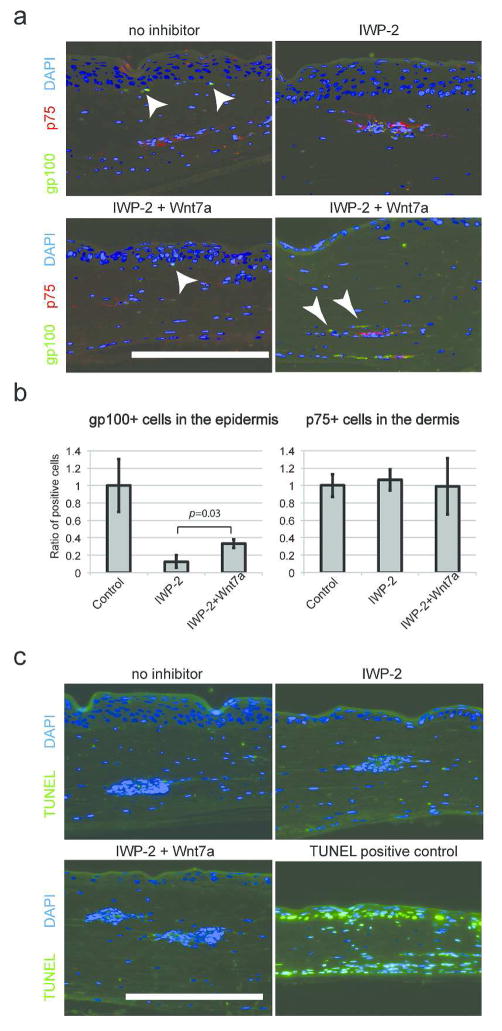
The Wnt pathway plays a variety of roles in neural crest development, including the specification of the melanocyte lineage. We hypothesized that the Wnt pathway is involved in melanocyte differentiation of NCSC-like cells in the adult human skin environment. Using 3D skin reconstructs, we tested the impact of Wnt inhibition in cell-fate determination of NCSC-like cells (Fig. 2a). Without Wnt pathway inhibition, gp100-positive melanocytes that differentiated from NCSC-like cells were observed at the basement membrane after two weeks of culture. Strikingly, the number of differentiated melanocytes was significantly decreased when the 3D skin reconstructs were treated with small molecule Wnt inhibitors, XAV-939 or IWP2, while the number of p75-positive cells remained similar compared to control 3D skin reconstructs without inhibitors (Fig. 2b). XAV-939 inhibits tankyrases, thereby stabilizing Axin and specifically promoting GSK-3β-dependent phosphorylation to inhibit canonical Wnt-β-catenin signaling (Huang et al., 2009). IWP2 inhibits secretion of Wnt ligands by preventing palmitylation of Wnt proteins by Porcupine (Chen et al., 2009). These results demonstrate that canonical Wnt ligands, which are secreted from cells inside 3D skin reconstructs, are essential for melanocyte differentiation from NCSC-like cells.
Figure 2. The canonical Wnt pathway promotes melanocytic differentiation of NCSC-like cells.
(a) Without inhibitors, p75-positive NCSC-like cells embedded in the dermis of 3D skin reconstructs are partially differentiated into gp100-positive melanocytes (arrows) and are localized at the basement membrane within 14 days, while the number of gp100-positive cells is reduced when 3D skin reconstructs are treated with XAV-939 (5 μM) or IWP-2 (5 μM). Nuclei are counterstained with DAPI (blue). Scale bars = 200 μm. (b) Quantification of the number of gp100-positive melanocytes located at the basal layer of the epidermis. The Y-axis indicates the relative ratio of marker-positive cells. Data represent means ± SD, n=4, *p ≤ 0.001.
UV-induced Wnt7a in keratinocytes promotes melanocytic differentiation of NCSC-like cells
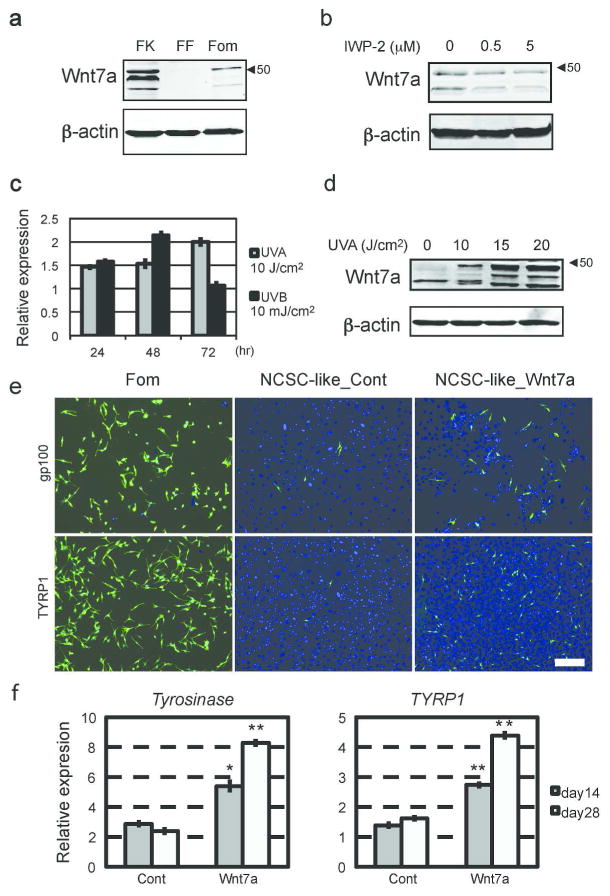
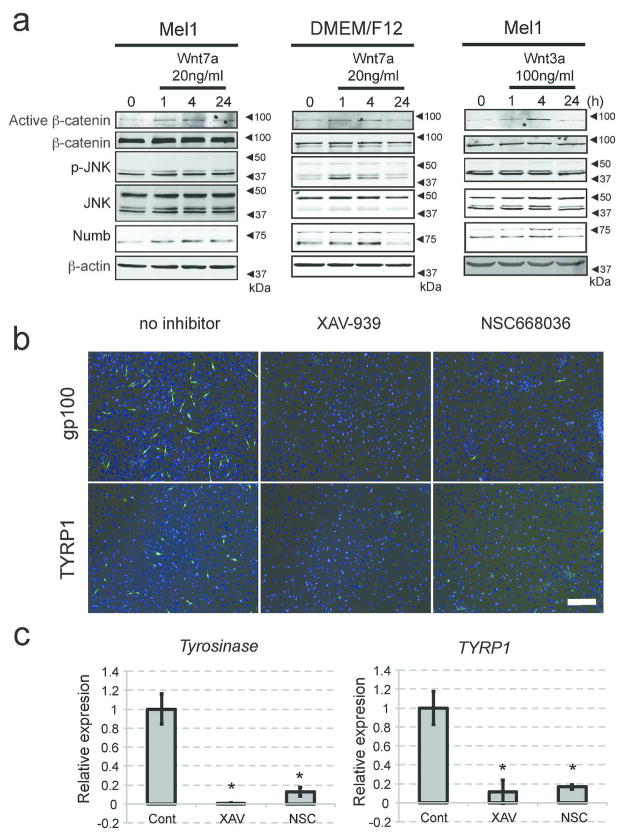
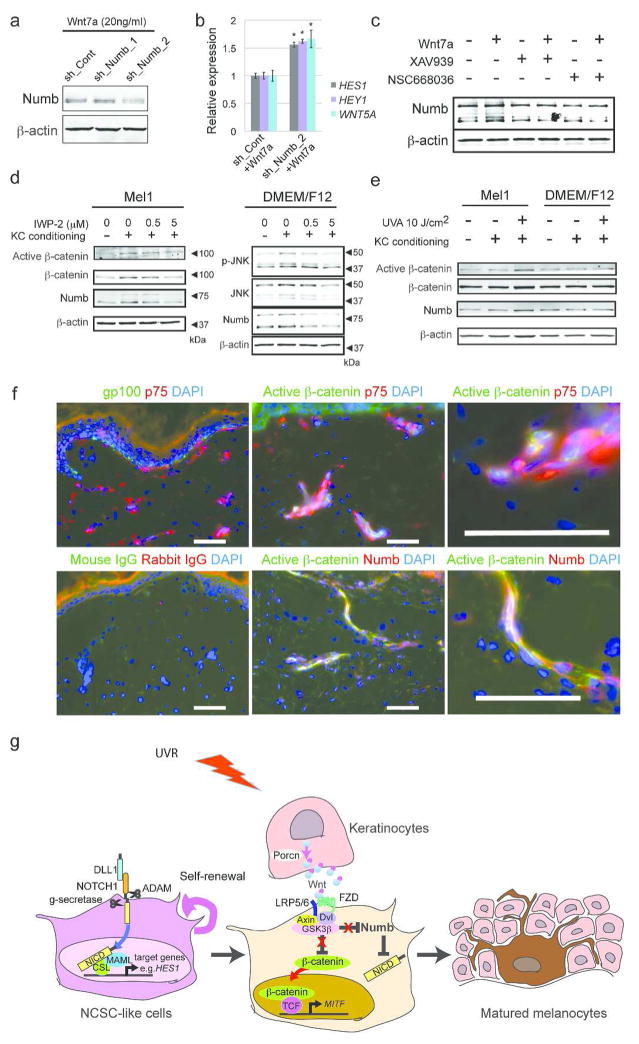
When NCSC-like cells are embedded into the skin reconstructs, they first interact with fibroblasts and are then attracted by keratinocytes, which promote their migration and differentiation; therefore, we postulated that Wnt ligands are expressed by human keratinocytes within the epidermal layer. Among those tested, we found that human keratinocytes express high levels of Wnt7a, which induces melanocyte differentiation in mouse hair follicles (Rabbani et al., 2011; Yamada et al., 2013), (Fig. 3a, Supplementary Fig. 3a). In contrast, other cell types, such as fibroblasts and NCSC-like cells, did not express Wnt7a. Secretion of Wnt7a from keratinocytes was blocked by IWP-2 (Fig. 3b). Yamada et al. reported in mouse skin that Wnt7a expression is induced on day 1 of ultraviolet B (UVB) irradiation, and returns to the basal level on day 3 (Yamada et al., 2013). Similarly in human skin, Wnt7a was rapidly up-regulated by UVB irradiation in keratinocytes and returned to basal levels after 3 days (Fig. 3c). Since the vast majority of solar UV radiation is UVA (Seite et al., 2010), we also tested the influence of UVA on Wnt7a production (Fig. 3c). Non-lethal doses of UVA irradiation were able to induce Wnt7a expression in human keratinocytes, and this induction was enhanced towards later time points, suggesting UVA induces a gradual, but more prolonged, up-regulation of Wnt signaling compared to UVB (Fig. 3c, d). Our previous study showed that NCSC-like cells differentiate into melanocytes in adherent cultures with melanocyte differentiation media (Mel1) containing recombinant Wnt3a (Li et al., 2010). When NCSC-like cells were treated with Mel1 media, in which Wnt3a was substituted with recombinant Wnt7a, the emergence of cells positive for melanocyte markers gp100 and tyrosinase-related protein 1 (TYRP1) increased, compared to control Wnt-free Mel1 media (Fig. 3e). Mel1 media supplemented with Wnt7a induced the expression of melanocyte-specific genes such as tyrosinase and MITF in a manner similar to Mel1 media supplemented with Wnt3a (Fig. 3f, Supplementary Fig. 3b). Constitutively activated Notch1 in NCSC-like cells inhibited induction of tyrosinase by Wnt7a, suggesting that the melanocyte differentiation by Wnt7a requires down-regulation of Notch signaling (Supplementary Fig. 3c). In Mel1 media, active β-catenin was increased in NCSC-like cells shortly after treatment with Wnt7a in a manner similar to treatment with Wnt3a (Fig. 4a), along with its translocation to the nucleus (Supplementary Fig. 3d). In basal media for stem cell cultures (DMEM/F12 with GlutaMAX™), Wnt7a up-regulated phosphorylation of the JNK p46 isoform as well as β-catenin, while phosphorylation of JNK p46 was not obvious in Mel1 media (Fig. 4a). This observation suggests that Wnt7a activates both the canonical and the non-canonical Wnt pathways in a context-dependent manner. We further determined whether Wnt7a could promote melanocyte differentiation in a canonical Wnt-dependent manner using two small molecule inhibitors, XAV-939 and NSC668036 (Shan et al., 2005). Both XAV-939 and NSC668036 significantly impaired the emergence of gp100/TYRP1-positive melanocytes, which were differentiated by Mel1 media supplemented with Wnt7a (Fig. 4b, c), suggesting that Wnt7a promotes the melanocyte differentiation of NCSC-like cells in a canonical Wnt pathway-dependent manner. Exogenous Wnt7a treatment partially rescued the emergence of epidermal melanocytes in the 3D skin reconstructs treated with IWP2, while also increasing ectopic gp100 positive melanocytes in the dermis (Fig. 5a, b). To determine whether Wnt7a also induces migration of NCSC-like cells, we performed Transwell® migration assays. Wnt7a treatment did not increase the migratory potential of NCSC-like cells, suggesting that a proper gradient of Wnt7a may be necessary for melanocyte differentiation in the epidermis, but not for the migration of NCSC-like cells (Supplementary Fig. 3e). Inhibition of Wnt signaling did not affect survival of NCSC-like cells in skin reconstructs (Fig. 5c), suggesting that the appearance of gp100-positive cells is not due to selection of a subpopulation in the starting cell population.
Figure 3. Wnt7a is produced by human keratinocytes and up-regulated by UV irradiation, and promotes melanocytic differentiation of NCSC-like cells.
(a) Immunoblot analysis of conditioned media confirming that Wnt7a (the most robust bands present around 48 kDa) is secreted by keratinocytes (FK), but barely by melanocytes (Fom) or fibroblasts (FF). Blotting for β-actin indicates the equivalent amount of cell volume used in conditioning media. (b) Immunoblot analysis showing that treatment with IWP-2 decreases Wnt7a secretion from keratinocytes. Blotting for β-actin indicates the equivalent amount of cell volume used in conditioned media. (c) qRT-PCR showing that mRNA relative expression of WNT7A in UV-irradiated keratinocytes compared to non-irradiated keratinocytes. (d) Immunoblot analysis of conditioned media confirming that UVA irradiation up-regulates Wnt7a production in keratinocytes in a dose-dependent manner. Blotting for β-actin indicates the equivalent amount of cell volume used in conditioning media. (e) Expression of gp100 and TYRP1 showing the efficacy of melanocyte differentiation from NCSC-like cells. Four weeks after differentiation induced by Mel1 media supplemented with Wnt7a, NCSC-like cells give rise to melanocytes (NCSC-like_Wnt7a) positive for gp100 and TYRP1 more efficiently compared to control Wnt-free Mel1 media (NCSC-like_Cont). Human melanocytes are stained as a positive control for gp100 and TYRP1. Scale bar = 200 μm. (f) qRT-PCR showing that Mel1 media supplemented with Wnt7a induces the expression of melanocyte-specific genes Tyrosinase and TYRP1 more efficiently than control Wnt-free Mel1 media. mRNA levels of target genes were normalized to GAPDH. Data represent means ± SD, n=4, *p ≤ 0.001, **p ≤ 0.0001.
Figure 4. Wnt7a promotes melanocytic differentiation in a canonical Wnt-dependent manner.
(a) Immunoblot analysis showing that brief Wnt7a treatment activates β-catenin, phosphorylates JNK, and increases Numb expression in NCSC-like cells. Note: Wnt3a activates β-catenin and increases Numb expression, but does not phosphorylate JNK. Blotting for β-actin serves as a loading control. (b) Expression of gp100 and TYRP1 showing the efficacy of melanocyte differentiation from NCSC-like cells four weeks after differentiation induced by Mel1 media supplemented with Wnt7a. Both XAV-939 and NSC668036 significantly impaired the emergence of gp100- and TYRP1-positive melanocytes, which are differentiated in Mel1 media supplemented with Wnt7a. Scale bar = 200 μm. (c) qRT-PCR showing that both XAV-939 and NSC668036 significantly reduce Tyrosinase and TYRP1 in cells differentiated from NCSC-like cells in Mel1 media supplemented with Wnt7a. mRNA levels of target genes were normalized to GAPDH. Data represent means ± SD, n=4, *p ≤ 0.01.
Figure 5. Wnt7a partially rescued the emergence of gp100 positive epidermal melanocytes.
(a) Wnt7a partially rescued the emergence of gp100 positive epidermal melanocytes and ectopic melanocytes in the dermis in the 3D skin reconstructs treated with a porcupine inhibitor IWP2 (arrowheads). Nuclei are counterstained with DAPI (blue). Scale bars = 200 μm. (b) Quantification of the number of gp100-positive melanocytes located at the basal layer of the epidermis. The Y-axis indicates the relative ratio of marker-positive cells. Data represent means ± SD, n=4. (c) TUNEL staining (green) showed that inhibition of Wnt signaling does not affect cell death of NCSC-like cells in the 3D skin reconstructs. Nuclei are counterstained with DAPI (blue). Scale bars = 200 μm.
Wnt7a interferes with the Notch pathway through up-regulation of Notch inhibitor Numb in NCSC-like cells
The crosstalk between the Wnt and Notch pathways has been reported in different cell systems (Chen et al., 2009). Depending on the cellular context, Wnt and Notch pathways can act either in a synergistic or antagonistic fashion. In human diseased liver, macrophage-derived Wnt signaling antagonizes the Notch pathway in hepatic progenitor cells through up-regulation of Numb, an endogenous Notch inhibitor, and induces hepatocyte differentiation (Boulter et al., 2012). Since we observed that the Notch pathway is essential for the self-renewal of NCSC-like cells and that Wnt7a induces melanocyte differentiation, we examined whether Wnt up-regulates Numb in NCSC-like cells. Treatment with either Wnt7a or Wnt3a up-regulated expression of Numb protein in NCSC-like cells (Fig. 4a, Supplementary Figure 4a). This up-regulation occurred rapidly (within 4 h) after adding the Wnt ligands, while the expression of NUMB mRNA was not altered up to 24 h (Supplementary Fig. 4b). Together, these data suggest that Numb expression is regulated at the protein level by the Wnt pathway. Expression of the Notch target gene HES1 was rapidly down-regulated in NCSC-like cells treated with Wnt7a (Supplementary Fig. 4c). In Wnt7a-treated NCSC-cells, Numb depletion increased the expression of Notch target genes HES1, HEY1, and WNT5A, suggesting that NUMB is mediating the inhibition of Notch signaling in NCSC-like cells (Figure 6a, b). Using Wnt inhibitors, we then tested whether Wnt7a up-regulates Numb expression in NCSC-like cells in a canonical Wnt-dependent manner. Immunoblot data showed that both XAV-939 and NSC668036 were able to inhibit the increase of Numb induced by Wnt7a (Fig. 6c, Supplementary Figure 4d), suggesting that Wnt7a up-regulates Numb in a canonical Wnt pathway-dependent manner. As expected, keratinocyte-conditioned media induced Numb expression and activated both the canonical and non-canonical Wnt pathways with the up-regulation of active β-catenin and phosphorylated-JNK (Fig. 6d, compare the first and second lanes of each blot, quantified in Supplementary Figure 4e). The conditioned media generated from IWP-2 treated keratinocytes did not induce either Wnt pathway activation or Numb expression (Fig. 6d, Supplementary Figure 4e). In contrast, the expression of Numb and active β-catenin were induced more robustly in NCSC-like cells treated with the Mel1 media conditioned from UVA-irradiated keratinocytes compared to NCSC-like cells treated with the Mel1 media conditioned from non-irradiated keratinocytes (Fig. 6e, Supplementary Figure 4f). Collectively, our data demonstrated that keratinocyte-conditioned medium up-regulates Numb in a canonical Wnt-dependent manner. In human skin explant cultures, active β-catenin positive cells were seen in the dermis regardless of UV-irradiation (Supplementary Fig. 5a). Wnt7a was expressed in the epidermis of untreated skin samples as well as UV-irradiated samples, suggesting that basal levels of Wnt7a expression is already high in ex vivo skin cultures. p75 positive cells in the dermis did not express melanocytic marker gp100, while a part of the p75 positive cells co-expressed active β-catenin (Fig. 6f). Active β-catenin was co-localized with Numb in those cells, suggesting that the regulation of both proteins occurs simultaneously in that portion of the p75 positive cells.
Figure 6. Keratinocyte-derived Wnt signaling inhibits the Notch pathway through up-regulation of the endogenous Notch inhibitor Numb in NCSC-like cells.
(a) Immunoblot analysis of Numb in control vector or shRNA’s targeting NUMB-transduced NCSC-like cells treated with Wnt7a. (b) qRT-PCR showing that Numb depletion increased the expression of Notch target genes HES1, HEY1, and WNT5A, in NCSC-like stem cells treated with Wnt7a. mRNA levels of target genes were normalized to GAPDH. Data represent means ± SD, n=4, *p ≤ 0.01. (c) Immunoblot analysis showing that both XAV-939 and NSC668036 decrease Numb expression induced by Wnt7a. Blotting for β-actin serves as a loading control. (d) Immunoblot analysis showing that keratinocyte-conditioned medium activates β-catenin, phosphorylates JNK and increases Numb expression in NCSC-like cells. The conditioned media from IWP-2 treated keratinocytes does not induce Wnt activation or Numb expression, suggesting that keratinocyte-conditioned media up-regulates Numb in a Wnt-dependent manner. Blotting for β-actin serves as a loading control. (e) Immunoblot analysis showing that the Mel1 media conditioned from UVA-irradiated keratinocytes induced the expression of Numb and active β-catenin in NCSC-like cells more robustly than the Mel1 media conditioned from non-irradiated keratinocytes. Blotting for β-actin serves as a loading control. (f) Human skin explants irradiated with 10 J/cm2 of UVA. Skin explants were harvested 48 hours after irradiation. At this time point, p75 positive cells in the dermis did not express melanocytic marker gp100 48h after UVA irradiation (left top panel), while a part of the p75 positive cells co-expressed active β-catenin (right top panel). Active β-catenin expression was co-localized with Numb expression (left bottom panel). Scale bar = 100 μm. (g) Crosstalk between the Wnt and Notch signaling pathways in NCSC-like cells differentiation. Notch signaling plays a role in the self-renewal of NCSC-like cells. Binding of Notch ligands such as DLL1 to Notch receptors results in the cleavage of the Notch intracellular domain (NICD), which then translocates to the nucleus and regulates the transcription of target genes. Wnt ligands such as Wnt7a are secreted by keratinocytes and activate the canonical Wnt pathway, which promotes melanocyte differentiation of NCSC-like cells. At the same time, the canonical Wnt pathway inhibits the Notch pathway through up-regulation of the endogenous Notch inhibitor Numb in NCSC-like cells.
Discussion
In this study, we investigated the roles of the Notch and Wnt signaling pathways and discovered the crosstalk between them in NCSC-like cells residing in the dermis of human skin (Fig. 6g). The activation of the Notch pathway is essential to maintain growth of NCSC-like cells. Wnt7a, which is produced by human keratinocytes, promotes melanocyte differentiation from NCSC-like cells in a canonical Wnt signaling-dependent manner. The production of Wnt7a is especially enhanced when keratinocytes are irradiated by UVR, implying that NCSC-like cells can potentially function as a reservoir for melanocytes in UV-irradiated skin. Furthermore, keratinocyte-derived canonical Wnt signaling induces up-regulation of the endogenous Notch inhibitor Numb, and inhibits Notch signaling in NCSC-like cells. Pharmacological inhibition of canonical Wnt signaling abrogated the melanocytic differentiation of NCSC-like cells in 3D human skin reconstructs.
The Wnt and Notch pathways regulate diverse cellular functions in a variety of stem/progenitor cells, depending on the tissue context (Molofsky et al., 2004). Our study reveals that the roles of these two pathways in NCSC-like cells are similar to those in melanocyte stem cells of mouse hair bulge regions (Moriyama et al., 2006; Rabbani et al., 2011; Yamada et al., 2013). Although melanocyte stem cells are considered to be lineage-committed progenitors, a recent study described an unexpected plasticity of melanocytic progenitors isolated from mouse skin (Motohashi et al., 2009). These findings raise the possibility that NCSC-like cells isolated from human dermis may be the in vivo equivalent of melanocyte stem cells in mouse hair follicles. Our data suggest that the non-canonical Wnt7a-JNK pathway is predominantly activated in stem cell culture conditions (Fig. 4a). Mouse melanocyte stem cells express Frizzled4 and Frizzled7 (Yamada et al., 2010), while human NCSC-like cells additionally express Frizzled10, which activates non-canonical Wnt7a-JNK signaling in endometrial cancer (Supplementary Fig. 5b) (Carmon and Loose, 2008). The biological significance of the Wnt7a-JNK pathway in melanocyte stem cells in hair follicles has not yet been determined. Further studies are required to elucidate whether NCSC-like cells in human glabrous skin are the counterpart of melanocyte stem cells in mouse hair follicles and whether Wnt7a-mediated non-canonical Wnt signaling plays a role in differentiation of both cell types.
Depending on the tissue context, the Wnt and Notch pathways interact either in synergistic or antagonistic manners. Similar to hepatic progenitors (Boulter et al., 2012), the two pathways act in opposing manners in NCSC-like cells, i.e. the canonical Wnt pathway inhibits the Notch pathway through the Notch inhibitor Numb. However, our data indicate that Numb is not transcriptionally up-regulated as seen in the hepatic system and in myoblasts (Liu et al., 2013), but is regulated in NCSC-like cells post-transcriptionally in a canonical Wnt pathway-dependent manner. Several studies also report the post-transcriptional regulation of Numb. The RNA-binding protein Musashi1/2 translationally down-regulates Numb expression (Imai et al., 2001; Ito et al., 2010; Sakakibara and Okano, 1997). In addition, Numb is a target of E3 ubiquitin-protein ligases, such as Siah-1 (seven in absentia homolog 1), LNX (ligand of Numb protein-X), and MDM2 (mouse double minute 2 homolog) (Nie et al., 2002; Sczaniecka et al., 2012; Susini et al., 2001). Thus, it is likely that the canonical Wnt pathway increases expression of Numb protein by regulating post-transcriptional modifiers.
The improper activation of developmental pathways is often involved in cancer pathogenesis (Izrailit and Reedijk, 2012; Janikova and Skarda, 2012). Our study shows that the biological antagonism of the Wnt/Notch signaling pathways is relevant to the homeostasis of somatic stem cells in the human skin microenvironment, implying that the disturbed interaction between these two pathways may contribute to human melanomagenesis. In NCSC-like cells, inhibition of the Notch pathway by Numb is likely a trigger to switch from the Wnt5a-high stem/migratory phenotype to the MITF-high differentiated phenotype. Previous studies indicate that Numb is a tumor suppressor in various human cancer types (Pece et al., 2011). Therefore, the function of Numb and the mechanism of its regulation in melanoma are worth investigating. Overall, our study revealed a crosstalk between the two conserved developmental pathways in postnatal human skin, and highlights the role of the skin microenvironment in specifying the fate of stem cells.
Material and Methods
Tissue culture
NCSC-like cells were isolated from human foreskins as described elsewhere (Li et al., 2010) and were cultured as spheres in human embryonic stem cell culture medium (StemPro® hESC SFM, Life Technologies) without basic fibroblast growth factor (bFGF). Human foreskin samples were obtained from surgical discards and approved for use by the Wistar Institute’s Institutional Review Board. Human melanoma cell lines (WM35, WM88, WM852 and WM3734) were isolated as previously described (Satyamoorthy et al., 1997) and were cultured in Mel 2% melanoma growth medium or grown in StemPro® hESC serum-free medium (http://www.wistar.org/lab/meenhard-herlyn-dvm-dsc/page/cell-culture-techniques). Normal human primary melanocytes, keratinocytes, and fibroblasts were isolated from the epidermis of neonatal foreskins and were cultured as previously described (Fukunaga-Kalabis et al., 2006). UV irradiation was performed with a Daavlin Research Series Irradiation Unit with UVA (range from 320 to 400 nm) and UVB (range from 290 to 320 nm) bulbs. Melanocyte differentiation assays were performed as described elsewhere (Li et al., 2010). Recombinant Wnt (R&D Systems) was added at a final concentration of 20 ng/ml. 3D human skin reconstructs with NCSC-like cells were generated as described previously (Li et al., 2011, 2013; Li et al., 2010). Bovine Pituitary Extract (BPE) was not added to skin reconstruct medium in order to avoid canonical Wnt ligands derived from the pituitary gland. Human foreskin explants were maintained in 45% DMEM, 45% Keratinocyte-SFM supplemented with epidermal growth factor and 10% calf serum. Skin explants were irradiated following the protocol of Arad et al. (Arad et al., 2007), incubated for 48h, frozen, and sectioned for staining.
Lentivial vectors
Lentiviral constructs encoding active Notch1 (Nic) and control GFP have been described elsewhere (Balint et al., 2005). Lentiviral constructs encoding shRNA sequences targeting NUMB were purchased from OpenBiosystems (oligo IDs: TRCN 0000007227 and TRCN0000007226; referred to as shNUMB_1 and shNUMB_2). A lentiviral construct encoding shRNA sequences targeting GFP (TRCN0000072196) served as vector control. 3
Cell viability assays
Primary isolated NCSC-like cells were cultured as spheres in 6-well plates for 14 days without adding new media, after which the cell suspensions were seeded in 96-well plates. CellTiter 96® AQueous MTS reagent (Promega) was added at 4 mg/ml to each well, and cells were incubated for 4 h at 37°C. The absorbance of each well was measured using an EL800 Microplate Reader (BioTek) at 490 nm. The ability for self-renewal was measured by secondary sphere formation assay. To obtain single cells, spheres were incubated in 1 mg/ml collagenase type I (Sigma) and 1 mg/ml collagenase type IV (Life Technologies) for 5 min, then mechanically dissociated by pipetting. Single cells were seeded into 96-well plates (2000 cells per well) in StemPro®. After 14 days, the relative growth of spheres in each well was measured using CellTiter 96® AQueous MTS reagent. Cell death was assessed with the red cell-impermeant viability indicator EthD-1 (Life Technologies). Dead cells, marked by bright red to orange fluorescence, were observed using a Nikon TE2000 inverted microscope (Nikon Instruments) using 10, 20 or 40x objective lenses and photo capture was performed with Q-Imaging Retiga EX digital camera. TUNEL assay was performed on sectioned skin reconstructs according to the manufacturer’s protocol using the Biotool™ TUNEL Apo-Green Detection Kit (Selleck Chemicals, Houston, TX).
Immunostaining
NCSC-like cells and differentiated cells were stained as described elsewhere (Li et al., 2010). Cells were fixed with either 4% paraformaldehyde in phosphate buffer solution (PBS) for 20 min at room temperature or in acetone-methanol at −20°C, then permeabilized with 0.5% Triton X-100 for 5 min. After blocking with 5% bovine serum albumin, cells were stained with the primary antibodies listed in Supplementary Table 1 or with isotype-matched IgG at 4°C. Cells were washed 3 times with PBS, and fluorochrome-conjugated secondary antibodies were added and incubated for 1 h at room temperature. Microscopy and photo capture was performed at room temperature on a Nikon E600 upright fluorescence microscope using 20, 40, or 60x objective lenses with a Spot RT Slider digital camera and ImagePro Plus software. Paraffin-embedded skin reconstructs were sectioned and deparaffinized, followed by antigen retrieval, and staining as described above.
Western blot analysis
To generate whole cell lysates, cells were washed with PBS and lysed in RIPA buffer. Nuclear fraction proteins were isolated using a NE-PER Nuclear Protein Extraction Kit (Thermo Scientific) according to the manufacturer’s instructions. For the detection of secreted Wnt7a, 48 h-conditioned media were collected and filtered with a Millex-GP Syringe Driven Filter Unit (Millipore, cat# SLGP033NS) to exclude cells and debris and were then concentrated to 100 μl by centrifugation at 6000 RPM for 30 min with Amicon Ultra-15 Centrifugal Filters with Ultracel-10K membranes (Millipore, cat# UFC901008). The primary antibodies are listed in Supplementary Table 1, and the signals were detected with the LICOR® Odyssey Infrared Imaging System after incubation with appropriate IRDye® secondary antibodies (LICOR Biosciences).
Quantitative real-time PCR (qRT-PCR)
Total mRNA was collected from cells using an RNeasy kit (Qiagen) according to the manufacturer’s instructions. cDNA was synthesized from each mRNA with a SuperScriptIII RT kit (Life Technologies) according to the manufacturer’s instructions. qRT-PCR was performed using the Power SYBR Green PCR Master Mix-kit (Applied Biosystems) on an ABI 7500 PRISM machine. Values were normalized to levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Sequences of the primers used are listed in Supplementary Table 1.
Statistics
All experiments were done in replicate samples and were repeated at least two or three times for validation. Two-sample t-tests with equal or unequal variances were used for data analysis. All statistical tests were performed under a two-sided hypothesis with a two-tailed p-value of less than or equal to 0.05 to reject the null hypothesis.
Supplementary Material
Acknowledgments
We thank J. Hayden and F. Keeney (Wistar Microscopy Facility) and R. Delgiacco (Wistar Histotechnology Facility) for technical support. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers P30CA10815, P01CA025874, R01CA076674, the Alfred Marchionini Foundation and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. M.V. Heppt was supported by the Böhringer Ingelheim Fonds (MD-fellowship). Support for Core Facilities utilized in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Abbreviations List
- NCSC
neural crest stem cell
- EDN3
endothelin-3
- SCF
stem cell factor
- hESC
human embryonic stem cell
- HES
hairy and enhancer of split
- HEY
hairy/enhancer-of-split related with YRPW motif
- DLL1
delta-like 1
- JAG1
Jagged 1
- DAPT
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- EthD-1
ethidium homodimer-1
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- Mel1
melanocyte differentiation media
- gp100
glycoprotein 100
- TYRP1
tyrosinase-related protein 1
- JNK
c-Jun NH(2)-terminal kinase
- Siah-1
seven in absentia homolog 1
- LNX
ligand of Numb protein-X
- bFGF
basic fibroblast growth factor
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Arad S, Konnikov N, Goukassian DA, et al. Quantification of inducible SOS-like photoprotective responses in human skin. J Invest Dermatol. 2007;127:2629–36. doi: 10.1038/sj.jid.5700893. [DOI] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–76. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Warneke JA, Nickoloff BJ, et al. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–70. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–28. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–8. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky WE, Curley DP, Santhanakrishnan M, et al. beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–54. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V, Beermann F, Martinozzi S, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–35. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–3. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Liu ZJ, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol. 2006;175:563–9. doi: 10.1083/jcb.200602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14:320–8. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, et al. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159:867–80. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23:746–59. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- Hu B, Lefort K, Qiu W, et al. Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes Dev. 2010;24:1519–32. doi: 10.1101/gad.1886910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Imai T, Tokunaga A, Yoshida T, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21:3888–900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kwon HY, Zimdahl B, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–8. doi: 10.1038/nature09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrailit J, Reedijk M. Developmental pathways in breast cancer and breast tumor-initiating cells: therapeutic implications. Cancer Lett. 2012;317:115–26. doi: 10.1016/j.canlet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Janikova M, Skarda J. Differentiation pathways in carcinogenesis and in chemo- and radioresistance. Neoplasma. 2012;59:6–17. doi: 10.4149/neo_2012_002. [DOI] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, et al. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–8. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- Kageshita T, Hamby CV, Ishihara T, et al. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001;145:210–6. doi: 10.1046/j.1365-2133.2001.04336.x. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Bushoven P, Iwasaki M, et al. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–45. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. Secrets to developing Wnt-age melanoma revealed. Pigment Cell Melanoma Res. 2009;22:520–1. doi: 10.1111/j.1755-148X.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp. 2011 doi: 10.3791/2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Herlyn M. Isolation, characterization, and differentiation of human multipotent dermal stem cells. Methods Mol Biol. 2013;989:235–46. doi: 10.1007/978-1-62703-330-5_18. [DOI] [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Yu H, et al. Human dermal stem cells differentiate into functional epidermal melanocytes. J Cell Sci. 2010;123:853–60. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Wu Y, Yao S, et al. Androgens Upregulate Transcription of the Notch Inhibitor Numb in C2C12 Myoblasts via Wnt/beta-catenin Signaling to Tcf Elements in the Numb Promoter. J Biol Chem. 2013 doi: 10.1074/jbc.M113.478487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–7. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Osawa M, Mak SS, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173:333–9. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi T, Yamanaka K, Chiba K, et al. Unexpected multipotency of melanoblasts isolated from murine skin. Stem Cells. 2009;27:888–97. doi: 10.1634/stemcells.2008-0678. [DOI] [PubMed] [Google Scholar]
- Nie J, McGill MA, Dermer M, et al. LNX functions as a RING type E3 ubiquitin ligase that targets the cell fate determinant Numb for ubiquitin-dependent degradation. Embo J. 2002;21:93–102. doi: 10.1093/emboj/21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos GN, Duarte M, Kubu CJ, et al. Soluble Jagged1 attenuates lateral inhibition, allowing for the clonal expansion of neural crest stem cells. Stem Cells. 2007;25:3133–42. doi: 10.1634/stemcells.2007-0327. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Saga Y. Notch signaling is required for the maintenance of enteric neural crest progenitors. Development. 2008;135:3555–65. doi: 10.1242/dev.022319. [DOI] [PubMed] [Google Scholar]
- Pece S, Confalonieri S, PRR, et al. NUMB-ing down cancer by more than just a NOTCH. Biochim Biophys Acta. 2011;1815:26–43. doi: 10.1016/j.bbcan.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Pinnix CC, Lee JT, Liu ZJ, et al. Active Notch1 confers a transformed phenotype to primary human melanocytes. Cancer Res. 2009;69:5312–20. doi: 10.1158/0008-5472.CAN-08-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourreyron C, Reilly L, Proby C, et al. Wnt5a is strongly expressed at the leading edge in non-melanoma skin cancer, forming active gradients, while canonical Wnt signalling is repressed. PLoS One. 2012;7:e31827. doi: 10.1371/journal.pone.0031827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–55. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–12. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, et al. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. Embo J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, DeJesus E, Linnenbach AJ, et al. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7(Suppl 2):S35–42. [PubMed] [Google Scholar]
- Sczaniecka M, Gladstone K, Pettersson S, et al. MDM2 protein-mediated ubiquitination of numb protein: identification of a second physiological substrate of MDM2 that employs a dual-site docking mechanism. J Biol Chem. 2012;287:14052–68. doi: 10.1074/jbc.M111.303875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seite S, Fourtanier A, Moyal D, et al. Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review. Br J Dermatol. 2010;163:903–14. doi: 10.1111/j.1365-2133.2010.10018.x. [DOI] [PubMed] [Google Scholar]
- Shan J, Shi DL, Wang J, et al. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- Susini L, Passer BJ, Amzallag-Elbaz N, et al. Siah-1 binds and regulates the function of Numb. Proc Natl Acad Sci U S A. 2001;98:15067–72. doi: 10.1073/pnas.261571998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Yamada T, Akamatsu H, Hasegawa S, et al. Melanocyte stem cells express receptors for canonical Wnt-signaling pathway on their surface. Biochem Biophys Res Commun. 2010;396:837–42. doi: 10.1016/j.bbrc.2010.04.167. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hasegawa S, Inoue Y, et al. Wnt/beta-catenin and Kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- Zabierowski SE, Baubet V, Himes B, et al. Direct reprogramming of melanocytes to neural crest stem-like cells by one defined factor. Stem Cells. 2011;29:1752–62. doi: 10.1002/stem.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier L, Homminga I, Calvert V, et al. NOTCH1 and/or FBXW7 mutations predict for initial good prednisone response but not for improved outcome in pediatric T-cell acute lymphoblastic leukemia patients treated on DCOG or COALL protocols. Leukemia. 2010;24:2014–22. doi: 10.1038/leu.2010.204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.