Abstract
Plasma membrane-derived vesicles are being used in biophysical and biochemical research as a simple, yet native-like model of the cellular membranes. Here we report on the characterization of vesicles produced via two different vesiculation methods from CHO and A431 cell lines. The first method is a recently developed method which utilizes chloride salts to induce osmotic vesiculation. The second is a well established chemical vesiculation method which uses DTT and formaldehyde. We show that both vesiculation methods produce vesicles which contain the lipid species previously reported in the plasma membrane of these cell lines. The two methods lead to small but statistically significant differences in two lipid species only; phosphatidylcholine (PC) and plasmalogen phosphatidylethanolamine (PEp). However, highly significant differences were observed in the degree of incorporation of a membrane receptor and in the degree of retention of soluble cytosolic proteins within the vesicles.
Introduction
The cellular plasma membrane, a complex assembly of lipids and proteins, plays a critical role in cell physiology (1-4). The membrane provides the barrier, and mediates the communication, between the cell and its environment. The processes that occur in the plasma membrane, such as ion conduction, nutrients uptake, and signal transduction, are critical for cell function (5-8). It is often difficult, however, to study these processes in the plasma membrane of living cells, and thus biophysicists and biochemists often rely on model membrane systems. One such model system is plasma membrane-derived vesicles, which bud off cells in response to external stress (9, 10). These vesicles are derived from the native cellular membrane, and are thus more native-like than vesicles made of synthetic lipids. They are increasingly used in studies of lipid-lipid, lipid-protein and protein-protein interactions, and have already yielded new knowledge about lipid domains and receptor interactions in the membrane (11-15). Often, however, they are not well characterized in terms of their lipid and protein content.
The most widely used vesiculation method, developed in the 1960s, utilizes the chemicals formaldehyde and dithiothreitol (DTT) to stress the cells and to induce an apoptosis-like response (9, 16, 17). Vesicles can be produced with this method from a variety of mammalian cells including human embryonic kidney (HEK) 293T cells, Chinese hamster ovary (CHO) cells, A431 human epidermoid carcinoma cells, 3T3 fibroblasts, endothelial cells, a variety of other cancer cells, and macrophages (9, 12, 16-21). These vesicles have been used widely in the literature to study lipid domains, but concerns may arise in some cases due to the presence of DTT, a reducing agent, as well as formaldehyde, a molecular cross-linker. Thus, an alternative vesiculation method, which utilizes osmotic stress rather than chemicals, was recently developed (10). In this method, vesiculation is induced by incubating cells with a buffer which contains high concentration of chloride salts. This osmotic method has been used to produce vesicles from CHO and A431 cells, in the absence of DTT and formaldehyde. The overall appearance of the vesicles produced with the osmotic stress method and the DTT/formaldehyde method is very similar (10), and both vesicle preparations have been used successfully in studies of protein interactions in membranes (18).
Here we sought to characterize and compare the vesicles produced by chemical and osmotic vesiculation and to identify differences that might exist between the two types of vesicles. In particular, we characterized and compared A431 chloride salt vesicles and A431 DTT/formaldehyde vesicles. As a control, we also characterized CHO DTT/formaldehyde vesicles, with the goal of comparing differences due to vesiculation method and differences due to cell type.
Materials and Methods
Cell culture and vesiculation
Chinese hamster ovary (CHO) and A431 cells were cultured in T75 flasks. These cells were vesiculated at 70% confluency using a DTT/formaldehyde buffer (9) or a chloride salt osmotic buffer (10).
Vesicle lipid pelleting
Centrifugation was performed at 125×g, 4 °C to pellet the cell debris. A second centrifugation was performed at 25000×g for 45 minutes at 4 °C to pellet the vesicles. The supernatant was discarded.
Liquid chromatography mass spectrometric (LC-MS) analysis of lipids and cholesterol
Three independent samples were prepared for each vesicle type. After pelleting, lipids and cholesterol were extracted as described previously using a modified Bligh/Dyer procedure, spiked with appropriate internal standards (22), and analyzed using a 6490 Triple Quadrupole LC-MS system (Agilent Technologies, Santa Clara, CA). Glycerophospholipids and sphingolipids were separated with normal-phase HPLC as described before (22), with a few changes. An Agilent Zorbax Rx-Sil column (inner diameter 2.1 × 100 mm) was used under the following conditions: mobile phase A (chloroform:methanol:1 M ammonium hydroxide, 89.9:10:0.1, v/v) and mobile phase B (chloroform:methanol:water:ammonium hydroxide, 55:39.9:5:0.1, v/v); 95% A for 2 min, linear gradient to 30% A over 18 min and held for 3 min, and linear gradient to 95% A over 2 min and held for 6 min. Sterols and glycerolipids were separated with reverse-phase HPLC using an isocratic mobile phase as before (22) except with an Agilent Zorbax Eclipse XDB-C18 column (4.6 × 100 mm). Quantification of lipid species was accomplished using multiple reaction monitoring (MRM) transitions (22) in conjunction with referencing of appropriate internal standards: PA 14:0/14:0, PC 14:0/14:0, PE 14:0/14:0, PG 15:0/15:0, PI 16:0/16:0, PS 14:0/14:0, BMP 14:0/14:0, APG 14:0/14:0, LPC 17:0, LPE 14:0, LPI 13:0, Cer d18:1/17:0, SM d18:1/12:0, dhSM d18:0/12:0, GalCer d18:1/12:0, GluCer d18:1/12:0, LacCer d18:1/12:0, D7-cholesterol, CE 17:0, MG 17:0, 4ME 16:0 diether DG, D5-TG 16:0/18:0/16:0 (Avanti Polar Lipids, Alabaster, AL).
Thin layer chromatography
The pelleted plasma membrane vesicles were resuspended in distilled water. The solution was dried in a rotory evaporator and the lipids were extracted using the Folch method with chloroform:methanol:distilled water (2:1:1 (v/v)) at room temperature (23). The extracts were dried under a stream of N2 gas and resuspended in a chloroform:methanol mixture (2:1 (v/v)). One dimensional thin layer chromatography (TLC) was used to analyze the lipid content. Whatman flexible silica gel G plates were spotted with solutions containing the extracted lipids, as well as the following lipid standards: 1-palmitoyl-2-deoyl-sn-glycerol-3-phosphocholine (POPC), 1-palmitoyl-2-deoyl-sn-glycerol-3-phospho-L-Serine (POPS), 1-palmitoyl-2-deoyl-sn-glycerol-3-phosphoethanolamine (POPE), Sphingomyelin (from Porcine brain), and cholesterol (Avanti Polar Lipids Inc). The plates were then placed in a chamber with a chloroform:methanol:7-N NH4OH (65:27:5 (v/v)) solution to separate the different lipid components. After separation, the lipid components were visualized in iodine vapor.
31P NMR phospholipid analysis
Vesicle pellets were resuspended in distilled water. The solution was dried in a rotory evaporator and the lipids were extracted using the Folch method with chloroform:methanol:distilled water (2:1:1 (v/v)) at room temperature (23). The extracts were dried under a stream of N2 gas and resuspended in a chloroform:methanol mixture (2:1 (v/v)). 20-30 mg of lipid extracts from each vesicle type were sent to Avanti Polar Lipids Analytical Services for 31P NMR analysis. A Bruker Avance™ III 400 mHz with a 5 mm BBFO Probe NMR spectrometer was used to characterize the phospholipid composition of the samples dissolved in 1 mL of detergent.
Annexin V binding to plasma membrane derived vesicles
Vesicles were incubated for 1 hour with fluorescein-conjugated Annexin V using the LI2004 Annexin V detection kit (Molecular Probes). Images were recorded in a Nikon confocal microscope.
Plasmids for vesicle content leakage assays
The wild type human fibroblast growth factor receptor 2 (FGFR2) plasmid was a gift from Dr. Moosa Mohammadi (NYU). The FGFR2-mCherry plasmid was constructed by fusing mCherry to the C-terminus of full length FGFR2. The plasmid encoding 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1-GFP (Plcδ1-PH-GFP) was obtained from Dr. Tamas Balla (NIH). The plasmids encoding Intersectin II-GFP, 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1-GFP (Plcγ-GFP) and Protein kinase C theta-GFP (PKCθ-GFP) were a kind gift from Dr. Christoph Wuelfing (University of Bristol). The Growth factor receptor-bound protein 2-Venus (Grb2-Venus) plasmid was a gift from Dr. Jin Zhang (Johns Hopkins). The VVVVVV (Venus × 6) plasmid was purchased from Addgene (courtesy of Dr. Steven Vogel, NIH).
Western blot analysis of EGFR in A431 vesicles
The vesicle pellets were lysed with lysis buffer (25 mM Tris-HCl, 0.5% Triton X-100, 20mM NaCl, 2 mM EDTA, phosphatase inhibitor and protease inhibitor, Roche Applied Science). The lysates were loaded onto 3–8% NuPAGE®Novex®Tris–Acetatemini gels (Invitrogen, CA). The proteins were transferred onto a nitrocellulose membrane, and blocked using 5% milk in TBS. EGFR was detected with anti-EGFR receptor antibodies (2232s, Cell Signaling Technology USA), followed by anti-rabbit HRP conjugated antibodies (W4011, Promega). The proteins were visualized with the Amersham ECL detection system (GE Healthcare) as described previously (24, 25).
EGF-Rhodamine binding to EGFR in A431 vesicles
Vesicles were incubated with 1ug/mL of Epidermal Growth Factor - Tetramethylrhodamine Conjugate (E3481, Molecular Probes) for 1h, and were then imaged in the confocal microscope.
Results
Different methods of vesicle production lead to small, but statistically significant differences in cholesterol and lipid composition
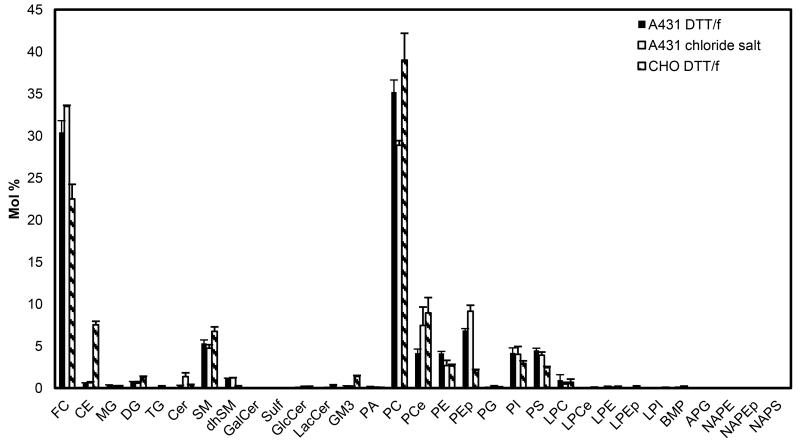
We first compared the lipid and cholesterol content of the three types of vesicles used in this study: A431 chloride salt vesicles, A431 DTT/formaldehyde vesicles, and CHO DTT/formaldehyde vesicles using liquid chromatography-mass spectrometry (LC-MS). Three independent samples were prepared for each type of vesicle preparation. The vesicles were pelleted and the lipids were extracted as described in Materials and Methods. The LC-MS results, shown in Figure 1, report on the relative abundance of cholesterol and lipids in the three vesicle preparations. We found that the three types of vesicles contain significant amounts of free cholesterol (FC), phosphatidylcholine (PC), sphingomyelin (SM), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI). Other substantial lipids are dhSM, PCe, PEp and LPC in A431 DTT/formaldehyde vesicles, Cer, dhSM, PCe, PEp and LPC in A431 chloride salt vesicles, and DG, GM3, dhSM, PCe, PEp and LPC in CHO DTT/formaldehyde vesicles (see lipid annotations in Figures 1).
Figure 1.
Cholesterol and lipid composition of A431 chloride salt vesicles, A431 DTT/formaldehyde vesicles, and CHO DTT/formaldehyde vesicles, determined by High Performance Liquid Chromatography Mass Spectrometry (LC-MS). Shown are averages for three independent preparations, and the standard errors. FC: free cholesterol; CE: cholesteryl ester; MG: monoacylglycerol; DG: diacylglycerol; TG: triacylglycerol; Cer: ceramide; SM: sphingomyelin; dhSM: dihydrosphingomyelin GalCer: galactosylceramide; Sulf: sulfatide; GlcCer: glucosylceramide; LacCer: lactosylceramide; GM3: monosialodihexosylganglioside 3; PA: phosphatidic acid; PC: phosphatidylcholine; PCe: etherphosphatidylcholine; PE: phosphatidylethanolamine; PEp: plasmalogen phosphatidylethanolamine; PG: phosphatidylglycerol; PI: phosphatidylinositol; PS: diacylglycerol; LPC: lysophosphatidylserine; LPCe: lysoetherphosphatidylcholine; LPE: lysophosphatidylethanolamine; LPEp: lysoplasmalogen phosphatidylethanolamine; LPI: lysophosphatidylinositol; BMP: bismonoacylglycerol; APG: acyl phosphatidylglycerol; NAPE: N-Acyl phosphatidylethanolamine; NAPEp: N-Acyl plasmalogen phosphatidylethanolamine; NAPS: N-Acyl phosphatidylserine. Cholesterol is significantly different between A431 and CHO vesicles.
The mole % of free cholesterol was 33.5 ± 0.1, 30.4 ± 1.4, and 22.5 ± 1.7 for A431 chloride salt, A431 DTT/formaldehyde, and CHO DTT/formaldehyde vesicles, respectively. There was no statistically significant difference between the free cholesterol mole% in A431 chloride salt and A431 DTT/formaldehyde vesicles (p = 0.09), based on a two-tailed t-test. However, there was a statistically significant difference between A431 DTT/formaldehyde vesicles and CHO DTT/formaldehyde vesicles (p = 0.02).
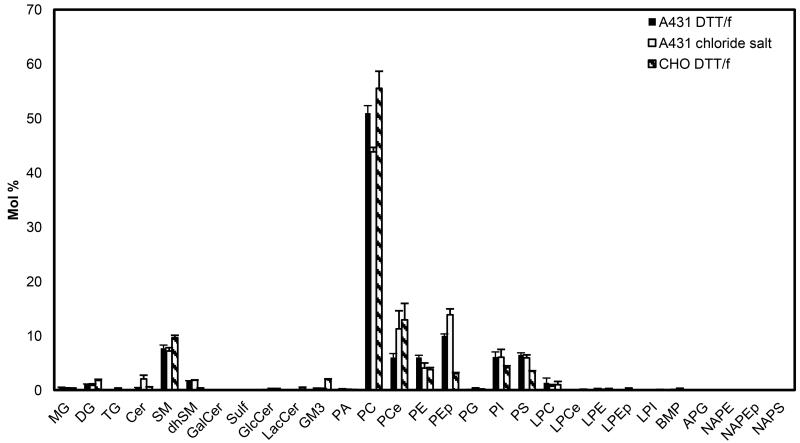
Figure 2 compares the lipid content of the three vesicle preparations. As expected, the major lipid component in the three vesicle preparations was PC. There was a statistically significant difference between PC mole % in A431 chloride salt vesicles (43.8 ± 0.8 mole %) and A431 DTT/formaldehyde vesicles (51.0 ± 1.4 mole %) (p = 0.01). On the other hand, the PC levels in A431 DTT/formaldehyde vesicles were not different from CHO DTT/formaldehyde levels (55.5 ± 3.1mole %) (p =0.2).
Figure 2.
Lipid composition of A431 chloride salt vesicles, A431 DTT/formaldehyde vesicles, and CHO DTT/formaldehyde vesicles, recalculated without cholesterol by re-scaling the LC-MS data shown in Figure 1. The major lipid component is PC, and there is a statistically significant difference between PC abundance in A431 vesicles produced with the two methods (chloride salt and DTT/formaldehyde). There are also significant differences in PEp abundance. Comparing A431 DTT/formaldehyde and CHO DTT/formaldehyde samples, statistically significant differences are observed between PE, PS, GM3, dhSM, and PEp levels.
The SM content in the A431 chloride salt vesicles, A431 DTT/formaldehyde vesicles, and CHO DTT/formaldehyde vesicles was 7.2 ± 0.6 mole %, 7.7 ± 0.6 mole %, and 9.6 ± 0.5 mole % respectively. No statistical differences were observed between SM mole % in A431 chloride salt and A431 DTT/formaldehyde vesicles (p = 0.6), or between A431 DTT/formaldehyde and CHO DTT/formaldehyde vesicles (p = 0.07). Similarly, PI mole % was not significantly different in the three vesicle types.
PS content was 5.9 ± 0.6 mole % in A431 chloride salt vesicles, 6.5 ± 0.4 mole % in A431 DTT/formaldehyde vesicles, and 3.5 ± 0.1 mole % in CHO DTT/formaldehyde vesicles. The PS content was different in A431 DTT/formaldehyde and in CHO DTT/formaldehyde vesicles (p = 0.001) but not in A431 DTT/formaldehyde and A431 chloride salt vesicles. Similarly, the PE content was similar in the two types of A431 vesicles, but different in the A431 and CHO vesicles.
Monosialodihexosylganglioside 3 (GM3) levels were not statistically different in A431 chloride salt vesicles (0.3± 0.06 mole %) and in A431 DTT/formaldehyde vesicles (0.3± 0.08 mole %) (p=0.7), but were significantly different when comparing A431 DTT/formaldehyde (0.3± 0.08 mole %) and CHO DTT/formaldehyde (1.9± 0.1 mole %) vesicles (p=0.0003). This difference was most likely due to differences in cellular membrane composition, as GM3 has been previously reported in the plasma membranes of intact CHO cells (26), but not in A431 cells (27).
Overall, the LC-MS results demonstrated that all vesicle preparations contain all the major lipids known to exist in plasma membranes of intact cells. While there were some statistically significant differences between lipids in A431 chloride salt vesicles and A431 DTT/formaldehyde vesicles, these differences appeared only in two lipid components, namely PC and plasmalogen phosphatidylethanolamine (PEp). On the other hand, significant differences were observed between free cholesterol, CE, GM3, dhSM, PE, PEp and PS levels when comparing A431 DTT/formaldehyde and CHO DTT/formaldehyde vesicles, due to differences in cell type.
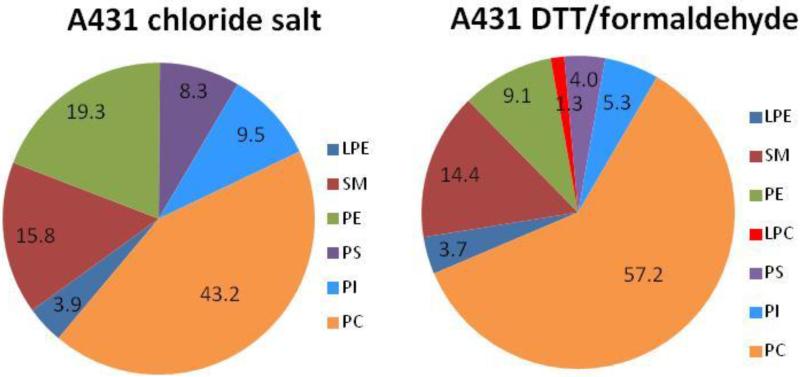
The lipid composition of the three types of vesicles was further studied using Thin Layer Chromatography (TLC) and 31P NMR. The TLC results, presented in Figure S1, show that cholesterol, PC, SM, PE, PS and PI are present in all vesicle types. We also used the Avanti analytical services to characterize the lipid composition of A431 chloride salt vesicles and A431 DTT/formaldehyde vesicles with 31P NMR (spectra shown in Figure S2). The analysis identified PC, PE, PI, PS, SM and LPE as the major species in the A431 chloride salt vesicles, and PC, PE, PI, PS, SM, LPE and LPC in the A431 DTT/formaldehyde vesicles (Figure 3).
Figure 3.
Phospholipid content of the vesicles, from 31P NMR experiments performed by Avanti Polar Lipids Analytical Services (see Figure S2 for spectra). Single samples were analyzed for each vesicle preparation. Qualitatively, the results are in agreement with the results in Figure 2, with PC being the most abundant phospholipid in the two samples.
Overall, 31P NMR and TLC results were in agreement with the LC-MS results, since they identified the same major lipids as the LC-MS experiments. They further confirmed that PC is the major phospholipid component in all vesicle preparations.
Vesicles bind annexin V, independent of production method
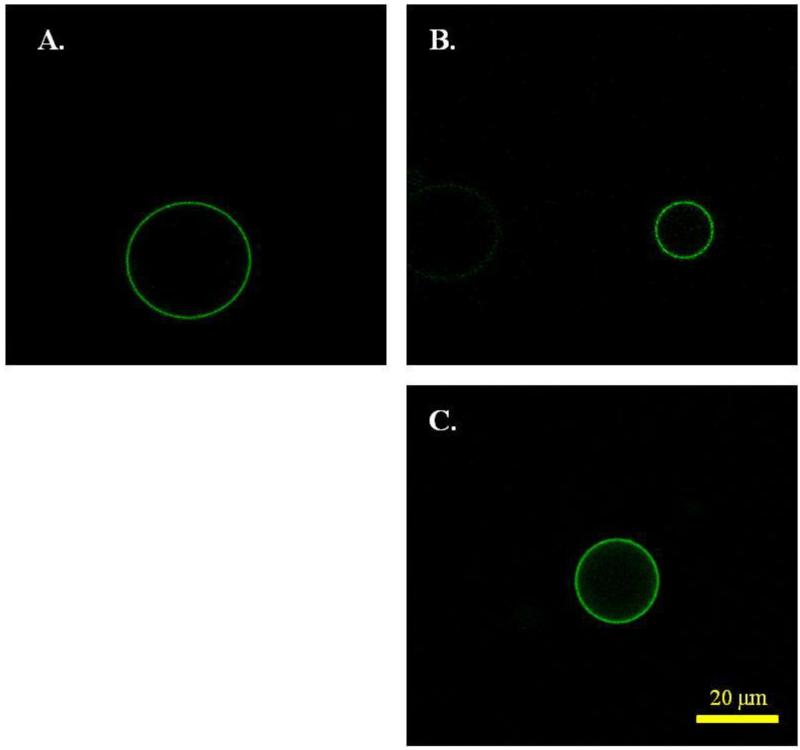
It is known that plasma membrane vesicles form as a result of cell stress which induces an apoptotic response (16, 17). Under stress, the cellular membrane loses some of its asymmetry, as PS, which is found in the cytoplasmic leaflet of intact cells, becomes exposed on the cell surface. As a result, the plasma membrane derived-vesicles are expected to have PS on their outer surfaces. Annexin V, which binds specifically to PS, is therefore expected to bind to the vesicles (28). Here we asked whether we can detect differences in Annexin V binding due to vesicle production. We thus incubated the vesicles with fluorescently labeled Annexin V. Fluorescence images, captured in the confocal microscope, are shown in Figure 4. We quantified and plotted the pixel intensities for 87 A431 DTT/formaldehyde vesicles and 52 A431 chloride salt vesicles. The difference in parameters of the pixel intensities distributions for the two types of vesicles was not statistically significant. Thus, there were no measurable differences in PS exposure to Annexin V due to production method. In these experiments, no Annexin V binding was observed to intact, non-apoptotic, un-vesiculated cells, which served as control.
Figure 4.
Annexin V binding to (A) A431 chloride salt vesicles, (B) A431 DTT/formaldehyde vesicles, and (C) CHO DTT/formaldehyde vesicles. The method of vesicle production does not have a significant effect on Annexin V binding.
Cytoplasmic proteins are not retained in vesicles produced via osmotic vesiculation
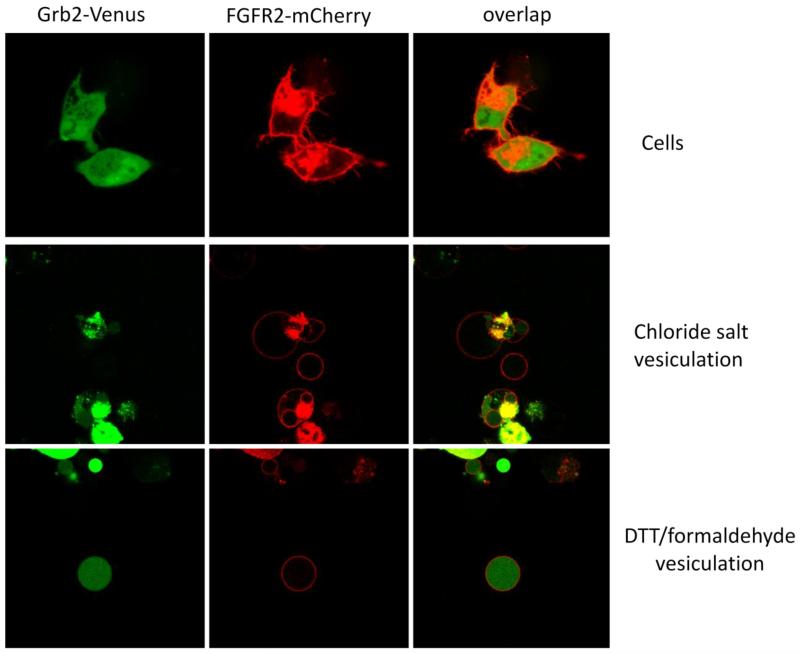
In our prior work, we have shown that soluble fluorescent proteins, expressed in CHO cells, fill the DTT/formaldehyde vesicles (29). In the course of the current work we noticed, however, that the fluorescent proteins are not retained within chloride salt vesicles. To further investigate this phenomenon, we studied the retention of several soluble cytoplasmic proteins of different molecular weight, within the vesicles. We labeled the membranes of cells and vesicles using Fibroblast Growth Factor Receptor-(FGFR2)-mCherry, a fluorescently tagged membrane protein.
Figure 5 shows the results for Grb2-Venus (molecular weight ~60 kDa). In this experiments, CHO cells were co-transfected with plasmids encoding for FGFR2-mCherry and Grb2-Venus. After expression, FGFR2-mCherry was located on the membane, while Grb2-Venus filled the cytoplasm. After vesiculation, Grb2-Venus (molecular weight ~60 kDa) was not found inside CHO chloride salt vesicles, but was found inside the CHO DTT/formaldehyde vesicles. We next performed experiments with proteins of higher molecular weight, PKCΘ-GFP (MW~120 kDa), Venus × 6 (MW~160 kDa), Intersectin II-GFP (MW~170 kDa), and PLCγ-GFP (MW~210 kDa). These are all soluble proteins that reside in the cytoplasm of intact cells (Figure S3, B-F). As shown in Figures S5-S7 and Figure 6, none of these proteins were found inside the vesicles produced with the osmotic stress method.
Figure 5.
Top panel: Chinese Hamster Ovary (CHO) expressing Grb2-Venus (MW ~ 60 kDa) and FGFR2-mCherry. Middle panel: Grb2-Venus (MW ~ 60 kDa) is not retained in CHO chloride salt vesicles. Bottom panel: Grb2-Venus is retained in DTT/formaldehyde vesicles.
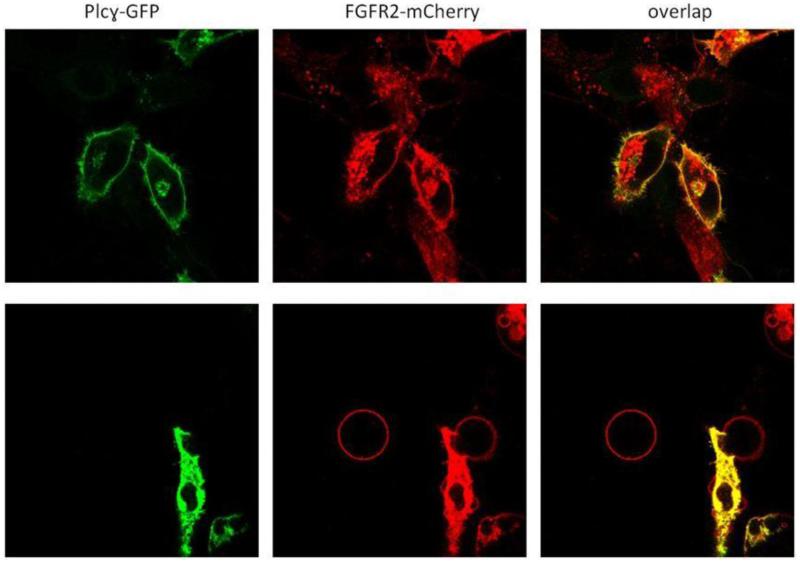
Figure 6.
Top panel: CHO cells expressing Plcγ-GFP and FGFR2-mCherry. Bottom panel: Plcγ-GFP (MW ~ 210 kDa) is not retained in CHO chloride salt vesicles.
In Figure 6, we see that PLCγ-GFP (MW~210 kDa) associates with the plasma membrane in cells when FGFR2-mCherry is present because it specifically interacts with it. Despite binding to FGFR2 in cells, however, PLCγ-GFP was not found in the choloride salt vesicles (Figure 6). This likely occurs because, upon vesiculation, PLCγ-GFP reaches a new equilibrium with the aqueous sample volume which is effectively infinite.
To confirm that interactions with the membrane do not lead to soluble protein retention in the chloride salt vesicles, we also worked with Plcδ1-PH domain-GFP (MW~45 kDa), which is known to bind to PIP2 in cells (Figure S3 A). As seen in Figure S6, Plcδ1-PH domain-GFP is not retained inside the chloride salt vesicles, either. All above experiments lead us to conclude that the chloride salt vesicles, unlike the DTT/formaldehyde vesicles, are ghost vesicles that lack cytoplasmic content.
EGFR incorporates very efficiently in chloride salt vesicles, but not in DTT/formaldehyde vesicles
Vesicles are derived from the plasma membranes of cells, and are thus expected to contain membrane proteins, not only lipids. However, it is not known if the incorporation of membrane proteins in the vesicles in affected by the method of vesicle production. Since A431 cells are known to express the epidermal growth factor receptor (EGFR) endogenously at high levels (30-32), we sought to compare the amount of EGFR in the A431 vesicles produced with the two methods using Western blotting.
After vesiculation, we loaded identical amounts of total protein on the gel, and we visualized EGFR using anti-EGFR antibodies as described in Materials and Methods. The results are shown in Figure 7. While we see intense anti-EGFR bands in vesicles produces with the osmotic stress method, we see very weak anti-EGFR staining in the vesicles produces with the DTT/formaldehyde method. This finding suggests that EGFR is not efficiently incorporated in the vesicles during DTT/formaldehyde vesiculation, but is efficiently incorporated during osmotic vesiculation.
Figure 7.
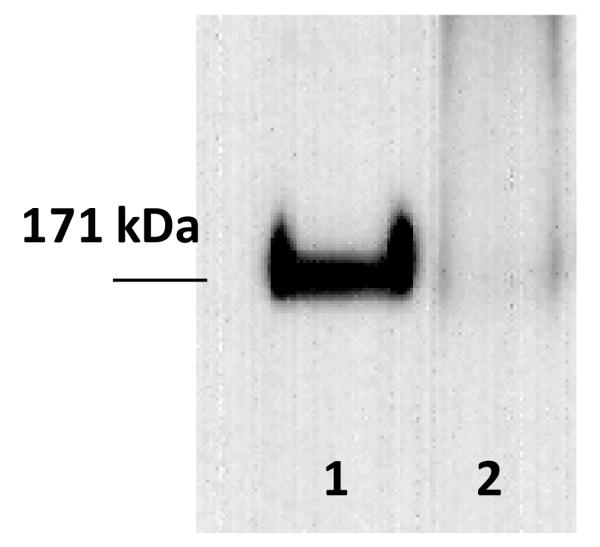
Western blots results for endogenous Epidermal Growth Factor Receptor (EGFR) in A431 vesicles. After vesicle lysis, identical amounts of total protein were loaded on the gel, and EGFR bands were visualized using anti-EGFR antibodies as described in Materials and Methods. Lane 1: A431 chloride salt vesicles. Lane 2: A431 DTT/formaldehyde vesicles.
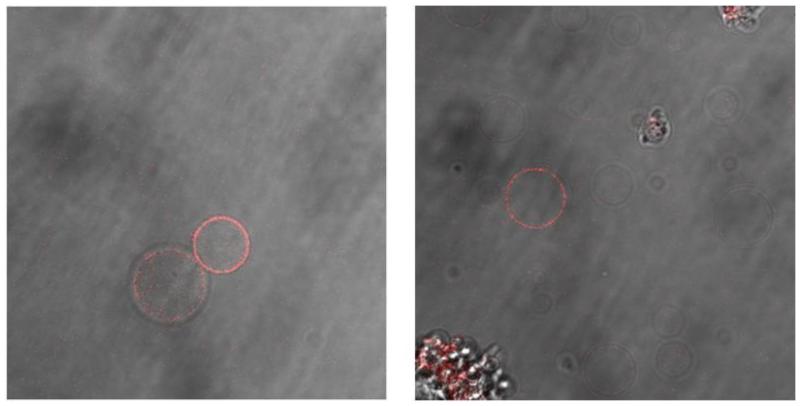
To assess the functionality of EGFR in the two types of vesicles, we investigated if the incorporated EGFR is capable of binding its ligand, EGF. The vesicles were therefore incubated with fluorescently labeled EGF (EGF-Rhodamine), see Figure 8. We observed that a large fraction (~50-90%) of the A431/chloride salt vesicles was labeled. On the other hand, only a small fraction of the A431 DTT/formaldehyde vesicles (~5%) bind EGF-Rhodamine. This finding is consistent with the observation that EGFR is not incorporated efficiently in the DTT/formaldehyde vesicles. Yet, EGF-Rhodamine binding in Figure 8 suggests that the receptors in the two types of vesicles are capable of ligand binding.
Figure 8.
EGF-Rhodamine binding to A431 vesicles. Left: A341 chloride salt vesicles; Right: A431 DTT/formaldehyde vesicles.
Discussion
Here we characterized and compared A431 vesicles produced by two different methods: osmotic vesiculation with chloride salts and chemical vesiculation using DTT and formaldehyde. We also characterized CHO DTT/formaldehyde vesicles. The goal of these experiments was to understand how differences due to production method compare with differences due to cell type.
Here we uncovered small but statistically significant differences in PC and PEp content, due to production method. On the other hand, statistically significant differences in FC, CE, dhSM, GM3, PE, PEp and PS were observed in A431 and CHO vesicles produced with the same chemical vesiculation method. Thus, differences in cholesterol and lipid content due to production method are quite modest, and smaller than differences due to cell type.
We further demonstrated that the vesiculation method affects the efficiency of incorporation of the membrane protein EGFR into the vesicles. In particular, EGFR incorporates easily into A431 chloride salt vesicles but is much less efficiently incorporated into DTT/formaldehyde vesicles. Yet, prior work has demonstrated that other membrane proteins such as GPA, Neu, and FGFR3 readily incorporate in DTT/formaldehyde vesicles (13, 14, 33-35). Thus, the incorporation efficiency of a membrane protein into the vesicles depends both on the production method and on the identity of the membrane protein itself.
The most striking difference between chloride salt and DTT/formaldehyde vesicles is in the degree of retention of soluble proteins inside the vesicles. While soluble proteins are retained within the DTT/formaldehyde vesicles, soluble proteins of molecular weight up to 210 kDa are not found inside the chloride salt vesicles. An explanation of this finding may be that formaldehyde cross-links cellular components and thus helps with the retention of soluble proteins in the cytoplasm.
Overall, we find that plasma membrane-derived vesicles produced by osmotic and chemical vesiculation are not identical models of the cellular membrane. We therefore propose that parallel biophysical characterization of membrane proteins in the two types of vesicles may be highly advantageous in some cases. Thus far, the interactions between TM helices have been characterized in the two types of vesicles and have been shown to be similar (10, 18). Soluble proteins, however, may interact with full-length membrane proteins and modulate their behavior. While such effects are difficult to quantify in live cells, they could be assessed by quantifying differences in membrane protein interactions in different vesicle preparations, in the presence and absence of soluble proteins.
Supplementary Material
Highlights.
Cell-derived vesicles are a model of the plasma membrane.
We characterized vesicles produced via osmotic and chemical methods.
Vesicles contain the lipid species known to exist in plasma membranes.
Osmotic A431 cell vesiculation leads to efficient EGFR incorporation.
Osmotic vesiculation produces ghost vesicles without cytosolic proteins.
Acknowledgments
This work was supported by NIH GM95930 and NSF MCB 1157687 to K.H., and NS056049 to G.D.P. We thank Drs. William Wimley, Ilya Levental, Mark Lemmon, Gerald Feigenson, Jeffrey Moore, Jose Luis Santos and Mr. Gregory Wiedman, Ms. Lolli Meeks and Ms. Lurong Pan for helpful discussions. We thank Dr. Lijuan He for the vesicle pelleting protocol.
Lipid abbreviations
- FC
free cholesterol
- CE
cholesteryl ester
- MG
monoacylglycerol
- DG
diacylglycerol
- TG
triacylglycerol
- Cer
ceramide
- SM
sphingomyelin
- dhSM
dihydrosphingomyelin
- GalCer
galactosylceramide
- Sulf
sulfatide
- GlcCer
glucosylceramide
- LacCer
lactosylceramide
- GM3
monosialodihexosylganglioside 3
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PCe
etherphosphatidylcholine
- PE
phosphatidylethanolamine
- PEp
plasmalogen phosphatidylethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
diacylglycerol
- LPC
lysophosphatidylserine
- LPCe
lysoetherphosphatidylcholine
- LPE
lysophosphatidylethanolamine
- LPEp
lysoplasmalogen phosphatidylethanolamine
- LPI
lysophosphatidylinositol
- BMP
bismonoacylglycerol
- APG
acyl phosphatidylglycerol
- NAPE
N-Acyl phosphatidylethanolamine
- NAPEp
N-Acyl plasmalogen phosphatidylethanolamine
- NAPS
N-Acyl phosphatidylserine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Coskun U, Simons K. Membrane rafting: From apical sorting to phase segregation. FEBS Lett. 2010;584:1685–1693. doi: 10.1016/j.febslet.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Maddy AH. The organization of protein in the plasma membrane. In: Warren KB, editor. In Formation and Fate of Cell Organelles. Academic Press; New York and London: 1967. pp. 255–273. [Google Scholar]
- 3.Stoeckenius W, Engelman DM. Current models for the structure of biological membranes. J. Cell Biol. 1969;42:613–646. doi: 10.1083/jcb.42.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson TE. In Cellular Membranes in Development. Academic Press; New York: 1964. The properties of bimolecular phospholipid membranes; pp. 83–96. [Google Scholar]
- 5.White SH, Ladokhin AS, Jayasinghe S, Hristova K. How membranes shape protein structure. J. Biol. Chem. 2001;276:32395–32398. doi: 10.1074/jbc.R100008200. [DOI] [PubMed] [Google Scholar]
- 6.Neher E. Ion channels for communication between and within cells. Neuron. 1992;8:605–612. doi: 10.1016/0896-6273(92)90083-p. [DOI] [PubMed] [Google Scholar]
- 7.Fantl WJ, Johnson DE, Williams LT. Signaling by Receptor Tyrosine Kinases. Annu. Rev. Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 8.Bockaert J, Dumuis A, Fagni L, Marin P. GPCR-GIP networks: A first step in the discovery of new therapeutic drugs? Current Opinion in Drug Discovery & Development. 2004;7:649–657. [PubMed] [Google Scholar]
- 9.Scott RE. Plasma membrane vesiculation: A new technique for isolation of plasma membrane. Science. 1976;194:743–745. doi: 10.1126/science.982044. [DOI] [PubMed] [Google Scholar]
- 10.Del Piccolo N, Placone J, He L, Agudelo SC, Hristova K. Production of plasma membrane vesicles with chloride salts and their utility as a cell membrane mimetic for biophysical characterization of membrane protein interactions. Anal. Chem. 2012;84:8650–8655. doi: 10.1021/ac301776j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levental I, Grzybek M, Simons K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11411–11416. doi: 10.1073/pnas.1105996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. Critical fluctuations in plasma membrane vesicles. Acs Chemical Biology. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Novicky L, Merzlyakov M, Hristov T, Hristova K. Measuring the Energetics of Membrane Protein Dimerization in Mammalian Membranes. J. Am. Chem. Soc. 2010;132:3628–3635. doi: 10.1021/ja910692u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Placone J, Hristova K. Direct Assessment of the Effect of the Gly380Arg Achondroplasia Mutation on FGFR3 Dimerization Using Quantitative Imaging FRET. PLoS ONE. 2012;7:e46678. doi: 10.1371/journal.pone.0046678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Piccolo N, Placone J, Hristova K. Effect of Thanatophoric Dysplasia Type I Mutations on FGFR3 Dimerization. Biophys. J. 2015;108:272–278. doi: 10.1016/j.bpj.2014.11.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott RE, Maercklein PB. Plasma-Membrane Vesiculation in 3T3-Cells and Sv3T3 Cells .2. Factors Affecting the Process of Vesiculation. J. Cell Sci. 1979;35:245–252. doi: 10.1242/jcs.35.1.245. [DOI] [PubMed] [Google Scholar]
- 17.Scott RE, Perkins RG, Zschunke MA, Hoerl BJ, Maercklein PB. Plasma-Membrane Vesiculation in 3T3-Cells and Sv3T3-Cells .1. Morphological and Biochemical Characterization. J. Cell Sci. 1979;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- 18.Sarabipour S, Hristova K. Glycophorin A transmembrane domain dimerization in plasma membrane vesicles derived from CHO, HEK 293T, and A431 cells. Biochim. Biophys. Acta. 2013;1828:1829–1833. doi: 10.1016/j.bbamem.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holowka D, Baird B. Structural Studies on the Membrane-Bound Immunoglobulin E-Receptor Complex .1. Characterization of Large Plasma-Membrane Vesicles from Rat Basophilic Leukemia-Cells and Insertion of Amphipathic Fluorescent-Probes. Biochemistry. 1983;22:3466–3474. doi: 10.1021/bi00283a025. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochimica et Biophysica Acta-Biomembranes. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui GH, Di Paolo G. Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease. J. Biol. Chem. 2012;287:2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloan Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 24.He L, Horton WA, Hristova K. The physical basis behind achondroplasia, the most common form of human dwarfism. J. Biol. Chem. 2010;285:30103–30114. doi: 10.1074/jbc.M109.094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, Hristova K. Pathogenic activation of receptor tyrosine kinases in mammalian membranes. J. Mol. Biol. 2008;384:1130–1142. doi: 10.1016/j.jmb.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Warnock DE, Roberts C, Lutz MS, Blackburn WA, Young WW, Baenziger JU. Determination of Plasma-Membrane Lipid Mass and Composition in Cultured Chinese-Hamster Ovary Cells Using High-Gradient Magnetic Affinity-Chromatography. J. Biol. Chem. 1993;268:10145–10153. [PubMed] [Google Scholar]
- 27.Payrastre B, Plantavid M, Etievan C, Ribbes G, Carratero C, Chap H, Dousteblazy L. Characterization of Plasma-Membranes from A431 Cells, Isolated by Self-Generating Percoll Gradient - A Rapid Isolation Procedure to Obtain Plasma-Membranes with Functional Epidermal Growth-Factor Receptors. Biochim. Biophys. Acta. 1988;939:355–365. doi: 10.1016/0005-2736(88)90081-8. [DOI] [PubMed] [Google Scholar]
- 28.Oling F, Sopkova-de Oliveira Santos J, Govorukhina N, Mazères-Dubut C, Bergsma-Schutter W, Oostergetel G, Keegstra W, Lambert O, Lewit-Bentley A, Brisson A. Structure of membrane-bound annexin A5 trimers: A hybrid cryo-EM-X-ray crystallography study. J. Mol. Biol. 2000;304:561–573. doi: 10.1006/jmbi.2000.4183. [DOI] [PubMed] [Google Scholar]
- 29.Li E, Placone J, Merzlyakov M, Hristova K. Quantitative measurements of protein interactions in a crowded cellular environment. Anal. Chem. 2008;80:5976–5985. doi: 10.1021/ac800616u. [DOI] [PubMed] [Google Scholar]
- 30.Haigler H, Ash JF, Singer SJ, Cohen S. Visualization by Fluorescence of Binding and Internalization of Epidermal Growth-Factor in Human Carcinoma Cells A-431. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpenter G, King L, Cohen S. Rapid Enhancement of Protein-Phosphorylation in A-431 Cell-Membrane Preparations by Epidermal Growth-Factor. J. Biol. Chem. 1979;254:4884–4891. [PubMed] [Google Scholar]
- 32.Carpenter G, Cohen S. Epidermal Growth-Factor. Annu. Rev. Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Placone J, Novicky L, Hristova K. The extracellular domain of fibroblast growth factor receptor 3 inhibits ligand-independent dimerization. Science Signaling 3. 2010:ra86. doi: 10.1126/scisignal.2001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Placone J, He L, Del Piccolo N, Hristova K. Strong dimerization of wild-type ErbB2/Neu transmembrane domain and the oncogenic Val664Glu mutant in mammalian plasma membranes. Biochim. Biophys. Acta. 2014;1838:2326–2330. doi: 10.1016/j.bbamem.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarabipour S, Hristova K. FGFR3 Transmembrane Domain Interactions Persist in the Presence of Its Extracellular Domain. Biophys. J. 2013;105:165–171. doi: 10.1016/j.bpj.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.