Abstract
During infection invading pathogens must acquire all essential nutrients, including first row transition metals, from the host. To combat invaders, the host exploits this fact and restricts the availability of these nutrients using a defense mechanism known as nutritional immunity. While iron sequestration is the most well-known aspect of this defense, recent work has revealed that the host restricts the availability of other essential elements, notably manganese, during infection. Furthermore, these studies have revealed that the host utilizes multiple strategies that extend beyond metal sequestration to prevent bacteria from obtaining these metals. This review will discuss the mechanisms by which bacteria attempt to obtain the essential first row transition metal ion manganese during infection, and the approaches utilized by the host to prevent this occurrence. In addition, this review will discuss the impact of host-imposed manganese starvation on invading bacteria.
Keywords: ABC transporter, manganese, zinc, nutritional immunity, calprotectin, infection
1. Metal ions in biology
First row transition metals such as iron (Fe), zinc (Zn) and manganese (Mn) are essential for all forms of life. In biological systems between one-third and one-half of all proteins require interaction with a metal ion to facilitate their activity (Andreini et al. 2008). Metal ions predominantly perform either structural roles, such as in Zn-finger DNA-binding proteins, or serve as catalytic centers, i.e., activation roles in proteins. The capacity for metal ions to facilitate cellular chemistry is reflected in the prominence of metalloproteins throughout the six Enzyme Commission classes of proteins (Andreini et al. 2008; Waldron and Robinson 2009). Iron is present in almost every cell in all domains of life and was adopted early in evolution (Dupont et al. 2010). Iron is utilized as a cofactor by numerous proteins and enzymes where it facilitates oxygen evolution, signaling, energy metabolism, and cell replication. Zinc, which in biological systems is present as the divalent cation Zn2+, is important for a variety of cellular process including transcriptional regulation and signaling. More recently, Mn has emerged as a transition metal of significance for its roles in a range of enzymatic processes, including phosphorylation, hydrolysis, carbon metabolism, decarboxylation, and oxidative stress response (Papp-Wallace and Maguire 2006). Manganese, which predominantly occurs as Mn2+ in biological systems, is also increasingly being recognized as having an essential role in facilitating the virulence of numerous pathogenic bacteria.
Even though transition metals are essential for viability, in excess they can mediate significant toxicity. Our current understanding of metal toxicity is almost entirely derived from studies examining the roles and relative contributions of metal homeostatic pathways. While the precise molecular bases for metal toxicity remain unclear, indirect studies indicate that there are four general phenomena associated with metal toxicity. These are the generation of intracellular oxidative stress, genotoxicity, membrane perturbation and the impairment of protein function (Lemire et al. 2013). Metalloproteins are often susceptible to metal toxicity via the acquisition of an inappropriate metal ion, a mechanism also known as mismetallation (Lemire et al. 2013). The bioinorganic chemical basis for this phenomenon is attributable to conformational and dynamic flexibility that result in imperfect selection of metal ions by metal-binding proteins. The interaction of metal ions with proteins also involves consideration of the hard-soft acid-base chemical properties of the metal ions and the coordinating amino acid side chains [for a detailed review see Lippard and Berg (1994)]. However, in general the affinities of metal ions for proteins are significantly governed by the ligand field stabilization energies of the metal ions. As a consequence, this creates a universal order of preference, which for divalent metals is known as the Irving–Williams series: Mg2+/Ca2+ < Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+ (Irving and Williams 1953). It has been proposed, on the basis of cellular analyses of metal concentrations, that the Irving-Williams series underpins the biological abundance of each metal, such that selective metal binding by nascent protein chains can be achieved (Williams 2002; Waldron and Robinson 2009). From the perspective of metal toxicity, the functions of metal-dependent proteins and enzymes are likely to be disrupted if the ability to acquire the correct metal is abrogated due to competition from non-facile metal ions as a result of their over-accumulation.
During infection, pathogenic microorganisms must acquire essential transition metal ions from the host. Vertebrates take advantage of this fact to combat invading microorganisms by restricting their ability to acquire these essential nutrients, a defense known as nutritional immunity (Hood and Skaar 2012). The most well appreciated aspect of nutritional immunity is the sequestration of Fe. However, recently it has become apparent that the host also restricts access to other essential metals such as Mn. There is also a growing body of evidence that sequestration is not the only mechanism used to prevent pathogens from obtaining essential nutrients. This review will discuss the mechanisms utilized by bacteria to acquire Mn during infection, the approaches utilized by the host to restrict access to this essential metal, and the impact of host-imposed Mn starvation on the pathogen during infection.
2. Manganese acquisition by bacteria
In order to obtain Mn from their environment, bacteria express dedicated Mn-import systems. The vast majority of bacteria appear to use Mn-specific transporters that belong to either the ATP-binding cassette (ABC) transporter superfamily or the natural resistance-associated macrophage protein (Nramp) family (Papp-Wallace and Maguire 2006). ABC transporters have a conserved four-domain architecture comprising two cytosolic nucleotide-binding domains that facilitate the binding and hydrolysis of ATP, linked to the two transmembrane domains that form the translocation pore [for a detailed review see Davidson et al. (2008)]. ABC transporters enable unidirectional transport of cargo across the cell membrane, and this has led to the division into two major subgroups, ABC importers and ABC efflux transporters. While ABC efflux transporters are present in all forms of life, ABC importers are almost exclusively found in bacteria. In addition to the ABC transport apparatus, Mn-specific ABC importers also require a high-affinity solute-binding protein (SBP), which scavenges Mn2+ ions from the host environment and delivers them to the transporter (Davidson et al. 2008; Lewis et al. 2012). Together, the ABC importer and its cognate SBP comprise an ABC permease. Nramp-type transporters are comprised of a single multipass membrane protein and facilitate the cotransport of protons and a divalent transition metal (Nevo and Nelson 2006; Papp-Wallace and Maguire 2006). Although there is limited data available on the affinity of Nramp transporters, recent work on the Nramp transporter from Staphylococcus capitis, ScaDMT, showed that it has an affinity (Kd) for Mn2+ of ~ 37 μM (Ehrnstorfer et al. 2014). In addition to these systems, there is a report of a Mn2+-specific P-type ATPase being used by Lactobacillus plantarum to obtain Mn, although additional investigations are needed (Groot et al. 2005). In general, almost all pathogenic bacteria employ at least one high-affinity Mn2+-acquisition pathway to facilitate acquisition of this metal during infection. Regardless of the mechanism utilized to acquire Mn, studies in numerous bacteria including Streptococcus pneumoniae, Streptococcus pyogenes, as well as other streptococci, Neisseria gonorrhoeae, Staphylococcus aureus and Salmonella species have demonstrated the critical importance of these systems to bacterial pathogenesis (Berry and Paton 1996; Horsburgh et al. 2002; Marra et al. 2002; Janulczyk et al. 2003; Paik et al. 2003; McAllister et al. 2004; Lim et al. 2008; Wichgers Schreur et al. 2011; Kehl-Fie et al. 2013).
Manganese-specific ABC permeases have a prominent role in the virulence of the notable major human pathogens Streptococcus pneumoniae, Streptococcus pyogenes, S. suis, S. aureus and Salmonellae species (Berry and Paton 1996; Horsburgh et al. 2002; Marra et al. 2002; Janulczyk et al. 2003; Paik et al. 2003; McAllister et al. 2004; Lim et al. 2008; Wichgers Schreur et al. 2011; Zheng et al. 2011; Kehl-Fie et al. 2013). Specificity of the ABC permeases is dictated by the SBP as, in general, the ABC transporter component of the permease does not contain internal binding sites for their cargo (Oldham et al. 2007; Davidson et al. 2008; Lewis et al. 2012). The SBPs are extra-cytosolic, ligand-binding proteins that function as receptors and bind to specific cargoes, delivering them to a cognate ABC transporter (Higgins 1992; Lewis et al. 2012) (Figure 1). In Gram-negative bacteria, SBPs are found in the periplasm and are often referred to as periplasmic-binding proteins (Neu and Heppel 1965; Adler 1975). In Gram-positive microorganisms, SBPs are tethered to the cell membrane via an N-terminal lipid moiety (Gilson et al. 1988; Sutcliffe and Russell 1995). Irrespective of whether they occur in Gram-negative or Gram-positive organisms, Mn-binding SBPs belong to the Cluster A-I (formerly Cluster IX) subgroup of ABC transporter-associated SBPs. Cluster A-I SBPs have a conserved fold, comprising a two-lobed organization with N- and C-terminal (β/α)4-domains bisected by a cleft in which the metal ion binding site is formed. The metal-binding site is completely buried under the protein surface upon ligand binding (Counago et al. 2012; Lewis et al. 2012). Cluster A-I SBPs are involved in the acquisition of Mn2+, Fe2+ or Zn2+ (Lawrence et al. 1998; Lee et al. 1999; Lee et al. 2002; Banerjee et al. 2003; Li and Jogl 2007; Loisel et al. 2008; Sun et al. 2009; Ilari et al. 2011; McDevitt et al. 2011; Couñago et al. 2014). In general, the binding sites of cluster A-I SBPs are defined by four coordinating ligands. Regardless of the metal ion imported, the same types of ligands are found in three of the four positions: two Nε2 atoms from absolutely conserved histidine residues and a carboxylate group from an aspartate or a glutamate residue. The fourth position has been postulated to be the key to receptor metal ion specificity. For Zn2+-specific SBPs, the ‘soft’ Nε2 atom from a histidine residue occupies this position (Banerjee et al. 2003; Chandra et al. 2007; Li and Jogl 2007; Ilari et al. 2011). By contrast, in Mn2+-specific SBPs a carboxylate group from a glutamate residue occupies this position (Adir et al. 2002; McDevitt et al. 2011; Gribenko et al. 2013). Crucially, the carboxylate groups can contribute one (monodentate) or two (bidentate) ligands each to metal ion coordination. Hence, the two carboxylate groups increase the coordination nature of the binding site in Mn2+-specific SBPs (up to 6), and this has been shown to be essential for Mn2+ binding, which has a preferred coordination number of 6. By contrast, the lower coordination number of the binding sites of Zn2+-specific SBPs (up to 5) is sufficient for Zn2+ binding, which has preferred coordination number of 4. Although other features also influence metal ion coordination, the nature of the amino acid side chains at the metal-binding sites have a major role in determining the metal specificity of ABC permeases in recruiting their cognate ions, with high affinity, from the host environment during colonization.
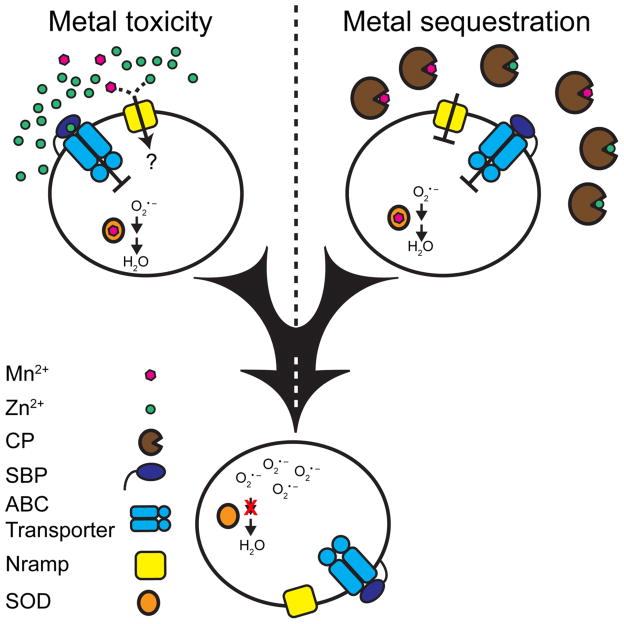
Figure 1. The two routes for host-mediated manganese starvation.
Cartoon representation of how the host imposes Mn starvation. The top left shows Mn2+ and Zn2+ competing with each other for the SBP of a Mn2+-specific ABC permease mismetallated by a Zn2+ ion. This competition inhibits the ABC transporter function and leads to Mn starvation in organisms that only express an ABC type Mn2+- acquisition system. Top right shows calprotectin sequestering and removing Mn and Zn from the local environment, starving the bacteria for these metals. The bottom shows the impact of Mn deprivation on a model bacterial cell. This starvation inactivates Mn-dependent superoxide dismutases resulting in increased sensitivity to oxidative stress. The two arrows indicate the two steps involved in detoxifying superoxide, the conversion to hydrogen peroxide by superoxide dismutase and subsequent conversion to water by other cellular factors. The red X indicates inactivation of the first step in this process during Mn starvation. This starvation also inactivates other unidentified essential bacterial processes as well. Inactivation of these processes may be mediated by a reduction in absolute intracellular metal levels or by altered metal ratios that lead to increased metalloprotein mismetallation.
In contrast to the carefully poised intracellular concentration of metals, bacteria are unable to exert control over the extracellular environment and, as such, the prevailing abundances can result in mismetallation and inhibition of extracellular metalloproteins. Recent studies investigating PsaA, the SBP of the S. pneumoniae Mn-specific ABC permease, have revealed that the SBPs of Mn2+-specific ABC transporters are acutely sensitive to mismetallation (McDevitt et al. 2011; Couñago et al. 2014; Eijkelkamp et al. 2014). This susceptibility arises from the presence of the N-ligands in the binding sites of Mn2+-specific SBPs, which makes them permissive for also binding other metal ions, such as Zn2+. These factors result in a protein that binds metal ions other than Mn, such as those at the higher end of the Irving-Williams stability series, e.g. Zn2+, with greater stability. Although both Mn2+ and Zn2+ have zero crystal field stabilization energies, the smaller ionic radius of Zn2+ leads to a general increase in metal-complex stability relative to Mn2+ (Ma et al. 2009). The significance of this has been shown by the ability of Zn2+ to compete for interaction with Mn2+-specific SBPs and thereby block Mn2+-uptake (McDevitt et al. 2011; Eijkelkamp et al. 2014). Thus, Zn2+ and other divalent cations are capable of competing for interaction with Mn2+-specific SBPs and can abrogate Mn acquisition via mismetallation of the SBP. In S. pneumoniae, which relies on PsaA for Mn acquisition and virulence, elevated extracellular Zn:Mn ratios prevent Mn acquisition and inhibit bacterial growth (Figure 1) (McDevitt et al. 2011; Eijkelkamp et al. 2014). These studies have revealed a potential weakness of Mn-specific ABC transporters that may be exploited by the host during infection.
3. Host mechanisms for withholding Mn from bacterial pathogens
3(a). Host sequestration of Mn
While Fe sequestration is the best characterized component of nutritional immunity, it has become apparent that Mn and Zn are also withheld by the host to combat invading pathogens during infection (Corbin et al. 2008; Kehl-Fie and Skaar 2010; Kehl-Fie et al. 2013). Human Nramp-1 removes Mn from the phagolysosome (Papp-Wallace and Maguire 2006). A series of studies using a combination of Nramp-1-deficient mice and bacteria lacking Mn uptake systems have shown that removing Mn from the phagolysosome contributes to controlling Salmonella Typhimurium infection (Janakiraman and Slauch 2000; Boyer et al. 2002; Zaharik et al. 2004; Papp-Wallace and Maguire 2006). More recently, the application of advanced elemental imaging modalities, which produce two-dimensional maps of metal distribution within tissue samples, revealed that Mn and Zn restriction occurred on a larger scale during infection than previously appreciated. In particular, laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) revealed that staphylococcal abscesses are rendered virtually devoid of detectable Mn and Zn ions (Corbin et al. 2008; Kehl-Fie et al. 2013). Notably, while the staphylococcal abscess is rendered devoid of these metal ions, there is little or no change in the total Mn and Zn content of the organ (Kehl-Fie et al. 2013). Subsequent investigations revealed that a critical component of this nutrient withholding response is the Mn and Zn binding protein calprotectin (CP) (Corbin et al. 2008; Kehl-Fie et al. 2011). This innate immune effector protein constitutes approximately 50% of the total protein in the neutrophil cytoplasm and can be present at sites of infection in excess of 1 mg.ml−1 (Clohessy and Golden 1995; Gebhardt et al. 2006). Additionally, epithelial cells can be induced by proinflammatory cytokines, such as interleukin-22, to produce CP (Blaschitz and Raffatellu 2010). LA-ICP-MS revealed that CP-deficient mice have defects in Mn sequestration during staphylococcal infection (Corbin et al. 2008). The loss of Mn sequestration closely correlates with increased staphylococcal burdens during infection. In addition to S. aureus, CP-deficient mice are also more susceptible to a range of bacterial and fungal pathogens, including Acinetobacter baumanii, Klebsiella pneumoniae, Candida albicans and Aspergillus nidulans (Urban et al. 2009; Bianchi et al. 2011; Achouiti et al. 2012; Hood et al. 2012). In vitro CP inhibits the growth of a wide range of pathogenic microorganisms, including both fungi and bacteria, by restricting their ability to acquire Mn and Zn (Figure 1) (Corbin et al. 2008; Urban et al. 2009; Bianchi et al. 2011; Kehl-Fie et al. 2011; Achouiti et al. 2012; Hood et al. 2012; Damo et al. 2013).
The crucial importance of restricting Mn bioavailability has been demonstrated by in vivo experiments using staphylococcal Mn uptake mutants and CP-deficient murine models of infection. S. aureus expresses two Mn2+ importers, MntABC, an ABC permease and, MntH, an Nramp family member (Horsburgh et al. 2002; Kehl-Fie et al. 2013). MntC is a cluster A-I SBP associated with the Mn2+-specific ABC transporter (MntAB) and binds Mn2+ with an affinity of ~4 nM (Gribenko et al. 2013). A staphylococcal mutant lacking both acquisition systems (ΔmntCΔmntH) is twice as sensitive to CP-mediated Mn starvation as wild-type S. aureus (Kehl-Fie et al. 2013). Both MntABC and MntH have been shown to contribute to staphylococcal infection (Horsburgh et al. 2002; Kehl-Fie et al. 2013; Diep et al. 2014). In a systemic infection model, mice infected with a ΔmntCΔmntH mutant lose significantly less weight than wild-type mice and have lower bacterial burdens in the kidneys and liver (Kehl-Fie et al. 2013). However, the livers of CP-deficient mice have comparable bacterial burdens when infected with either wild type S. aureus or the ΔmntCΔmntH mutant (Kehl-Fie et al. 2013). As CP-deficient mice do not sequester Mn away from staphylococcal liver abscesses (Corbin et al. 2008), this latter finding indicates that, in the absence of Mn sequestration, the Mn abundance of liver tissue is sufficient to support the full virulence of S. aureus. This result also further highlights the crucial importance of restricting Mn bioavailability during infection and the ongoing struggle between host and pathogen for this metal. Surprisingly, these studies also revealed that the kidneys of CP-deficient mice do not have increased bacterial burdens (Kehl-Fie et al. 2013). This result is explained by the subsequent observation that loss of CP does not prevent the host from removing Mn from staphylococcal kidney abscesses (Kehl-Fie et al. 2013). As CP is found in both kidney and liver abscesses, this result indicates that the host possesses redundant organ-specific mechanisms for sequestering Mn (Corbin et al. 2008; Kehl-Fie et al. 2013). This latter observation also highlights the importance of directly assessing Mn levels at sites of infection.
CP is a member of the S100 EF-hand family of calcium binding proteins. While members of this family are typically homodimers, CP is a heterodimer of S100A8 and S100A9. In addition to being a heterodimer, CP differs from other S100 proteins in that it has two non-identical transition metal binding sites as a result of its heterodimeric nature (Korndorfer et al. 2007; Kehl-Fie et al. 2011). Typical transition metal binding S100 proteins, such as S100A7 and S100A12, contain two identical binding sites, each composed of three histidines and one aspartic acid residue. Two of the histidines come from one monomer and are arranged in an HXXXH motif, while the third histidine and the aspartic acid are contributed by the other monomer (Brodersen et al. 1999; Moroz et al. 2003; Moroz et al. 2009). The first site of CP, based on homology with other S100 proteins, was originally predicted to be comprised of H17 and H27 from S100A8 and H91 and H95 from S100A9 (Korndorfer et al. 2007; Kehl-Fie et al. 2011). However, subsequent investigations, discussed below, revealed that two additional histidines (H103 and H105) from S100A9 also contribute to transition metal binding (Damo et al. 2013). The second site resembles the canonical transition metal binding site present in other S100 proteins and is comprised of H83 and H87 from S100A8 and H20 and D30 from S100A9 (Korndorfer et al. 2007; Kehl-Fie et al. 2011). Unexpectedly, initial investigations revealed that CP bound two Zn2+ ions but only one Mn2+ ion tightly (Kehl-Fie et al. 2011).
Subsequent studies have found that Site I binds both Mn2+ and Zn2+ tightly (subsequently referred to as the Mn/Zn site), while Site II only binds Zn2+ tightly (subsequently referred to as the Zn site) (Brophy et al. 2012; Damo et al. 2013; Hayden et al. 2013). High-resolution structural studies have also revealed that in addition to the four predicted histidine residues, the Mn/Zn site contains two additional histidine residues, H103 and H105, contributed by the C-terminal extension of S100A9 (Damo et al. 2013). The C-terminal extension is unique to S100A9 amongst the S100 proteins, and all other S100 proteins studied to date do not bind Mn2+ (Brophy et al. 2013; Damo et al. 2013). The six histidine residues bind Mn2+ with near perfect octahedral geometry, with the tail of S100A9 wrapping around the Mn2+ ion (Damo et al. 2013). Constructs lacking residues H103 and H105 but otherwise possessing an intact Mn/Zn site do not bind Mn2+ tightly, confirming their crucial importance in binding of this metal (Brophy et al. 2013; Damo et al. 2013). The same variants, however, retain the ability to bind two Zn2+ atoms tightly (Damo et al. 2013). The hexahistidine coordination represents a new mode of Mn2+ binding that has not been previously reported for proteins. Subsequent EPR-based studies have confirmed the initial finding that the tail of CP has a crucial role in the high-affinity binding of Mn2+ and facilitating the octahedral coordination of the metal ion (Brophy et al. 2013).
Isothermal titration calorimetry (ITC) indicated that CP binds Mn2+ and Zn2+ with affinities (Kd) of 10 nM or less (Kehl-Fie et al. 2011; Damo et al. 2013; Lisher and Giedroc 2013). Later dye competition studies revealed that the Zn site binds Zn2+ with a Kd of less than 10 pM, while the Mn/Zn site has an affinity of less than 240 pM (Brophy et al. 2012). The lack of available techniques for measuring high affinity Mn2+ binding has hampered attempts to obtain a more precise affinity, and other groups have observed weaker Mn2+ binding than the low/subnanomolar affinities observed by ITC (Hayden et al. 2013). However, the in vitro Mn2+-binding affinities obtained by ITC are consistent with the ability of CP to abrogate Mn acquisition by bacteria expressing Mn2+-specific ABC permeases, which also bind Mn2+ with low nanomolar or subnanomolar affinities (McDevitt et al. 2011; Gribenko et al. 2013; Lisher and Giedroc 2013; Abate et al. 2014). Interestingly, the affinity of CP for Mn2+ and Zn2+ is influenced by calcium availability (Brophy et al. 2012; Hayden et al. 2013). In the absence of calcium the affinity of CP for Mn2+ decreases to ~ 5 μM and the Kd for Zn increases to ~133 pM for the Zn site and ~219 nM for the Mn/Zn site (Brophy et al. 2012; Hayden et al. 2013). The calcium dependence of transition metal binding is likely due to the location of the Mn/Zn and Zn sites at the end of α-helices that are predicted to become elongated upon calcium binding (Hsu et al. 2009). Relative to the extracellular space, the host cell cytoplasm is calcium-deplete. Thus, it seems highly probable that the calcium-dependent metal binding functions as a switching mechanism (Brophy et al. 2012; Hayden et al. 2013) that prevents CP from binding Mn2+ and Zn2+ ions until secreted into the calcium rich extracellular space.
A major challenge to studying the impact of nutritional immunity is the ability to specifically withhold individual metals in a biologically relevant manner. While much has been learned through the use of chemical chelators, their use has significant caveats. They are frequently membrane permeable, have off target cellular effects, and bind more than one metal (Shumaker et al. 1998; Graham et al. 2009; Veyrier et al. 2011). The generation of CP variants with altered metal binding properties has allowed the individual impact of Mn and Zn sequestration to be evaluated using a highly specific and biologically relevant molecule. Similar to wild-type CP, the staphylococcal ΔmntCΔmntH mutant is twice as sensitive as wild-type S. aureus to the ΔZn site mutant, which retains Mn binding. However, the ΔmntCΔmntH mutant and wild-type S. aureus are equally sensitive to the ΔMn/Zn site mutant, which cannot bind Mn (Damo et al. 2013; Kehl-Fie et al. 2013). This result demonstrates that these CP variants can be used to interrogate the respective contributions of Mn and Zn starvation to host defense. Utilization of these reagents revealed that both Mn and Zn sequestration contribute to the antimicrobial activity of CP. Surprisingly, these investigations also revealed that maximal antimicrobial activity towards a wide range of pathogens, including S. aureus, S. epidermidis, Enterococcus faecalis, Pseudomonas aeruginosa, Acinetobacter baumanii, and Shigella flexneri, requires Mn2+ sequestration (Damo et al. 2013). This observation further emphasizes the importance of restricting the ability of pathogens to acquire Mn during infection.
3(b). Metal intoxication as a mechanism to starve invaders
Zinc is the second most abundant transition element in humans and is of critical importance for immune development and function (Haase and Rink 2009). Zn deficiency results in compromised immune defense and an increased susceptibility to infection (Haase and Rink 2009). Although severe Zn deficiency is rare, mild to moderate Zn deficiency affects about 2 billion people worldwide (Prasad 2003). Diseases associated with Zn deficiency account for ~800,000 deaths per year (1.4% of global mortalities) making it the 11th greatest risk factor for global burden of disease (Guilbert 2003; WHO 2003). Bacterial infections associated with Zn deficiency are predominantly acute respiratory and enteric infections (i.e., pneumonia or diarrhea) (Zaidi et al. 2009). Clinical trials of Zn supplementation therapies have shown that the morbidity and mortality of pneumococcal pneumonia and enteric disease can be significantly reduced (Bhutta et al. 1999; Lassi et al. 2010; Valavi et al. 2011). The observation that Zn2+ irreversibly binds to the SBP of Mn2+-specific importers and prevent acquisition of this metal has led to the proposition that Zn may not only promote proper immune development but also contribute to nutritional immunity (McDevitt et al. 2011; Gribenko et al. 2013; Abate et al. 2014; Couñago et al. 2014; Eijkelkamp et al. 2014).
Analyses of tissue metal ion concentrations have revealed that in niches colonized by S. pneumoniae, Zn concentrations increased by more than 3-fold during infection, whereas almost all other transition metals had negligible changes (McDevitt et al. 2011). These changes in extracellular metal ion abundance closely correlate with alterations in the expression profiles of the metal ion uptake pathways of S. pneumoniae (McDevitt et al. 2011; Eijkelkamp et al. 2014; Plumptre et al. 2014). Notably, the observed in vivo Zn:Mn ratios were sufficient to inhibit Mn2+ uptake via PsaBCA and inhibit the in vitro growth of S. pneumoniae (McAllister et al. 2004; Eijkelkamp et al. 2014). More recent studies have also implicated Zn2+ mobilization as a major component of phagolysosomal killing of bacterial pathogens within immune effector cells. Here, the immune cell depletes essential metal ions, notably Mn2+ and Fe2+, by active efflux, while importing ‘toxic’ ions such as Zn2+ and Cu2+ (Jabado et al. 2000; Cellier et al. 2007; White et al. 2009; Botella et al. 2011). Recent in vitro studies of S. pyogenes M1T1 have shown Zn co-localized with the bacterial pathogen within human neutrophils (Ong et al. 2014). Although the precise molecular mechanism of bacterial killing by these immune effector cells has not been elucidated, the bacteria could be rescued by depleting the host cell of Zn using chemical chelators (Ong et al. 2014). This finding represents an additional way in which local changes in metal abundance may be manipulated by the host to starve bacteria, and thereby aid their clearance. Collectively, there is a growing body of evidence suggesting that the host actively manipulates local Zn concentrations, in turn altering the Zn2+ to Mn2+ ratio in an attempt to prevent bacteria from obtaining host Mn.
4. The impact of manganese starvation on bacterial pathogens
Whether by removing it from sites of infection or using the toxicity of other metals, it is clear that preventing pathogens from acquiring Mn is an important aspect of nutritional immunity. Given the numerous processes that Mn is involved in, restricting access to this metal has the potential to disrupt many processes in invading microbes. However, the specific Mn-dependent processes that are disrupted by this host defense are largely unknown. This uncertainty stems from difficulties in predicting metal dependency a priori and the ability of many metal-dependent enzymes to be activated by multiple metals in vitro and in vivo. This latter observation is exemplified by E. coli, which replaces Fe with Mn when experiencing oxidative stress in order to preserve enzyme function and reduce the formation of hydroxyl radicals via Fenton chemistry (Anjem et al. 2009; Sobota and Imlay 2011; Imlay 2013). While the specific processes that are disrupted by host-imposed metal starvation are largely unknown, they can be divided into two categories: those that are essential for bacterial viability and those that are non-essential, such as virulence factors. Although the specific factors that are disrupted by host-imposed Mn limitation are unknown, the reduced antimicrobial activity of the ΔMn/Zn site variant of CP indicates that essential processes are inhibited by host-meditated Mn sequestration (Damo et al. 2013; Kehl-Fie et al. 2013). Similarly, studies employing S. pneumoniae have demonstrated that Zn2+ toxicity inhibits Mn-dependent processes that are essential for viability (McDevitt et al. 2011; Eijkelkamp et al. 2014). Potential essential processes that may be disrupted by host-imposed Mn-starvation include enzymes involved in central metabolism, cell wall synthesis and DNA synthesis (Keele et al. 1970; Andreini et al. 2008; Ogunniyi et al. 2010; Wu et al. 2010; Cotruvo and Stubbe 2011; Aguirre and Culotta 2012).
By contrast, considerable advances have been made in elucidating the virulence factors that are inhibited by host-imposed Mn starvation. Studies utilizing S. pneumoniae and S. aureus have investigated the impact of Mn sequestration and Zn inhibition of Mn uptake on the ability to resist oxidative stress and neutrophil-mediated killing (Figure 1). In the case of S. aureus, Mn sequestration by CP inhibits the activity of the two superoxide dismutases (SOD) expressed by the bacterium, both of which are reported to be Mn-dependent (Clements et al. 1999; Valderas and Hart 2001; Kehl-Fie et al. 2011; Damo et al. 2013; Kehl-Fie et al. 2013). This, in turn, results in increased sensitivity to chemically-induced oxidative stress and neutrophil-mediated killing (Kehl-Fie et al. 2011; Damo et al. 2013; Kehl-Fie et al. 2013). Infection experiments utilizing SOD mutants and CP-deficient mice revealed that staphylococcal SOD activity is inhibited by host-imposed Mn starvation during infection (Kehl-Fie et al. 2011). Similarly, toxic levels of Zn2+ reduce pneumococcal SOD activity and render S. pneumoniae more sensitive to oxidative stress and neutrophil-mediated killing (McDevitt et al. 2011; Eijkelkamp et al. 2014). For both S. aureus and S. pneumoniae, inhibition of SOD activity does not explain the general growth defect observed in the presence of CP or toxic levels of Zn, as SOD mutants in both organisms have minimal if any growth defects in the absence of stress (Yesilkaya et al. 2000; Karavolos et al. 2003; Kehl-Fie et al. 2011; Eijkelkamp et al. 2014). It seems likely that in addition to inhibition of SOD activity other virulence factors may also be disrupted by host-imposed Mn starvation, however these systems have yet to be identified.
5. Conclusions
Nutritional immunity has long been recognized as a critical defense utilized by the host to combat invading microorganisms. Recently, the scope of nutrients sequestered has evolved beyond the ‘Fe-withholding’ response of the host to now include other transition metal ions, notably Mn and Zn. Simultaneously, it has become apparent that the host can also leverage the chemical properties of metals, such as Zn, to aid in mediating nutrient starvation. However, despite the imposition of metal restriction on invaders, bacteria remain capable of causing devastating disease. This indicates that successful pathogens must have specific adaptations that allow them to circumvent the impact of host-imposed metal starvation.
The increasing prevalence and spread of antibiotic resistance has lead the Centers for Disease Control and World Health Organization to state that the end of the antibiotic era is upon us and call for the development of new approaches to treat infectious diseases (CDC 2013; WHO 2014). Elucidating how successful pathogens circumvent nutritional immunity has the potential to identify new therapeutic targets, the disruption of which will enhance the efficacy of the innate immune response. Essential to developing these new therapies is a comprehensive understanding of the temporal and spatial aspects of how the host utilizes metal abundance, i.e., sequestration or intoxication, as weapons against invasive bacterial pathogens. From the perspective of the pathogen, elucidation of the mechanisms that mitigate host-mediated metal stress, i.e., nutrient restriction and protein mismetallation, will potentially reveal new targets for novel antimicrobials.
Acknowledgments
We apologize to our colleagues whose work we were unable to cite due to length restrictions. J.R.M. is supported by an Australian Postgraduate Award. This work was supported by the Australian Research Council grants DP120103957 and DP150104515 to C.A.M., the National Health & Medical Research Council Project grants 1022240 and 1080784 to C.A.M., and a K22 AI104805 from the National Institutes of Health as well as by Research Grant No. 5-FY15-30 from the March of Dimes Foundation to T.E.K..
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
This work is solely the responsibility of the authors and does not reflect the views of the National Institutes of Health.
References
- Abate F, Malito E, Cozzi R, Lo Surdo P, Maione D, Bottomley MJ. Apo, Zn2+-bound and Mn2+-bound structures reveal ligand-binding properties of SitA from the pathogen Staphylococcus pseudintermedius. Biosci Rep. 2014;34(6):e00154. doi: 10.1042/BSR20140088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achouiti A, et al. Myeloid-related protein-14 contributes to protective immunity in Gram-negative pneumonia derived sepsis. PLoS Pathog. 2012;8(10):e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adir N, Rukhman V, Brumshtein B, Anati R. Preliminary X-ray crystallographic analysis of a soluble form of MntC, a periplasmic manganese-binding component of an ABC-type Mn transporter from Synechocystis sp. PCC 6803. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 9):1476–1478. doi: 10.1107/S0907444902010922. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975:44341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13(8):1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72(4):844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Wei B, Bhattacharyya-Pakrasi M, Pakrasi HB, Smith TJ. Structural determinants of metal specificity in the zinc transport protein ZnuA from Synechocystis 6803. J Mol Biol. 2003;333(5):1061–1069. doi: 10.1016/j.jmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Berry AM, Paton JC. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64(12):5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta ZA, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators’ Collaborative Group. J Pediatr. 1999;135(6):689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127(5):1243–1252. e1247. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30(2):196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella H, et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10(3):248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70(11):6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen DE, Nyborg J, Kjeldgaard M. Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2+-bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2+-free states. Biochemistry. 1999;38(6):1695–1704. doi: 10.1021/bi982483d. [DOI] [PubMed] [Google Scholar]
- Brophy MB, Hayden JA, Nolan EM. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc. 2012;134(43):18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy MB, Nakashige TG, Gaillard A, Nolan EM. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc. 2013;135(47):17804–17817. doi: 10.1021/ja407147d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Antibiotic resistance threats in the United States, 2013. Center for Disease Control and Prevention; 2013. [Google Scholar]
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9(14–15):1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Chandra BR, Yogavel M, Sharma A. Structural analysis of ABC-family periplasmic zinc binding protein provides new insights into mechanism of ligand uptake and release. J Mol Biol. 2007;367(4):970–982. doi: 10.1016/j.jmb.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MO, Watson SP, Foster SJ. Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J Bacteriol. 1999;181(13):3898–3903. doi: 10.1128/jb.181.13.3898-3903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy PA, Golden BE. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42(5):551–556. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Corbin BD, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319(5865):962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Cotruvo JA, Stubbe J. Escherichia coli class Ib ribonucleotide reductase contains a dimanganese(III)-tyrosyl radical cofactor in vivo. Biochemistry. 2011;50(10):1672–1681. doi: 10.1021/bi101881d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counago RM, McDevitt CA, Ween MP, Kobe B. Prokaryotic substrate-binding proteins as targets for antimicrobial therapies. Curr Drug Targets. 2012;13(11):1400–1410. doi: 10.2174/138945012803530170. [DOI] [PubMed] [Google Scholar]
- Couñago RM, et al. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10(1):35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- Damo SM, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110(10):3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72(2):317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, et al. Identifying potential therapeutic targets of methicillin-resistant Staphylococcus aureus through in vivo proteomic analysis. J Infect Dis. 2014;209(10):1533–1541. doi: 10.1093/infdis/jit662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont CL, Butcher A, Valas RE, Bourne PE, Caetano-Anolles G. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc Natl Acad Sci U S A. 2010;107(23):10567–10572. doi: 10.1073/pnas.0912491107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol. 2014;21(11):990–996. doi: 10.1038/nsmb.2904. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp BA, Morey JR, Ween MP, Ong CL, McEwan AG, Paton JC, McDevitt CA. Extracellular zinc competitively inhibits manganese uptake and compromises oxidative stress management in Streptococcus pneumoniae. PLoS One. 2014;9(2):e89427. doi: 10.1371/journal.pone.0089427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72(11):1622–1631. doi: 10.1016/j.bcp.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Gilson E, Alloing G, Schmidt T, Claverys JP, Dudler R, Hofnung M. Evidence for high affinity binding-protein dependent transport systems in Gram-positive bacteria and in Mycoplasma. EMBO J. 1988;7(12):3971–3974. doi: 10.1002/j.1460-2075.1988.tb03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AI, et al. Severe zinc depletion of Escherichia coli: roles for high affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J Biol Chem. 2009;284(27):18377–18389. doi: 10.1074/jbc.M109.001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribenko A, et al. Three-dimensional structure and biophysical characterization of Staphylococcus aureus cell surface antigen-manganese transporter MntC. J Mol Biol. 2013;425(18):3429–3445. doi: 10.1016/j.jmb.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Groot MN, Klaassens E, de Vos WM, Delcour J, Hols P, Kleerebezem M. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology. 2005;151(Pt 4):1229–1238. doi: 10.1099/mic.0.27375-0. [DOI] [PubMed] [Google Scholar]
- Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16(2):230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Hayden JA, Brophy MB, Cunden LS, Nolan EM. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc. 2013;135(2):775–787. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992:867–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hood MI, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012;8(12):e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44(5):1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- Hsu K, et al. Anti-infective protective properties of S100 calgranulins. Antiinflamm Antiallergy Agents. Med Chem. 2009;8(4):290–305. doi: 10.2174/187152309789838975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilari A, Alaleona F, Petrarca P, Battistoni A, Chiancone E. The X-ray structure of the zinc transporter ZnuA from Salmonella enterica discloses a unique triad of zinc-coordinating histidines. J Mol Biol. 2011;409(4):630–641. doi: 10.1016/j.jmb.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11(7):443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving H, Williams RJP. The stability of transition-metal complexes. J Chem Soc. 1953:3192–3210. [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35(5):1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Janulczyk R, Ricci S, Bjorck L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun. 2003;71(5):2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149(Pt 10):2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- Keele BB, Jr, McCord JM, Fridovich I. Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970;245(22):6176–6181. [PubMed] [Google Scholar]
- Kehl-Fie TE, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10(2):158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14(2):218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, et al. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun. 2013;81(9):3395–3405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370(5):887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- Lassi ZS, Haider BA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2010;12:CD005978. doi: 10.1002/14651858.CD005978.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MC, Pilling PA, Epa VC, Berry AM, Ogunniyi AD, Paton JC. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure. 1998;6(12):1553–1561. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Lee YH, Deka RK, Norgard MV, Radolf JD, Hasemann CA. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat Struct Biol. 1999;6(7):628–633. doi: 10.1038/10677. [DOI] [PubMed] [Google Scholar]
- Lee YH, Dorwart MR, Hazlett KR, Deka RK, Norgard MV, Radolf JD, Hasemann CA. The crystal structure of Zn(II)-free Treponema pallidum TroA, a periplasmic metal-binding protein, reveals a closed conformation. J Bacteriol. 2002;184(8):2300–2304. doi: 10.1128/JB.184.8.2300-2304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- Lewis VG, Ween MP, McDevitt CA. The role of ATP-binding cassette transporters in bacterial pathogenicity. Protoplasma. 2012;249(4):919–942. doi: 10.1007/s00709-011-0360-8. [DOI] [PubMed] [Google Scholar]
- Li H, Jogl G. Crystal structure of the zinc-binding transport protein ZnuA from Escherichia coli reveals an unexpected variation in metal coordination. J Mol Biol. 2007;368(5):1358–1366. doi: 10.1016/j.jmb.2007.02.107. [DOI] [PubMed] [Google Scholar]
- Lim KH, et al. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun. 2008;76(8):3569–3576. doi: 10.1128/IAI.01725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippard SJ, Berg JM. Principles of bioinorganic chemistry. University Science Books; California: 1994. [Google Scholar]
- Lisher JP, Giedroc DP. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol. 2013:391. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel E, et al. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J Mol Biol. 2008;381(3):594–606. doi: 10.1016/j.jmb.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109(10):4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A, Lawson S, Asundi JS, Brigham D, Hromockyj AE. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology. 2002;148(Pt 5):1483–1491. doi: 10.1099/00221287-148-5-1483. [DOI] [PubMed] [Google Scholar]
- McAllister LJ, Tseng HJ, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53(3):889–901. doi: 10.1111/j.1365-2958.2004.04164.x. [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 2011;7(11):e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz OV, et al. Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr. 2003;59(Pt 5):859–867. doi: 10.1107/s0907444903004700. [DOI] [PubMed] [Google Scholar]
- Moroz OV, Blagova EV, Wilkinson AJ, Wilson KS, Bronstein IB. The crystal structures of human S100A12 in apo form and in complex with zinc: new insights into S100A12 oligomerisation. J Mol Biol. 2009;391(3):536–551. doi: 10.1016/j.jmb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Neu HC, Heppel LA. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240(9):3685–3692. [PubMed] [Google Scholar]
- Nevo Y, Nelson N. The NRAMP family of metal-ion transporters. Biochim Biophys Acta. 2006;1763(7):609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Ogunniyi AD, et al. Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J Bacteriol. 2010;192(17):4489–4497. doi: 10.1128/JB.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450(7169):515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis. 2014;209(10):1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol. 2003;185(20):5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006:60187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Plumptre CD, et al. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol Microbiol. 2014;91(4):834–851. doi: 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc deficiency. BMJ. 2003;326(7386):409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker DK, Vann LR, Goldberg MW, Allen TD, Wilson KL. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium. 1998;23(2–3):151–164. doi: 10.1016/s0143-4160(98)90114-2. [DOI] [PubMed] [Google Scholar]
- Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A. 2011;108(13):5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Baker HM, Ge R, Sun H, He QY, Baker EN. Crystal structure and metal binding properties of the lipoprotein MtsA, responsible for iron transport in Streptococcus pyogenes. Biochemistry. 2009;48(26):6184–6190. doi: 10.1021/bi900552c. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IC, Russell RR. Lipoproteins of Gram-positive bacteria. J Bacteriol. 1995;177(5):1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavi E, Hakimzadeh M, Shamsizadeh A, Aminzadeh M, Alghasi A. The efficacy of zinc supplementation on outcome of children with severe pneumonia. A randomized double-blind placebo-controlled clinical trial. Indian J Pediatr. 2011;78(9):1079–1084. doi: 10.1007/s12098-011-0458-1. [DOI] [PubMed] [Google Scholar]
- Valderas MW, Hart ME. Identification and characterization of a second superoxide dismutase gene (sodM) from Staphylococcus aureus. J Bacteriol. 2001;183(11):3399–3407. doi: 10.1128/JB.183.11.3399-3407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrier FJ, Boneca IG, Cellier MF, Taha MK. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog. 2011;7(9):e1002261. doi: 10.1371/journal.ppat.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7(1):25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO Position Paper - Streptococcus pneumoniae. WER. 2003:78110–118. [Google Scholar]
- WHO. Antimicrobial Resistance Global Report on Surveillance. World Health Organization; 2014. [Google Scholar]
- Wichgers Schreur PJ, Rebel JM, Smits MA, van Putten JP, Smith HE. TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol. 2011;193(19):5073–5080. doi: 10.1128/JB.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ. The fundamental nature of life as a chemical system: the part played by inorganic elements. J Inorg Biochem. 2002;88(3–4):241–250. doi: 10.1016/s0162-0134(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Wu HJ, et al. Manganese regulation of virulence factors and oxidative stress resistance in Neisseria gonorrhoeae. J Proteomics. 2010;73(5):899–916. doi: 10.1016/j.jprot.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun. 2000;68(5):2819–2826. doi: 10.1128/iai.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, et al. The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72(9):5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi AK, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J. 2009;28(1 Suppl):S10–18. doi: 10.1097/INF.0b013e3181958769. [DOI] [PubMed] [Google Scholar]
- Zheng B, et al. Insight into the interaction of metal ions with TroA from Streptococcus suis. PLoS One. 2011;6(5):e19510. doi: 10.1371/journal.pone.0019510. [DOI] [PMC free article] [PubMed] [Google Scholar]