Abstract
Background
Levels of lipoprotein(a), Lp(a), a genetically regulated independent cardiovascular risk factor present in humans and Old World monkeys, are impacted by the apolipoprotein(a), apo(a), gene. Allele-specific apo(a) levels, taking both the apo(a) genotypic and phenotypic characteristics into account, are useful markers to determine atherosclerotic cardiovascular risk.
Methods
We determined (1) the genetic variability of apo(a), (2) Lp(a) levels, and (3) allele-specific apo(a) levels in rhesus monkeys (n=95).
Results
Lp(a) levels differed substantially between animals (range: 4–247 nmol/L) with a skewed distribution towards lower levels. Lp(a) and allele-specific apo(a) levels were inversely related to the number of apo(a) Kringle 4 (K4) repeats. The median apo(a) size was 23 K4 repeats and the prevalence of a small size apo(a) (≤22 K4) was 43%.
Conclusions
Distribution of Lp(a) and allele-specific apo(a) levels in rhesus monkeys reflected the corresponding human patterns, but with a high prevalence of smaller apo(a) sizes.
Keywords: apo(a) phenotype, K4 repeat, nonhuman primate, Old World monkey
INTRODUCTION
Over the last decades, an elevated level of plasma Lipoprotein (a), Lp(a), has emerged as an important clinical risk factor for the development of atherosclerotic cardiovascular diseases (CVD) such as coronary heart disease, myocardial infarction, peripheral arterial disease, and ischemic stroke [9, 11, 12, 16, 31]. Recent studies using Mendelian randomization design have provided support for the causative role of Lp(a) in CVD [7, 14, 15]. Despite these advancements and recognition in clinical guidelines [30], the underlying mechanisms for an atherogenic role, as well as metabolic properties and any potential physiological functions of Lp(a), remain poorly understood.
Lp(a) consists of a cholesteryl-ester rich lipid core and one molecule each of two different apolipoproteins, apoB-100 and apo(a). Apo(a) represents an extensive size polymorphism (i.e., copy number variation) due to a variable number of repeated loop structures, referred to as Kringles (K) [5, 27, 35]. In general, smaller apo(a) sizes with fewer number of K repeats are associated with higher Lp(a) levels and considered more atherogenic than larger apo(a) sizes [12]. Yet, there is considerable variability in Lp(a) levels for any given apo(a) size [25, 26, 28, 33]. To better understand Lp(a) genotype/phenotype relations in humans, allele-specific apo(a) levels have been used to gain insights into the risks associated with Lp(a) [1, 2, 10, 31–33]. In the majority of individuals, the plasma Lp(a) level represents the sum of two different Lp(a) populations with likely different size apo(a) particles. The relative contribution of each apo(a) size isoform to the overall Lp(a) level may vary substantially depending on the dominance pattern of apo(a), placing individuals at a variable degree of risk. Thus, Lp(a)-associated cardiovascular risk for two individuals with the same plasma Lp(a) level and carrying the same size apo(a) can differ substantially. For example, individuals who have smaller apo(a) alleles as their dominating apo(a) will have more Lp(a) particles with atherogenic smaller apo(a) sizes in their plasma (i.e., higher allele-specific apo(a) levels with smaller apo(a) sizes) and are consequently at a greater Lp(a)-associated CVD risk compared to individuals who have more Lp(a) particles with less-atherogenic larger apo(a) sizes (i.e., higher allele-specific apo(a) levels with larger apo(a) sizes).
The apo(a) gene is thought to have evolved from the plasminogen (PLG) gene, which has five K domains (K1 through K5), during primate evolution, although the evolutionary aspects of the size variability in the apo(a) gene are not well understood. Notably, Lp(a) has a limited species distribution, and is exclusively found in humans, apes, and Old World monkeys, including rhesus monkey (Macaca mulatta) [23, 35] and in a deviant form in the European hedgehog [19]. The human apo(a) contains two K domains, K4 and K5 [27, 36], whereas the rhesus monkey apo(a) lacks the K5 domain [34]. The protease domain of the PLG gene is preserved in both humans and rhesus monkey apo(a), although considered inactive due to a Ser561-Ile562 substitution for Arg561-Val562 [27]. In contrast, apo(a) in the European hedgehog consists of multiple K3-like structures with an attached protease domain reflecting its independent evolution from Lp(a) in human and nonhuman primates [19]. There is limited knowledge about rhesus monkey Lp(a) and apo(a) properties, one of the few species with the potential to serve as a precision model for humans with regard to Lp(a). Characterizing size polymorphism, i.e., copy number variation in the nonhuman primate apo(a) gene not only will enhance understanding of the evolutionary history of these species, but will also help to advance our general knowledge of the potential functional and evolutionary significance of human copy number variations. Therefore, in the current study, we determined the genetic variability of apo(a) (i.e., number of K4 repeats), expressed as apo(a) isoforms together with Lp(a) and allele-specific apo(a) levels in a large number of rhesus monkeys within a broad age spectrum.
MATERIALS AND METHODS
Humane Care Guidelines
All animal procedures conformed to the requirements of the current edition of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act, and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Blood samples (2–3 mL) were collected from rhesus monkeys (Macaca mulatta) (n=95) including infants and juveniles (8 months to 3 years, n=8), young adults (4 to 6 years, n=14), mature adults (7 to 18 years, n=70) and aged adults (19 to 22 years, n=3). Samples were collected from sedated animals (ketamine hydrochloride ~10 mg/kg) after an overnight fast and placed into glass tubes with EDTA for assay (see below). Activities related to animal care (e.g., diet and housing) were performed according to Primate Center standard operating procedures. Animals are fed commercial monkey chow twice daily and are supplemented with fruit or vegetables twice weekly. Water is provided by automatic lixits and a variety of enrichment options are available daily.
Measurement of plasma Lp(a) level
Plasma samples from rhesus monkeys were separated and stored in aliquots at ≤−80°C prior to analysis. Plasma Lp(a) levels were measured by an apo(a) size-insensitive sandwich ELISA (Mercodia Inc., Uppsala, Sweden) [8], and the interassay coefficient of variation was consistently less than 10%.
Determinations of apo(a) isoform size, apo(a) dominance pattern, and allele-specific apo(a) level
Apo(a) isoform sizes were determined by Western blotting technique with sodium dodecyl sulfate-agarose gel electrophoresis of plasma samples, followed by immonoblotting as previously described [18, 33]. The protein isoform dominance pattern was assessed as described [2, 33], where animals with two different apo(a) isoforms were classified into three groups (larger isoform dominating, smaller isoform dominating, and co-dominating). Allele-specific apo(a) levels in the monkeys were determined as previously reported for humans [33]. Briefly, for each of the protein bands, Lp(a) levels were apportioned according to the degree of the intensity of the bands on the Western blot, using 10% increments. For example, an animal with an apo(a) level of 40 nmol/L, carrying 15 and 31 K4 repeats, with the smaller protein dominating by 90%, had 36 nmol/L apportioned to the 15 and 4 nmol/L to the 31 K4 repeat protein.
Statistical analysis
Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL). Results were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). Lp(a) and allele-specific apo(a) levels were square root transformed to achieve normal distributions. Group means were compared using the Student’s t-test. Univariate relationships between Lp(a), allele-specific apo(a) levels, and apo(a) size variability was assessed by the Pearson correlation coefficients. Both the larger and smaller apo(a) isoforms of monkeys expressing two distinguishable bands and one isoform of monkeys with a single expressed isoform were considered for statistical analyses. An analysis of variance and post hoc analysis with the Bonferroni test were performed for three or more independent samples. All analyses were two-tailed, and p-values less than 0.05 were considered statistically significant.
RESULTS
Lp(a) level and distribution pattern
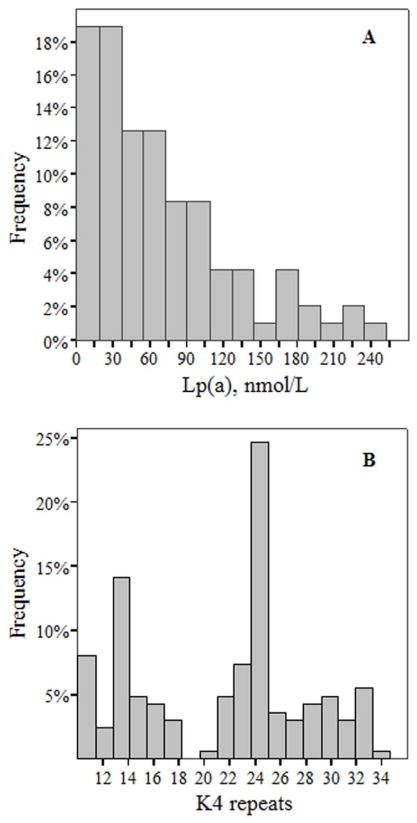
Plasma Lp(a) levels in rhesus monkeys ranged between 4 nmol/L to 247 nmol/L with a median level of 54 nmol/L (IQR: 22–97 nmol/L) (Table 1). The prevalence of animals with Lp(a) levels ≥72 nmol/L (~30 mg/dL), a commonly accepted cut-off point for elevated Lp(a) level, was 37%. As seen in Figure 1A, the distribution of plasma Lp(a) level was broad and skewed towards the lower level. Analysis of results across the rhesus monkey age groups indicated that plasma Lp(a) did not differ significantly between the age groups (Table 2), although Lp(a) levels tended to be elevated in the young and mature adult groups. When analyzed within each age group, plasma Lp(a) or allele-specific apo(a) levels were not correlated with body weight (p>0.3).
Table 1.
Lp(a) levels and apo(a) isoform distribution and dominance patterns
| Characteristics | Number/Relative frequency |
|---|---|
| Number, n | 95 |
| Females, n (%) | 83 (87%) |
| Lp(a), nmol/L | |
| 25th percentile | 22 |
| 50th percentile (median) | 54 |
| 75th percentile | 97 |
| Prevalence of Lp(a) level ≥72 nmol/L1 | 35 (37%) |
| Apo(a) distribution and dominance patterns2 | |
| No isoform, n (%) | 0 (0%) |
| Single isoform, n (%) | 28 (29%) |
| Double isoform, n (%) | 67 (71%) |
| Larger dominating | 6 (9%) |
| Co-dominating | 30 (45%) |
| Smaller dominating | 31 (47%) |
| Prevalence of small size apo(a) ≤22 K4 | 43% |
Lp(a) levels in nmol/L can be converted to mg/dL by use of a conversion factor of 2.4 nmol/L = 1 mg/dL [3]. A concentration 72 nmol/L corresponds to approximately 30 mg/dL.
Apo(a) isoform sizes were determined using Western blotting technique as described in Methods. Apo(a) isoform dominance pattern was assessed as previously described [16].
Figure 1. Distribution of plasma Lp(a) level (A) and apo(a) isoform size (B).
The histogram of plasma Lp(a) level shows a broad and skewed distribution towards lower level (Panel A). The histogram of apo(a) sizes shows a range of apo(a) sizes (10 to 35 K4 repeats) with two distinctive frequency peaks at 14 and 24 K4 repeats (Panel B). Apo(a) isoform size was determined using Western blotting technique as described [18, 33].
Abbreviations: K, Kringle; Lp(a), lipoprotein(a)
Table 2.
Characteristics of rhesus monkeys across age groups
| Characteristics | Infants/Juveniles | Adults
|
||
|---|---|---|---|---|
| Young | Mature | Aged | ||
| Number, n (%) | 8 (8%) | 14 (15%) | 70 (74%) | 3 (3%) |
| Female, n (%) | 5 (63%) | 13 (93%) | 63 (90%) | 2 (67%) |
| Age (years) | 2.0 ± 1.1 | 5.1 ± 0.4 | 11.0 ± 3.2 | 20.1 ± 1.3 |
| Body weight (kg) | 3.0 ± 1.5 | 6.6 ± 2.2 | 8.5 ± 2.2 | 8.1 ± 1.4 |
| Lipoprotein(a), nmol/L | 32 (23–82) | 58 (29–72) | 55 (23–108) | 21 (11–49) |
| Allele-specific apo(a), nmol/L | 18 (13–36) | 34 (9–57) | 32 (14–73) | 15 (6–27) |
| Kringle 4 repeats | 23 (15–25) | 22 (14–25) | 24 (14–26) | 26 (21–28) |
Data are reported as mean ± SD for age and body weight, and median (IQR) for Lp(a), allele-specific apo(a), and K4 repeats. Relative frequencies are shown in parentheses for numbers and females. Infants/Juveniles (8 months to 3 years), Young adults (4 to 6 years), Mature adults (7 to 18 years), and Aged adults (19 to 22 years). Allele-specific apo(a) level reflects the amount of Lp(a) associated with each individual protein isoform as described in the Methods section.
Apo(a) isoform distribution and dominance patterns
As shown in Figure 1B, the apo(a) size ranged between 10 to 35 K4 repeats in rhesus monkeys with two distinctive frequency peaks at 14 and 24 K4 repeats. The median apo(a) size was 23 K4 repeats, and the presence of small apo(a) sizes (≤22 K4 repeats), previously shown to be associated with CVD in humans [4], was 43% (Table 1). Furthermore, there was no significant difference in the number of K4 repeats between the four age groups (Table 2).
To characterize Lp(a) genotype/phenotype relations in more detail, we next analyzed the frequency of animals with double, single, or no circulating apo(a) protein isoforms. As noted in Table 1, at least one apo(a) isoform was detected in each animal. Thus, there were no animals lacking expressed apo(a) protein isoforms. Seventy-one percent of animals expressed double protein isoforms on Western blots. The frequency of dominating apo(a) isoforms was 9%, 45%, and 47% for larger, co-dominating (as assessed by a similar expression level of both protein isoforms) and smaller apo(a) isoforms, respectively. A representative image of a Western blot displaying the apo(a) dominance pattern is shown in Figure 2.
Figure 2. Determination of apo(a) dominance pattern by Western blotting.
Western blotting was done as described previously [18, 33]. This representative image of apo(a) Western blots from four animals demonstrates a varying apo(a) dominance pattern. For animals #1 and #3, the smaller apo(a) isoform is dominating (the expression level for the smaller allele is greater than that for the larger allele), whereas for animal #4, the larger isoform is dominating. For animal #2, both the larger and smaller alleles are expressed at a similar level, indicating a co-dominance pattern.
Abbreviations: Apo(a), apolipoprotein(a), K, Kringle number; M, Marker (apo(a) isoform size); L, larger allele dominating, S, smaller allele dominating, Co, Co-dominating alleles
Apo(a) size polymorphism and Lp(a) and allele-specific apo(a) levels
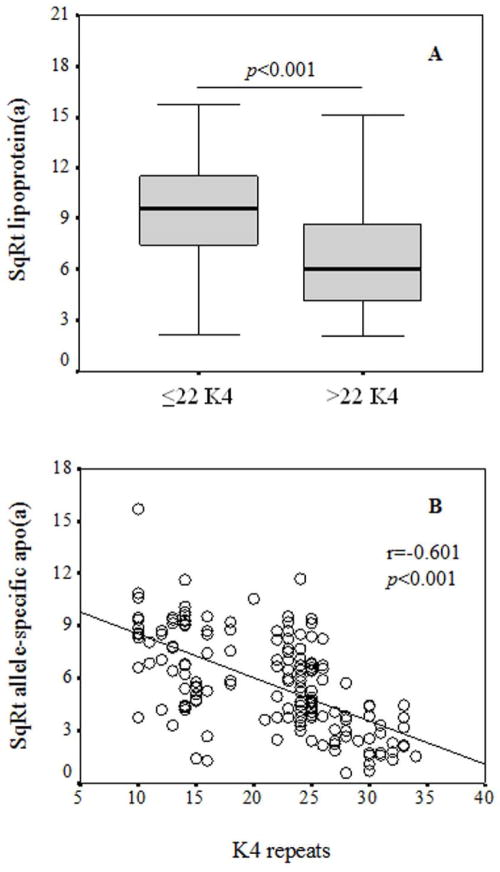
Lp(a) levels were significantly higher in rhesus monkeys carrying smaller apo(a) sizes (≤22 K4 repeats) compared to rhesus monkeys carrying larger apo(a) sizes (>22 K4 repeats) (Figure 3A, p<0.001). There was an inverse correlation between apo(a) size (number of K4 repeats) and allele-specific apo(a) levels (p<0.001) (Figure 3B). Furthermore, variability in allele-specific apo(a) levels for any given apo(a) size was observed. The median allele-specific apo(a) level was 29 nmol/L (IQR: 14–66 nmol/L) in all animals, and the levels did not differ significantly across age groups (Table 2).
Figure 3. Relationship between Lp(a) (A) and allele-specific apo(a) levels (B) with apo(a) isoform sizes.
The box plots show the median and interquartile range of the Lp(a) levels for animals carrying smaller (≤22 K4 repeats) and larger apo(a) sizes (>22 K4 repeats), respectively (Panel A). The median Lp(a) level was significantly elevated in animals carrying smaller apo(a) sizes compared to animals carrying larger apo(a) sizes. The relationship between the number of K4 repeats and square root transformed allele-specific apo(a) levels are described by Pearson’s correlation coefficients (Panel B). Allele-specific apo(a) levels were inversely associated with the number of K4 repeats (i.e., apo(a) sizes).
Abbreviations: K, Kringle; SqRt, square root
DISCUSSION
In contrast to humans where a wealth of information on Lp(a) and apo(a) properties can be found, limited information is available on Old World monkey Lp(a) and apo(a). In the present study, we characterized the distributions of both Lp(a) and allele-specific apo(a) levels and apo(a) size heterogeneity as a reference to humans in a relatively large number of rhesus monkeys within a broad age spectrum. Our findings therefore contribute to enhance our general understanding of Lp(a) and apo(a) evolution across primate populations.
Overall, our findings demonstrated that the rhesus monkey Lp(a) properties and apo(a) genetic variability closely reflected the previously reported corresponding patterns in humans. Thus, as in humans [5], there was extensive inter-animal variability in plasma Lp(a) levels with a broad and skewed distribution towards lower levels. A considerable proportion of animals had plasma Lp(a) levels ≥72 nmol/L, a generally accepted cut-off point for increased Lp(a) levels in humans. The apo(a) isoform distribution in rhesus monkeys was heterogeneous, and similar to humans [31] was inversely associated with allele-specific apo(a) levels. In addition, consistent with our previous observations in humans [31], allele-specific apo(a) levels exhibited a large variability for any given apo(a) size in rhesus monkeys. Further, the findings of this study confirm and extend prior observations of Lp(a) and apo(a) in this species [23, 24, 29]. An important observation was that the frequency of small size apo(a) (≤22 K4) was higher in rhesus monkeys compared to previously reported frequency in humans (43% versus 25–35%) [17]. Among humans, this phenotypic pattern has been associated with increased cardiovascular risk. A recent meta-analysis of 40 studies on Lp(a) indicated a two-fold increased risk of coronary heart disease in subjects carrying smaller apo(a) isoforms versus subjects carrying larger apo(a) isoforms [12]. This risk estimation indicates that Lp(a) with small size apo(a) is the strongest single genetic risk factor for CVD known to date, and a 25% to 35% prevalence of small apo(a) isoforms in the population poses high clinical relevance. Given the high frequency of small size apo(a), studies in rhesus monkeys can provide a host of opportunities to investigate atherogenic properties of apo(a). This provides several advantages over transgenic mice [3, 6, 20, 22] and rabbit [13] models representing a single or a very limited spectrum of apo(a) alleles/isoforms (versus ~40 different isoforms in humans). As apo(a) is a major predictor of plasma Lp(a) levels, the variability in Lp(a) concentration in mouse and rabbit models is considerably more limited than in natural settings. In humans, up to 1000-fold difference in plasma Lp(a) levels have been reported [5].
In the current study, we were able to detect 24 different sized apo(a) isoforms in rhesus monkeys with the largest apo(a) size being 35 K4 repeats (versus >40 K4 repeats in humans). A previous study by Neven at al. [29] that included 19 rhesus monkeys with and without LDL receptor deficiency identified six apo(a) phenotypes and found a wide variation in Lp(a) levels for any given apo(a) phenotype. Furthermore, Makino at el. [24] reported at least five different apo(a) isoforms among 13 rhesus monkeys originating from the same group described [29]. The prevalence of a single apo(a) protein band was approximately 25% in the latter study, which was closely aligned with our findings (29%). Lp(a) levels in rhesus monkeys ranged from 1–58 mg/dL, and 42% (8 of 19) exhibited Lp(a) levels >30 mg/dL in the study of Neven et al. [29]. In our study, the rhesus monkey Lp(a) levels ranged between 4–247 nmol/L (1.7–103 mg/dL), and the prevalence of animals with Lp(a) levels >30 mg/dL was 37%. In a smaller study, Makino et al. reported a slightly lower prevalence of Lp(a) levels >30 mg/dL (23%, 3 of 13 animals) [24].
Lp(a) is a primary carrier of proinflammatory and proatherogenic oxidized phospholipids (OxPL) in the circulation, and Lp(a) particles with smaller apo(a) sizes contain a greater amount of OxPL than those with larger apo(a) sizes [4]. Thus, an enhanced content of OxPL suggests that small size apo(a) are even more proinflammatory and proatherogenic. A previous study by Bergmark et al. [4] reported K5 as the site for OxPL binding in human apo(a), and that rhesus monkey apo(a) lacking K5 had no immunoreactivity to an antibody specific for the phosphocholine headgroup of OxPL. However, a recent study suggested that the presence of the K5 domain was not necessary for OxPL binding, and that an intact lysine-binding site on K4 type 10 was crucial for the binding of OxPL to Lp(a) [21].
The current study has some limitations as we did not have apo(a) genotyping data in rhesus monkeys and did not assess the prevalence of homozygotes and unexpressed apo(a) allele distributions. However, we phenotyped apo(a) in every animal. A relatively large number of monkeys within a wide age range allowed us to comprehensively characterize the distributions of Lp(a) levels, apo(a) isoform sizes and dominance patterns, as well as the prevalence of high Lp(a) levels and small atherogenic apo(a) isoforms to an extent that has not previously been reported. In addition, for the first time, we have determined allele/isoform-specific apo(a) levels in rhesus monkeys. Based on allele-specific apo(a) levels among humans, we have previously shown a greater predictive power of Lp(a) levels combined with apo(a) isoform sizes for coronary artery disease risk assessment [31]. Furthermore, although we did not observe significant differences in both Lp(a) and allele-specific apo(a) levels across age groups in rhesus monkeys, these levels tended to be lower in the younger animals and the more mature adults. We recognize that the number of animals in some age groups was relatively modest, thus further studies are needed to explore a potential effect of age (or sex) on Lp(a) in rhesus monkeys.
In summary, we determined apo(a) genetic variability, Lp(a), and allele-specific apo(a) levels in rhesus monkeys. Our findings demonstrate similarities in Lp(a) and apo(a) properties in rhesus monkeys and humans, but also a smaller apo(a) size spectrum and a higher frequency of atherogenic smaller apo(a) in this species. Overall, the findings underscore cross-species similarities and support the usefulness of this nonhuman primate model for studies to better understand Lp(a) and apo(a) properties relevant to human health and disease.
Acknowledgments
Funding:
These studies were supported by the National Institutes of Health (NIH) UC Davis Clinical and Translational Center (CTSC) base operating grant (#TR000002, #RR024146), NIH National Heart, Lung, and Blood Institute (NHLBI) grants (#HL062705, #HL085794), and the California National Primate Research Center base operating grant (#OD011107). Dr. Enkhmaa Byambaa is a recipient of the UC Davis CTSC Highly Innovative Pilot Award and the Mentored Clinical and Population Research Program Award from the American Heart Association (#14CRP17930014), and is a current Building Interdisciplinary Research Careers in Women’s Health/K12 Training Program scholar (#NIH 2K12HD051958). Adnan Abbuthalha participated as a trainee in the UC Davis CTSC Highly Innovative Pilot Award.
These studies were supported by the UC Davis Clinical and Translational Science Center (NIH grant (#TR000002, #RR024146), NIH National Heart, Lung, and Blood Institute (NHLBI) grants (#HL062705, #HL085794), and the base operating grant of the California National Primate Research Center (#OD011107).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors who have taken part in this study declare that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.Anuurad E, Lu G, Rubin J, Pearson TA, Berglund L. ApoE genotype affects allele-specific apo[a] levels for large apo[a] sizes in African Americans: the Harlem-Basset Study. J Lipid Res. 2007;48:693–8. doi: 10.1194/jlr.M600431-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Anuurad E, Rubin J, Chiem A, Tracy RP, Pearson TA, Berglund L. High Levels of Inflammatory Biomarkers Are Associated with Increased Allele-Specific Apolipoprotein (a) Levels in African-Americans. J Clin Endocrinol Metab. 2008;93:1482–8. doi: 10.1210/jc.2007-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg K, Svindland A, Smith AJ, Lawn RM, Djurovic S, Alestrom A, Alestrom P, Eliassen K. Spontaneous atherosclerosis in the proximal aorta of LPA transgenic mice on a normal diet. Atherosclerosis. 2002;163:99–104. doi: 10.1016/s0021-9150(01)00772-9. [DOI] [PubMed] [Google Scholar]
- 4.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–9. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90:52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callow MJ, Stoltzfus LJ, Lawn RM, Rubin EM. Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc Natl Acad Sci U S A. 1994;91:2130–4. doi: 10.1073/pnas.91.6.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 8.Dembinski T, Nixon P, Shen G, Mymin D, Choy PC. Evaluation of a new apolipoprotein(a) isoform-independent assay for serum Lipoprotein(a) Mol Cell Biochem. 2000;207:149–55. doi: 10.1023/a:1007079223546. [DOI] [PubMed] [Google Scholar]
- 9.Dieplinger B, Lingenhel A, Baumgartner N, Poelz W, Dieplinger H, Haltmayer M, Kronenberg F, Mueller T. Increased serum lipoprotein(a) concentrations and low molecular weight phenotypes of apolipoprotein(a) are associated with symptomatic peripheral arterial disease. Clin Chem. 2007;53:1298–305. doi: 10.1373/clinchem.2007.088013. [DOI] [PubMed] [Google Scholar]
- 10.Enkhmaa B, Anuurad E, Zhang W, Abbuthalha A, Li XD, Dotterweich W, Pollard RB, Asmuth DM, Berglund L. HIV disease activity as a modulator of lipoprotein(a) and allele-specific apolipoprotein(a) levels. Arterioscler Thromb Vasc Biol. 2013;33:387–92. doi: 10.1161/ATVBAHA.112.300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, Danesh J. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010:2160–7. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Shimoyamada H, Sun H, Marcovina S, Honda K, Watanabe T. Transgenic rabbits expressing human apolipoprotein(a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler Thromb Vasc Biol. 2001;21:88–94. doi: 10.1161/01.atv.21.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–7. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1732–41. doi: 10.1161/ATVBAHA.112.248765. [DOI] [PubMed] [Google Scholar]
- 16.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 18.Lackner C, Cohen JC, Hobbs HH. Molecular definition of the extreme size polymorphism in apolipoprotein(a) Hum Mol Genet. 1993;2:933–40. doi: 10.1093/hmg/2.7.933. [DOI] [PubMed] [Google Scholar]
- 19.Laplaud PM, Beaubatie L, Rall SC, Jr, Luc G, Saboureau M. Lipoprotein[a] is the major apoB-containing lipoprotein in the plasma of a hibernator, the hedgehog (Erinaceus europaeus) J Lipid Res. 1988;29:1157–70. [PubMed] [Google Scholar]
- 20.Lawn RM, Wade DP, Hammer RE, Chiesa G, Verstuyft JG, Rubin EM. Atherogenesis in transgenic mice expressing human apolipoprotein(a) Nature. 1992;360:670–2. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- 21.Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis E, Witztum JL, Koschinsky ML, Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–30. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linton MF, Farese RV, Jr, Chiesa G, Grass DS, Chin P, Hammer RE, Hobbs HH, Young SG. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a) J Clin Invest. 1993;92:3029–37. doi: 10.1172/JCI116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino K, Abe A, Maeda S, Noma A, Kawade M, Takenaka O. Lipoprotein(a) in nonhuman primates. Presence and characteristics of Lp(a) immunoreactive materials using anti-human Lp(a) serum. Atherosclerosis. 1989;78:81–5. doi: 10.1016/0021-9150(89)90161-5. [DOI] [PubMed] [Google Scholar]
- 24.Makino K, Noma A, Scanu AM. Rhesus monkey lipoprotein (a). Relationship between Apo(a) isoforms and Lp(a) density in plasma. Ann N Y Acad Sci. 1995;748:603–5. [PubMed] [Google Scholar]
- 25.Marcovina SM, Albers JJ, Jacobs DR, Jr, Perkins LL, Lewis CE, Howard BV, Savage P. Lipoprotein(a) concentrations and apolipoprotein(a) phenotypes in Caucasians and African Americans. The CARDIA study. Arterioscler Thromb. 1993;13:1037–45. doi: 10.1161/01.atv.13.7.1037. [DOI] [PubMed] [Google Scholar]
- 26.Marcovina SM, Albers JJ, Wijsman E, Zhang Z, Chapman NH, Kennedy H. Differences in Lp(a) concentrations and apo(a) polymorphs between black and white Americans. J Lipid Res. 1996;37:2569–85. [PubMed] [Google Scholar]
- 27.McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, Scanu AM, Lawn RM. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–7. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 28.Mooser V, Scheer D, Marcovina SM, Wang J, Guerra R, Cohen J, Hobbs HH. The Apo(a) gene is the major determinant of variation in plasma Lp(a) levels in African Americans. Am J Hum Genet. 1997;61:402–17. doi: 10.1086/514851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neven L, Khalil A, Pfaffinger D, Fless GM, Jackson E, Scanu AM. Rhesus monkey model of familial hypercholesterolemia: relation between plasma Lp[a] levels, apo[a] isoforms, and LDL-receptor function. J Lipid Res. 1990;31:633–43. [PubMed] [Google Scholar]
- 30.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen A European Atherosclerosis Society Consensus P. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–24. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 32.Rubin J, Kim HJ, Pearson TA, Holleran S, Ramakrishnan R, Berglund L. Apo(a) size and PNR explain African American-Caucasian differences in allele-specific apo(a) levels for small but not large apo(a) J Lipid Res. 2006;47:982–9. doi: 10.1194/jlr.M500359-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Rubin J, Paultre F, Tuck CH, Holleran S, Reed RG, Pearson TA, Thomas CM, Ramakrishnan R, Berglund L. Apolipoprotein (a) genotype influences isoform dominance pattern differently in African Americans and Caucasians. J Lipid Res. 2002;43:234–44. [PubMed] [Google Scholar]
- 34.Tomlinson JE, McLean JW, Lawn RM. Rhesus monkey apolipoprotein(a). Sequence, evolution, and sites of synthesis. J Biol Chem. 1989;264:5957–65. [PubMed] [Google Scholar]
- 35.Utermann G. The mysteries of lipoprotein(a) Science. 1989;246:904–10. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 36.van der Hoek YY, Wittekoek ME, Beisiegel U, Kastelein JJ, Koschinsky ML. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum Mol Genet. 1993;2:361–6. doi: 10.1093/hmg/2.4.361. [DOI] [PubMed] [Google Scholar]