Abstract
Introduction
Several treatment options for clinically localized prostate cancer currently exist under established guidelines. We aim to assess nationally-representative trends in treatment over time and determine potential geographic variation using two large national claims registries.
Methods
Men with prostate cancer insured by Medicare (1998–2006) or a private insurer (Ingenix database, 2002–2006) were identified using ICD-9 and CPT-4 codes. Geographic variation and trends in the type of treatment utilized over time were assessed. Geographic data was mapped using the GeoCommons online mapping platform. Predictors of any treatment were determined using a hierarchical generalized linear mixed model using the logit link function.
Results
The use of radical prostatectomy (RP) increased, 33% to 48%, in the privately insured i3 database, while remaining stable at 12% in the Medicare population. There was a rapid uptake in the use of newer technologies over time in both the Medicare and i3 cohorts. The use of laparoscopic assisted prostatectomy increased from 1% in 2002 to 41% in 2006 in i3 patients, while the incidence increased from 3% in 2002 to 35% in 2006 for Medicare patients. The use of neoadjuvant/adjuvant androgen deprivation therapy (ADT) was lower in the i3 cohort and has decreased over time in both i3 and Medicare. Physician density had an impact on type of primary treatment received in the New England region, however, this trend was not seen in the Western or Southern regions of the United States.
Conclusion
Using two large national claims registries, we have demonstrated trends over time and substantial geographic variation in the type of primary treatment used for localized prostate cancer. Specifically, there has been a large increase in the use of newer technologies, (i.e. laparoscopic-assisted prostatectomy and IMRT). These results elucidate the need for improved data collection on prostate cancer treatment outcomes to reduce unwarranted variation in care.
Introduction
Prostate cancer is estimated to account for nearly a third of all incident cancers in men.1 With many therapeutic options available, localized prostate cancer treatment patterns are quite diverse. Some of this variation in treatment is likely explained by a lack of sufficient evidence to suggest a greater benefit of one treatment approach over another.2,3
Variation in treatment attributable to local practice site has been shown to range from 13% for primary androgen deprivation therapy to 74% for cryotherapy.4 This suggests factors other than cancer risk and patient characteristics may influence treatment decisions.5 Furthermore, advances in both radiation and surgical technology have also led to changes in treatment patterns over time.
The aim of the current study was to confirm nationally representative trends in the use of primary treatment for localized prostate cancer in two large claims-based registries representing men across the age spectrum, and assess demographic, geographic, and clinical predictors of treatment.
Methods
Using the Medicare claims database, 77,216 men were identified with the diagnosis of prostate cancer between 1998 and 2006. Men were identified by having an International Classification of Diseases (ICD-9, 9th revision) code of 185 and a Current Procedural Terminology code (CPT-4, 4th edition) for either a prostate biopsy or transurethral resection of the prostate within 180 days of the ICD-9 code date. Men were excluded if they were < 66 years of age at the time of diagnosis (n=10,157), not continuously covered by Medicare Part A&B (n=6,753), had Medicare managed care coverage (n=4,171), had underwent primary orchiectomy (n=1,073), and/or primary chemotherapy (n=90). After exclusion criteria, 54,322 men remained in the final Medicare analysis.
The i3 database (Ingenix, Salt Lake City, UT), a subsidiary of UnitedHealth Group, is a large medical claims database providing information on privately insured individuals. The i3 database contains clinical information on patients from all 50 states and includes demographic characteristics, diagnoses and procedures performed via ICD-9 and CPT codes as well as radiologic and laboratory tests. We had access to data from 2002 through 2006 where 63,150 men were identified with prostate cancer using the previously mentioned ICD-9 and CPT-4 codes. Men were excluded if they had discontinuous insurance coverage from 6 months before to 18 months following primary treatment (n=33,909), or treated primarily with orchiectomy (n=109) or chemotherapy (n=41). Thus, 16,161 men were included in the i3 database analysis.
Primary forms of surgical therapy included retropubic radical prostatectomy (RRP), perineal radical prostatectomy (PRP), and laparoscopic (+/− robot assist) radical prostatectomy (LRP). Primary radiotherapy included external-beam radiation therapy (EBRT), intensity-modulated radiation therapy (IMRT), brachytherapy (BT), and cyberknife/proton beam therapy. Other primary treatments included cryotherapy, primary androgen deprivation monotherapy (PADT) and watchful waiting/active surveillance (WW/AS). These treatments were assigned using CPT codes identified in the 180 days following the index diagnosis. Those men assigned to WW/AS were required to have a CPT code for prostate biopsy, transrectal ultrasound, or PSA 30 to 180 days from their index diagnosis date.
Demographic data available for both cohorts included age, race, place of residence, and primary treatment. Differences between the two cohorts were assessed with Pearson chi-square methods. Summary statistics were used to assess differences in utilization of primary treatment. Geographic variation in radical prostatectomy, radiation therapy, and primary ADT utilization by urologist and radiation oncologist density was mapped in three distinct regions of the United States: California, New England, and Florida using the GeoCommons online mapping platform. These regions were selected due to the data being most robust in these locations and also being distinct geographic regions of the country.
To estimate the fixed effects of various clinical and demographic predictors of primary treatment, a hierarchical generalized linear mixed model using the logit link function accounting for the random effects of the counties was used to account for correlation of the county level data. Only counties with 30 or more prostate cancer patients were included in the final model to eliminate treatment outliers due to low volume counties. The model outcome was primary treatment of any type. All other analyses were performed using SAS (SAS Institute, Cary, NC) and Stata (STATA, College Station, TX) statistical software.
Results
Table 1 compares patient demographics between the two cohorts. By nature of age-based eligibility requirements, Medicare patients were older at diagnosis (mean age 75 vs. 64 in i3 men). The predominant race in both Medicare and i3 patients was Caucasian. A plurality of patients in both cohorts resided in Southern states. As expected given the age distribution, i3 patients received surgery at a higher proportion than Medicare patients, where as Medicare patients received more radiation.
Table 1.
Baseline patient demographics of I3 and Medicare sample.
| Medicare (%) |
Ingenix (%) |
p-value | |
|---|---|---|---|
| Age at diagnosis: | <0.01 | ||
| Mean Age (SD) | 75 (6.1) | 64 (10.1) | |
| <60 | N/A | 5,600 (35) | |
| 60–64 | N/A | 3,577 (22) | |
| 65–69 | 11,532 (21) | 2,222 (14) | |
| 70–74 | 16,517 (30) | 1,935 (12) | |
| 75 or older | 26,273 (49) | 2,827 (17) | |
| Race: | <0.01 | ||
| White | 47,313 (87) | 11,202 (69) | |
| Black | 4,841 (9) | 807 (5) | |
| Asian | 547 (1) | 183 (1) | |
| Hispanic | 1,072 (2) | 627 (4) | |
| Other | 493 (1) | 84 (1) | |
| Unknown | 56 (0) | 3,258 (20) | |
| Region of Residence: | <0.01 | ||
| Northeast | 10,849 (20) | 2,094 (13) | |
| South | 20,376 (38) | 6,811 (42) | |
| Midwest | 13,739 (25) | 5,070 (31) | |
| West | 8,515 (15) | 2,173 (14) | |
| Other* | 843 (2) | 13 (0) | |
| Primary Treatment: | <0.01 | ||
| RP | 5,132 (10) | 5,030 (31) | |
| EBRT | 12,519 (23) | 2,629 (16) | |
| Brachtherapy | 3,838 (7) | 1,269 (8) | |
| Cryotherapy | 271 (1) | 66 (1) | |
| ADT | 8,554 (16) | 1,040 (6) | |
| WW/AS | 7,287 (14) | 1,400 (9) | |
| None/Other | 16,381 (29) | 4,571 (29) | |
Puerto Rico, Guam, etc.
RP-Radical Prostatectomy, EBRT-External-Beam Radiotherapy, ADT-Androgen Deprivation Therapy, WW/AS-Watchful Waiting, Active Surveillance
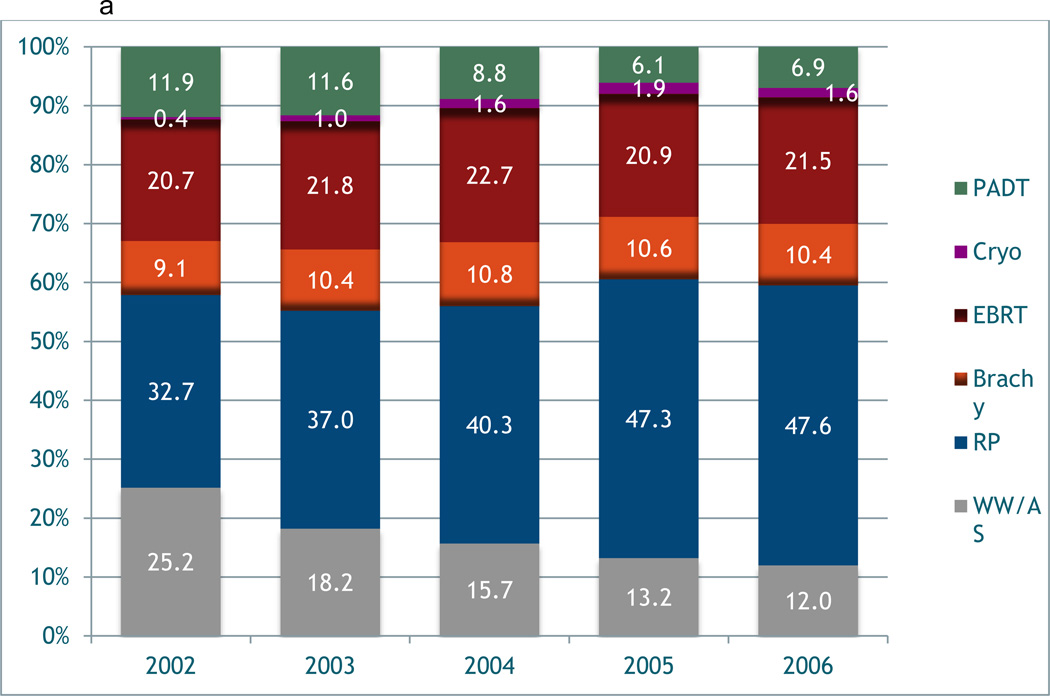
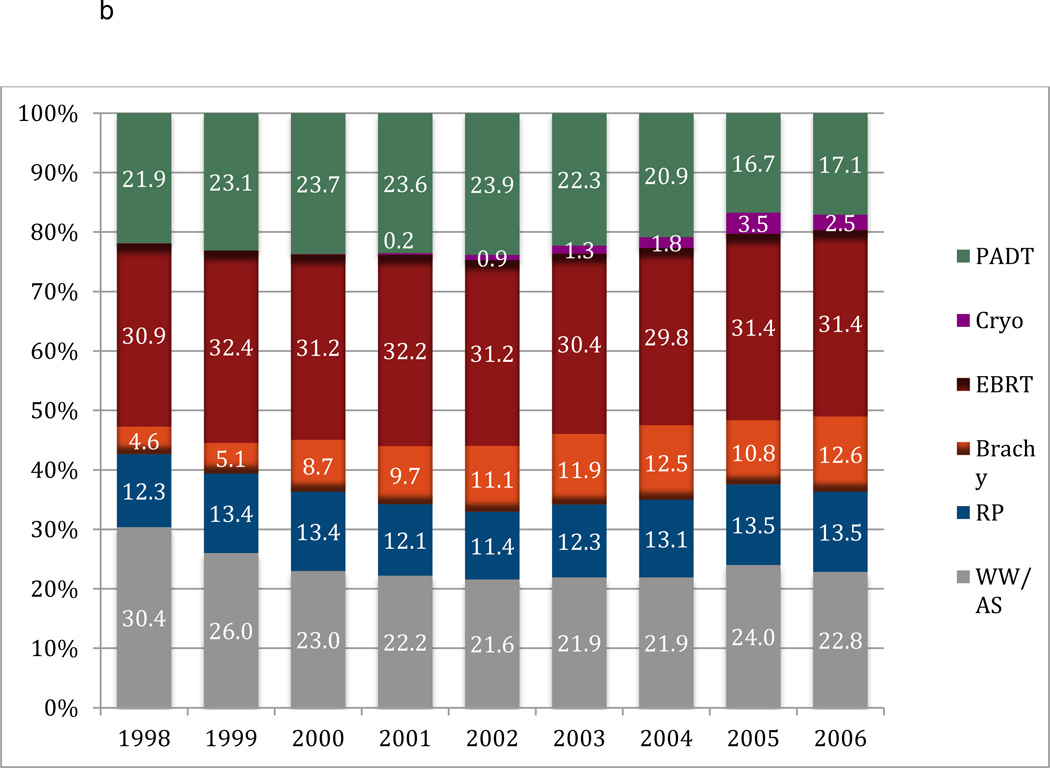
Figures 1a and 1b depict the temporal trends in the type of primary treatment between each cohort. In the i3 population, the use of radical prostatectomy (RP) increased over time from 33% in 2002 to 48% by 2006; the use of EBRT remained stable at around 20%. Of those undergoing RP, only 0.8% had minimally invasive surgery (MIS) (i.e. laparoscopic/robotic) in 2002, which had increased to 40.8% by 2006. Despite the use of EBRT remaining stable, there was an increase in the use IMRT from 21.8% to 71.1% during this time period. The use of BT remained stable at around 10% in the i3 cohort. Conversely, the use of RP (~12%) and EBRT (~31%) in the Medicare cohort remained stable during the study period. Similar to the i3 cohort, the utilization of MIS (0% to 35%) and IMRT (0% to 74.8%) increased in the Medicare sample from 1998 to 2006. There was an increase in the use of BT in Medicare patients from 4.6% to 12.6% over time. In both cohorts there was a decrease in the utilization of WW/ AS, however, this was more pronounced in the i3 cohort. There was a decreased use of PADT in both cohorts. There was also limited utilization of newer radiation technologies such as cyberknife and proton beam therapy in both cohorts.
Figure 1.
a. Variation in primary treatment of prostate cancer over time in i3 registry.
b. Variation in primary treatment of prostate cancer over time in the Medicare sample.
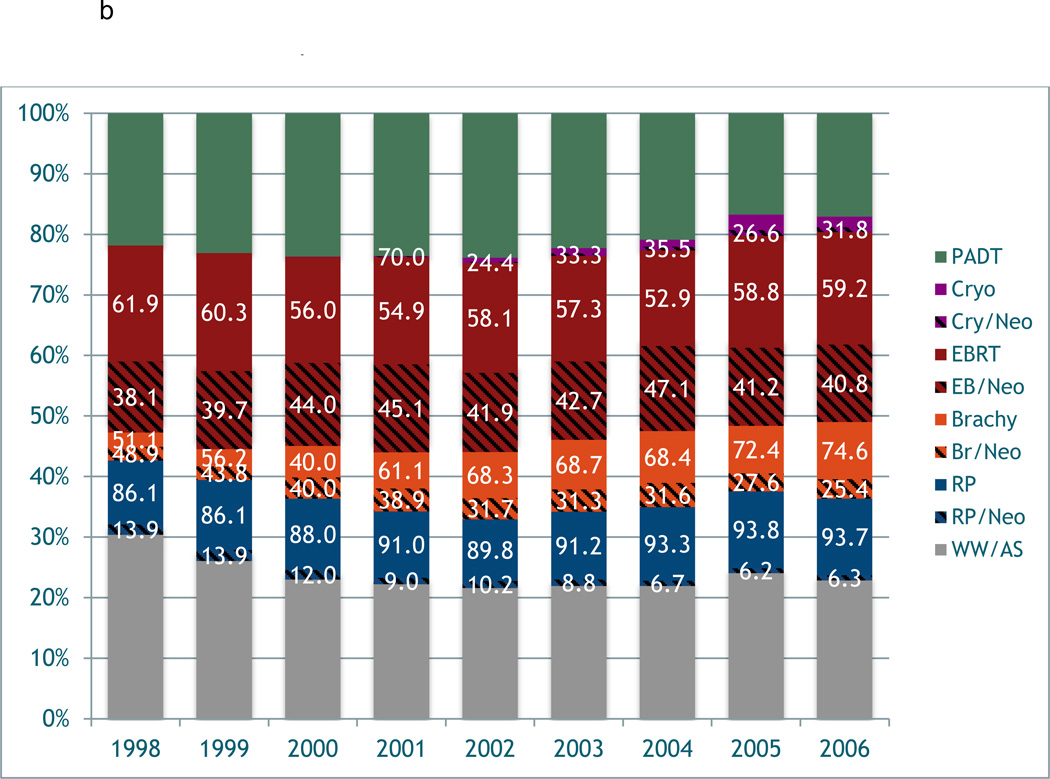
Variation in the use of neoadjuvant/adjuvant (neo/adjuvant) androgen deprivation therapy around the time of primary prostate cancer treatment was observed between the Medicare and i3 cohorts over time (Figure 2). In general, men in i3 received neo/adjuvant ADT at a lower proportion than men in Medicare regardless of type of primary treatment (i.e. RP, EBRT, cryotherapy). The use of neo/adjuvant ADT with primary EBRT reached its highest incidence in 2004, then declined through the end of the study period in both cohorts. The use of neo/adjuvant ADT for RP, BT, and cryotherapy declined over the entire study period.
Figure 2.
a. Variation in the use of neoadjuvant/adjuvant treatment in prostate cancer over time in the i3
b. Variation in the use of neoadjuvant/adjuvant treatment in prostate cancer over time in the Medicare sample.
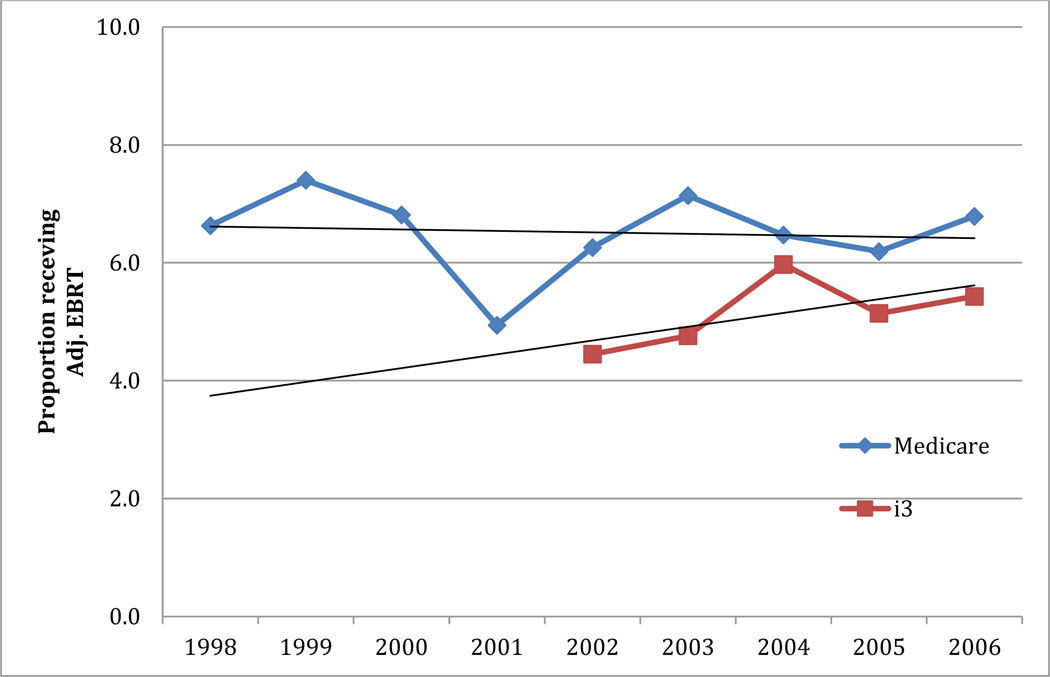
Figure 3 shows the trends of adjuvant EBRT following RP over time in both i3 and Medicare. In both cohorts, the utilization of adjuvant radiation therapy is quite low; in Medicare it is approximately 6.5% and i3 approximately 5%. There was an increasing trend in the use of adjuvant EBRT in the i3 men, while relatively stable incidence in Medicare men over time. In the years directly comparable between Medicare and i3 (2002–2006), the trends differed the most between 2003 and 2004. During these years, incidence of adjuvant EBRT increased in i3 men and decreased in Medicare men.
Figure 3.
Variation in the use of adjuvant EBRT following RP over time in both i3 and Medicare samples.
Figure 4 demonstrates geographic variation in prostate cancer treatment (i.e. radical prostatectomy, radiation therapy, and primary ADT) by urologist and radiation oncologist density in three distinct regions of the United States; California, New England, and Florida. Proportion receiving each treatment is categorized into quintiles with lighter shades representing lower quintiles and darker shades higher quintiles. In New England, the counties with the highest urologist density had lower proportions receiving RP or PADT. Conversely, the counties with the highest density of radiation oncologist received higher proportions of radiation therapy. In California and Florida, there did not seem to be any discernable pattern between treatment received and urologist or radiation oncologist density. In the hierarchical multivariable analysis of those counties with at least 30 prostate cancer patients, age, comorbidity, urologist density, income level, and year of diagnosis were significant predictors of treatment.(Table 2)
Figure 4.
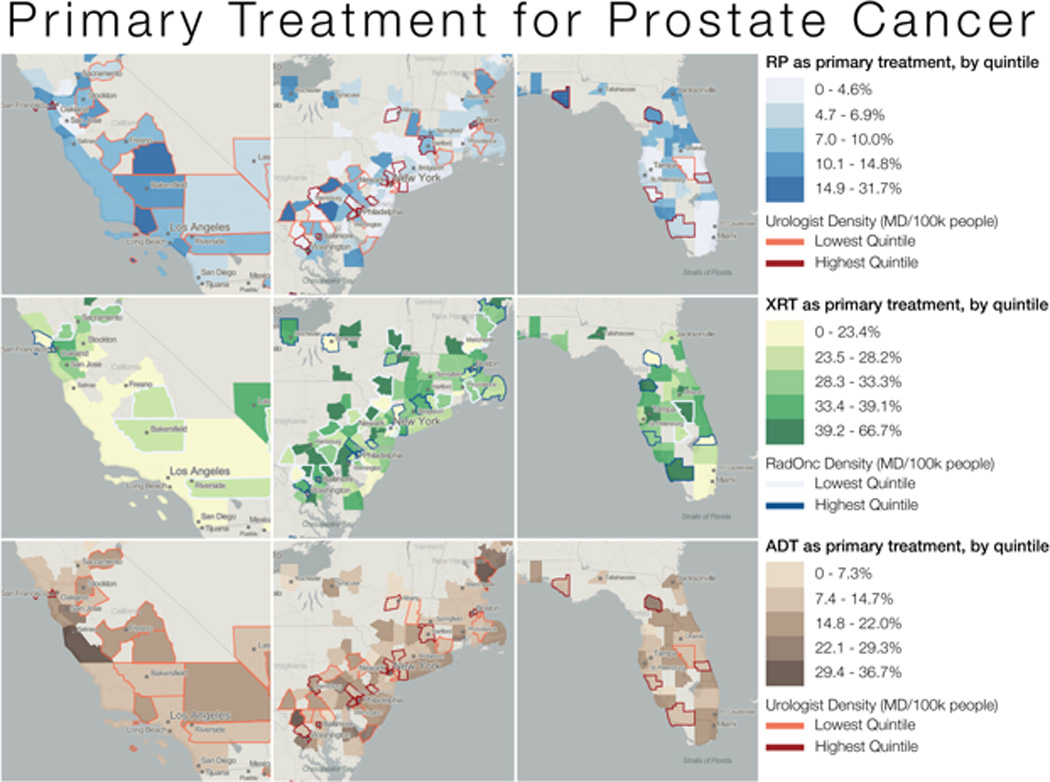
Geographic variation in prostate cancer treatment by urologist and radiation oncologist density in California, Florida, and the Northeast
Table 2.
Hierarchical multivariate logistic regression model for primary treatment of any type.
| Odds Ratio (95% CI) |
P-value | |
|---|---|---|
| Age at diagnosis |
0.96 (0.95–0.96) |
<0.0001 |
| Year of Diagnosis |
1.05 (1.04–1.06) |
<0.0001 |
| Race | ||
| Caucasian vs. African-American | 0.94 (0.87–1.01) |
0.10 |
| Other vs. African-American | 0.94 (0.82–1.07) |
0.33 |
| Charlson comorbidity | ||
| 1 vs. 0 |
0.79 (0.74–0.83) |
<0.0001 |
| 2+ vs. 0 |
0.53 (0.50–0.57) |
<0.0001 |
| Rural* | 1.04 (0.86–1.26) |
0.67 |
| Density of Primary care physicians (PCP) per county | ||
| <100 vs. >300 | 0.89 (0.77–1.03) |
0.12 |
| 100–300 vs. >300 | 1.06 (0.94–1.19) |
0.37 |
| Density of Urologists per county | ||
| <10 vs. >50 |
1.47 (1.25–1.72) |
<0.0001 |
| 10–50 vs. >50 |
1.28 (1.13–1.44) |
<0.0001 |
| Density of Radiation Oncologist per county | ||
| <5 vs. >25 | 1.04 (0.89–1.22) |
0.65 |
| 5–25 vs. >25 | 0.93 (0.82–1.06) |
0.28 |
| Median Household Income per county (per $1000) |
1 (1.0–1.0) |
0.001 |
| % uninsured per county | 0.98 (0.97–0.99) |
0.38 |
| % Unemployment per county | 0.99 (0.98–1.02) |
0.87 |
| % college educated per county | 1.01 (0.99–1.02) |
0.25 |
CI: Confidence interval
Base group is Urban
Discussion
This study assessed national trends in the primary treatment of localized prostate cancer using two large claims-based registries. In both cohorts there was a decrease over time in the use of PADT and an increased use of newer technologies such as IMRT for radiation and minimally invasive surgery. There was also a reduction in the use of WW/AS in both cohorts, which was more pronounced in the i3 cohort. In general, men in I3 received neoadjuvant ADT treatments at a lower rate then men in Medicare, while both cohorts underwent low rates of adjuvant radiation after RP. Finally, there was substantial geographic variation in primary treatment selection for men with localized prostate cancer.
When comparing the Medicare and i3 cohorts we noticed that a greater proportion of men in i3 were receiving active local treatment compared to those managed with PADT or WW/AS. This is most likely related to the younger age of the I3 cohort compared to men in Medicare. Previous studies have shown an association between younger age at diagnosis and the selection of more aggressive primary treatment.4,6 In addition, studies have shown that men with private insurance were more likely to undergo active treatment primarily with surgery.4 This is similar to our findings of increased active treatment, and more specifically surgery in the i3 cohort, who may have better access to health care and more available options for management of their prostate cancer. Without data of stage and grade of disease, we were unable to assess the affect of disease risk on treatment selection. In both cohorts we noticed an increased uptake of newer technologies such as IMRT over EBRT for radiation and laparoscopic radical prostatectomy over open radical prostatectomy for surgery. This is comparable to other studies that have shown a similar adoption of newer technologies replacing older approaches in the contemporary management of localized prostate cancer.7
This study also assessed temporal trends in the use of neo-adjuvant ADT and found a greater utilization among men in Medicare, compared to those in I3 regardless of primary treatment modality. This finding may reflect the older age of men on Medicare. Studies has shown that older men are more likely to harbor aggressive disease8,9, potentially resulting in a higher utilization of ADT in this population. We noticed a decline over time in the use of neo-adjuvant ADT among men undergoing RP, BT and cryotherapy in both i3 and Medicare. This may reflect the publication of several studies in the late 1990’s warning of the risk of ADT on several parameters including quality of life, cognition, bone heath and eventually cardiovascular and endocrine risk.10–13 Increased awareness of the potential harms of ADT coupled with the publication of trials questioning its benefit as a neo-adjuvant therapy in patients undergoing RP14 may have resulted in its declining use in these patients.
A recent study looking at trends in ADT use among men in Ontario, Canada reported a similar pattern of increasing ADT utilization from 1995 – 2001, followed by a sharp decline thereafter.15 The authors hypothesized that increased awareness of the potential adverse affects of ADT may have resulted in the observed decline in its use over time. Despite the declining use of ADT in most clinical settings that were explored in this study we did observe an increased utilization of neo-adjuvant ADT among men undergoing EBRT in both Medicare and i3. This may be impact of practice changing trials such as Bolla et al. published in the late 1990’s, which showed a benefit of ADT use in high-risk patients undergoing radiation and subsequent studies which showed similar benefits in intermediate risk patients.16,17
This study observed a low rate of adjuvant radiotherapy after RP in both the Medicare and i3 cohorts. A similar trend has been seen in other studies. In the CaPSURE database adjuvant radiation after surgery was uncommon and declining (7.3% in 1990–1994 to 2.3% in 2004–2007) after surgery, even among men with high-risk features.18 Despite the relatively low use of adjuvant radiotherapy in both cohorts, there was a trend towards increase utilization in the i3 population, which may reflect a more aggressive approach to management in this younger group of men with private insurance. The lack of a similar increase in adjuvant radiotherapy among men in Medicare may reflect the older age of the population, who may be more troubled by secondary adverse affects of their primary surgery (i.e incontinence) and a greater willingness to accept a less aggressive approach to management.
This study found a considerable variation in primary treatment patterns between different geographic regions. Other studies have noticed similar variation in primary treatment patterns for prostate cancer. For instance, a study evaluating 36 clinical sites contributing to the CaPSURE registry found marked variation in primary treatment patterns across different sites, which the authors felt was not explained by case-mix variability or known patient factors.4 Another study evaluating 96,769 men in the SEER dataset found significant geographic variation nationwide in surgical, radiation, and watchful waiting treatment rates.19 The authors felt that non-clinical factors such as ethnicity and income had a significant association with watchful waiting over definitive treatment. Despite imbalances in access to care, patient and clinician preferences can often lead to unwarranted variation in primary treatment patterns.20 This supports the need for high quality comparative effectiveness research assessing alternatives for the primary treatment of localized prostate cancer.
This study has several strengths including the use of two large nationwide registries including thousands of men over a long period of time. By utilizing both datasets, insights are gained into treatment patterns for all age groups and various health insurance coverage plans. Many studies using only Medicare data are limited by the age restriction of men >65 years of age, however, the inclusion of the i3 patient population allows the evaluation of treatment utilization in younger age categories. Interestingly, many of the treatment patterns in the two databases parallel one another, however, there were some differences between the groups. This study had some limitations that should be disclosed. One major limitation of the dataset was the lack of risk assessment of men in either cohort. Without knowledge of risk profile it is difficult to interpret any temporal or geographic variation in treatment pattern. However, one would expect that if geographic variation in risk profile existed, it would be subtle at best and would not explain all the variety seen in primary treatment selection. Secondly, the data included in the dataset only included information up to 2006. However, most newer technologies and thought processes guiding treatment have been introduced by this time period and trends in uptake of novel treatments could be expected to continue. In fact, in a 5% Medicare sample analysis, the proportion of men receiving intensity modulated radiation therapy (IMRT) in 2006 compared to 2008 increased from 66.7% to 80.1%; for laparoscopic radical prostatectomy (LRP) the proportion increased from 46% to 59%.21 Geographic variation is unlikely to have changed substantially in the last few years. Finally, the dataset does not differentiate between active surveillance and watchful waiting. These limitations are inherent to observational research in claims-based datasets.
Conclusion
Using two large national claims registries, we have demonstrated significant trends over time and geographic variation in the type of primary treatment used for localized prostate cancer. Specifically, there has been a large increase in the use of newer technologies, (i.e. laparoscopic-assisted prostatectomy and IMRT) and decrease in the proportion of patients undergoing PADT and WW/AS. Additionally, substantial sociodemographic and geographic variation exists in primary treatment selection. These results elucidate the need for better comparative effectiveness research to allow the consideration of the relative effectiveness and toxicity of various treatments to prevent unwarranted variation in care.
Acknowledgments
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN276201200016C; to M.S.L.)
Footnotes
Conflict of Interest:
Drs. Cary, Punnen, and Odisho have nothing to disclose. Dr. Cooperberg is a consultant for Genomic Health, Myriad, GenomeDx, Dendreon, Eli Lilly, Abbott Labs, Janssen, and Amgen. Dr. Saigal is a cofounder of Wiser Care LLC.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Carroll PR. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of Androgen Deprivation Therapy Use for Prostate Cancer: Role of the Urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambudiri VE, Landrum MB, Lamont EB, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79:537–545. doi: 10.1016/j.urology.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Kawachi MH. Counterpoint: robot-assisted laparoscopic prostatectomy: perhaps the surgical gold standard for prostate cancer care. J Natl Compr Canc Netw. 2007;5:689–692. [PubMed] [Google Scholar]
- 8.Bechis SK, Carroll PR, Cooperberg MR. Impact of Age at Diagnosis on Prostate Cancer Treatment and Survival. J Clin Oncol. 2011;29:235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Caire AA, Robertson CN, et al. Men older than 70 years have higher risk prostate cancer and poorer survival in the early and late prostate specific antigen eras. J Urol. 2009;182:2242–2248. doi: 10.1016/j.juro.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Herr HW, O'Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163:1743–1746. [PubMed] [Google Scholar]
- 11.Newton C, Slota D, Yuzpe AA, et al. Memory complaints associated with the use of gonadotropin-releasing hormone agonists: a preliminary study. Fertil and steril. 1996;65:1253–1255. doi: 10.1016/s0015-0282(16)58351-4. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian VB, Kuo Y-F, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 13.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 14.Cookson MS, Sogani PC, Russo P. Pathological staging and biochemical recurrence after neoadjuvant androgen deprivation therapy in combination with radical prostatectomy in clinically localized prostate cancer: results of a phase II study. BJU Int. 1997;79:432–438. doi: 10.1046/j.1464-410x.1997.00022.x. [DOI] [PubMed] [Google Scholar]
- 15.Krahn M, Bremner KE, Tomlinson G, et al. Androgen deprivation therapy in prostate cancer: are rising concerns leading to falling use? BJU Int. 2011;108:1588–1596. doi: 10.1111/j.1464-410X.2011.10127.x. [DOI] [PubMed] [Google Scholar]
- 16.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 17.D'Amico AV, Manola J, Loffredo M. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer. JAMA. 2004;292(7):821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Cowan J, Broering JM, et al. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26:211–218. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krupski TL, Kwan L, Afifi AA, et al. Geographic and Socioeconomic Variation in the Treatment of Prostate Cancer. J Clin Oncol. 2005;23:7881–7888. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 20.Kramer KM, Bennett CL, Pickard AS, et al. Patient preferences in prostate cancer: a clinician's guide to understanding health utilities. Clin Prostate Cancer. 2005;4:15–23. doi: 10.3816/cgc.2005.n.007. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor DA, Zimberg SH, Ohrin LM, et al. Utilization Trends in Prostate Cancer Therapy. J Urol. 2011;186:860–864. doi: 10.1016/j.juro.2011.04.075. [DOI] [PubMed] [Google Scholar]