Abstract
Background
More than 3.8 million children sustain traumatic brain injuries annually. Treatment of posttraumatic headache (PTH) in the emergency department (ED) is variable, and benefits are unclear.
Objective
The objective of the study is to determine if intravenous migraine therapy reduces pain scores in children with PTH and factors associated with improved response.
Methods
This was a retrospective study of children, 8 to 21 years old, presenting to a tertiary pediatric ED with mild traumatic brain injury (mTBI) and PTH from November 2009 to June 2013. Inclusion criteria were mTBI (defined by diagnosis codes) within 14 days of ED visit, headache, and administration of one or more intravenous medications: ketorolac, prochlorperazine, metoclopramide, chlorpromazine, and ondansetron. Primary outcome was treatment success defined by greater than or equal to 50% pain score reduction during ED visit. Bivariate analysis and logistic regression were used to determine predictors of treatment success: age, sex, migraine or mTBI history, time since injury, ED head computed tomographic (CT) imaging, and pretreatment with oral analgesics.
Results
A total of 254 patients were included. Mean age was 13.8 years, 51% were female, 80% were white, mean time since injury was 2 days, and 114 patients had negative head CTs. Eighty-six percent of patients had treatment success with 52% experiencing complete resolution of headache. Bivariate analysis showed that patients who had a head CT were less likely to respond (80% vs 91%; P = .008).
Conclusions
Intravenous migraine therapy reduces PTH pain scores for children presenting within 14 days after mTBI. Further prospective work is needed to determine long-term benefits of acute PTH treatment in the ED.
1. Introduction
1.1. Background
An estimated 3.8 million recreation- and sports-related concussions occur each year in the United States [1]. In children younger than 15 years, the estimated number of emergency department (ED) visits for mild traumatic brain injury (mTBI) is 500000 per year [2]. In the pediatric ED, children commonly present either with their initial injury or subsequently with postconcussive symptoms. Headache is the most common initial symptom and has been reported in up to 71.5% of children hospitalized with concussion [3]. Not only is posttraumatic headache (PTH) common in the acute period, but it may persist in up to 43% of children 3 months after the traumatic brain injury [4]. Chronic daily headaches lead to physical distress and impairment in school and emotional functioning [5].
The American Academy of Pediatrics guidelines note that medication use for the treatment of PTH is an option but there is little evidence to support its efficacy [6]. Systematic reviews from Watanabe et al [7] and Lucas [8] recommend treatment of PTH based on headache characteristics. They summarize the literature on medications such as tricyclic antidepressants, topiramate, and triptans; however, studies are limited to the adult population and outpatient settings.
Up to 38% to 55% of children with PTH have characteristics similar to migraines, which may help physicians tailor their treatments [9]. Kuczynski et al [10] demonstrated improvement in chronic PTH in children treated with oral medications, amitriptyline and topiramate, in the outpatient setting. Effective medications commonly used to treat acute migraine headache in the ED are ketorolac, prochlorperazine, and metoclopramide [11–14]. However, the benefits of intravenous (IV) migraine therapies for PTHs in children within the first few weeks after an mTBI have not been studied.
The primary objective of our study was to estimate the proportion of patients with PTHs who were successfully treated in the ED with IV migraine therapies within 14 days after an mTBI. Our secondary objective was to evaluate which clinical predictors are associated with treatment responders and nonresponders. We hypothesized that children treated for PTH within 14 days of an mTBI with IV migraine therapies will have reduced pain scores.
2. Materials and methods
2.1. Study design and setting
This was a retrospective, cross-sectional study of children (ages 8–21 years) presenting to the ED at a tertiary care children’s hospital for a PTH within 14 days after an mTBI. Our children’s hospital has 2 campuses: a level 1 trauma center and a satellite campus. There are approximately 118000 visits annually at both sites. Approximately 1225 visits between both sites are mTBI related. Visits from both sites were combined into the analysis. The study was approved by our institutional review board.
2.2. Participants
Participants with a billing International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis code of mTBI (800.0–801.9, 803.0–804.9, 850–854.1, and 959.01) were identified in the electronic medical record. Records from November 2009 to June 2013 were included. In preparation to initiating the study, we reviewed the number of patients seen in our ED with International Classification of Diseases, Ninth Revision, Clinical Modification, codes meeting our proposed study criteria. This review allowed us to estimate the number of expected participants. Based on that preliminary data, we estimated that 300 patients would meet inclusion criteria. A sample this size would provide an estimate of the proportion of patients successfully treated within a maximum margin of error, depending on the estimated proportion, of ±4.5 percentage points assuming a 95% confidence interval (CI).
Eligible participants also received one or more of the following IV medications for PTH: ketorolac, prochlorperazine, metoclopramide, chlorpromazine, or ondansetron. In addition, patients presented to the ED within 14 days of their mTBI and reported persistent headaches since the injury. Exclusion criteria included Glasgow Coma Scale less than 14 on ED presentation, significant neurologic/neurosurgical history (eg, seizure disorder, ventriculoperitoneal shunt, nonverbal, developmental delay, or malignancy), positive head computed tomography (CT), multisystem trauma (eg, presence of long-bone fractures, or intra-abdominal injury), or inadequate pain score documentation. Pain score documentation was deemed inadequate if a pretreatment and posttreatment score was not available or if a pain scale other than the Numerical Rating Scale (NRS) was used.
2.3. Variables/data sources/measurements
Our primary outcome was the NRS pain score, which has been validated for use in children 8 years and older [15,16]. Treatment success was defined by at least a 50% improvement from the patient’s initial triage pain score to the last documented pain score after medications were administered and before final disposition. This threshold was chosen because it is a frequently used measure of treatment success in the migraine literature [17–19]. We also conducted a subanalysis to specifically look at the rate of treatment success in the 4 medication groups: (1) ketorolac only, (2) ketorolac plus metoclopramide or prochlorperazine, (3) prochlorperazine or metoclopramide only, and (4) ondansetron only.
Factors associated with response or nonresponse to treatment were explored in secondary analyses. The factors of interest were extracted from the electronic medical record and included age, race, sex, history of prior mTBI(s), history of migraine headaches, number of days since initial mTBI, ordering of ED head CT imaging, and pretreatment in the ED with oral analgesics (acetaminophen or ibuprofen). These factors were chosen because they have previously been studied and are thought to be associated with concussion-related recovery [4,20,21]. In addition, patients with adverse reactions to metoclopramide or prochlorperazine were identified through chart review as having akathisia and subsequent diphenhydramine administration.
2.4. Statistical methods
Descriptive statistics, including frequency distributions and mean values for categorical and continuous variables, respectively, were performed. A bivariable analysis was completed to determine the association of demographic and treatment characteristics with treatment success. χ2 and t tests were conducted for categorical and continuous variables, respectively. A multivariable logistic regression model was developed to further explore factors associated with treatment success. The dependent variable was treatment success defined by at least a 50% reduction in pain scores. The independent variables listed above were the factors of interest. In addition, we tested for interaction effects between the following variables: age, sex, history of mTBI, and history of migraines. Knowing these factors are associated with concussion recovery, we wanted to assess whether the association of one factor with the treatment outcome is dependent on a second factor.
We conducted a similar analysis, in which the variable age was divided into 2 subgroups: 8 to 12 and 13 to 21 years old [22]. The rationale for this was to try to account for the different treatment responses that, we hypothesized, may occur in different age subgroups. In addition, we tested whether different predictors such as history of mTBI were significant in different subgroups.
3. Results
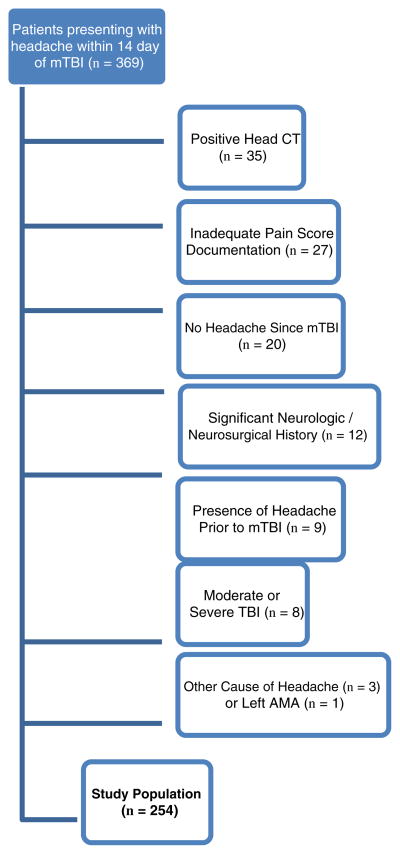
During the study period, 369 patients presented to the ED within 14 days after an mTBI for PTH and were given at least one of the following IV medications: ketorolac, prochlorperazine, chlorpromazine, metoclopramide, or ondansetron. Patients were excluded for the following reasons: head CT showing evidence of intracranial injury (n = 35), incomplete pain scores or use of pain scales other than NRS (n = 27), absence of headache (n = 20), significant neurologic or neurosurgical history (n = 12), presence of headache before mTBI occurred (n = 9), characteristics consistent with moderate or severe traumatic brain injury (n = 8), other identifiable cause of headache (n = 3), and leaving against medical advice (n = 1). Fig. 1 demonstrates the final study population of 254 patients.
Fig. 1.
Flow diagram.
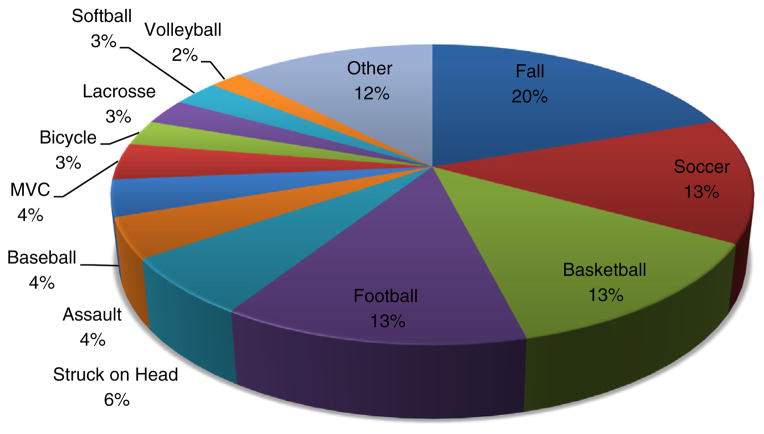
Table 1 shows the baseline demographics and patient characteristics. The mean age was 13.8 years old, 51% were female, 80% were white, and average time since injury was 2 days. Only 14% of patients had a history of prior mTBI, whereas 21% had a history of migraine headaches. Fig. 2 summarizes the different mechanisms of injury with “falls” being the most common. Soccer, football, and basketball were the 3 most common sports-related injuries.
Table 1.
Patient characteristics (n = 254)
| Mean age in years (SD) | 13.8 (2.3) |
| Male | 49% |
| Race | |
| White | 80% |
| African American | 17% |
| Other | 3% |
| History of prior mTBI | 14% |
| History of migraine headaches | 21% |
Fig. 2.
Mechanisms of injury.
Of the 254 patients who were treated for PTH, 86.2% (95% CI, 82.0–90.4) experienced treatment success defined as a reduction of pain score greater than or equal to 50%. In addition, 52.4% (95% CI, 46.3–58.5) had complete resolution of their headache.
Table 2 shows head CTs were obtained in 114 (45%) of patients. One hundred eighty-seven patients (74%) were treated with ketorolac, 214 patients (84%) were treated with an antiemetic (ondansetron, metoclopramide, or prochlorperazine), and 147 patients (58%) received a combination of both. No patients in our study population received chlorpromazine. Ninety-four patients (37%) received pretreatment with oral analgesics in the ED (acetaminophen or ibuprofen). A majority of patients were discharged from the ED (95%). The rate of akathisia in our population was 6% (12/201 patients received diphenhydramine for documented symptoms after treatment with prochlorperazine or metoclopramide).
Table 2.
Care and treatment characteristics (n = 254)
| Imaging | |
| Head CT obtained | 45% |
| Medications | |
| Oral analgesics before IV medsa | 37% |
| Ketorolac | 74% |
| Antiemeticb | 84% |
| Ketorolac + antiemeticb | 58% |
| Disposition | |
| Discharged to home | 95% |
Oral analgesics: acetaminophen or ibuprofen.
Antiemetic: ondansetron, metoclopramide, or prochlorperazine.
A subanalysis of 4 specific medication combinations is shown in Table 3. The combination of medications most frequently given was ketorolac and metoclopramide/prochlorperazine (n = 152), with 89% experiencing treatment success. Fifty-five patients received only ketorolac, with 80% experiencing treatment success. Thirty patients received metoclopramide or prochlorperazine, with 93% experiencing treatment success. Finally, 37 patients received only ondansetron, with 79% experiencing treatment success. All patients who received prochlorperazine/metoclopramide also received a bolus IV infusion of normal saline, whether in combination with ketorolac or not.
Table 3.
Treatment success by medication
| Medication groups | Treatment success |
|---|---|
| Ketorolac only (n = 55) | 80% |
| Metoclopramide or prochlorperazine only (n = 30) | 93% |
| Ketorolac plus (metoclopramide or prochlorperazine) (n = 132) | 89% |
| Ondansetron only (n = 37) | 78% |
The bivariable analysis evaluated factors associated with treatment success (Table 4). We found that patients who received a head CT were less likely to respond to treatment compared to those who did not receive head imaging (80% vs 91%, P = .008). All other factors were not significantly associated with treatment success. The multivariable analysis showed similar results (P = .006). Patients who did not receive a head CT were more likely to experience treatment success (odds ratio, 2.95; 95% CI, 1.39–6.56) compared to those who did. In addition, we assessed for 2-way interactions between age, sex, history of migraine, and history of mTBI and found that none were significant. Finally, the bivariable and multivariable models that included age as a categorical variable provided very similar results.
Table 4.
Bivariable analysis of the association of potential predictors to treatment success
| Patient and treatment characteristics | Treatment not successful | Treatment successful | P |
|---|---|---|---|
| Age, y | 14.5 | 14.4 | .718 |
| Days since mTBI | 2.2 | 1.8 | .232 |
| Male sex | 43% | 50% | .447 |
| Race | .645 | ||
| White | 77% | 80% | |
| African American | 17% | 16% | |
| Other | 6% | 3% | |
| History of migraine | 17% | 21% | .559 |
| History of mTBI | 6% | 16% | .068 |
| Received oral analgesic | 37% | 37% | .986 |
| Head CT obtained | 66% | 42% | .008 |
4. Discussion
Our study is the first to our knowledge to evaluate the potential benefit of IV migraine medications on PTHs in children presenting to the ED within 14 days after an mTBI. Most patients experienced treatment success (86%) and had complete headache resolution (52%), with a small incidence of adverse reactions (6%). These findings indicate that acute treatment of PTH with commonly used IV migraine medications may be beneficial in children and should be considered as a treatment option.
Because most previous works have focused on adults, in an outpatient or inpatient setting, and have been limited by small sample sizes, our study fills a critical gap in the literature by examining the benefits of acute treatment of PTH with typical IV migraine medications in children. A recent randomized controlled trial of children presenting to the ED with PTHs showed that hypertonic saline was more effective compared to normal saline in the treatment of PTHs in the ED [23]. They demonstrated a statistically significant mean improvement in pain scores in children treated with hypertonic saline compared to normal saline in the ED as well as during a 2- to 3-day follow-up period. This study was done in a similar population and setting to our study but did not address the use of IV migraine medications. They also used the Wong-Baker FACES pain rating scale as a continuous variable in their primary outcome as opposed to setting a threshold and definition for treatment success; therefore, we are unable to directly compare their outcomes to our study. Studies that evaluate the comparative and combined benefits of IV medications and saline are needed in the future.
Triptans, topiramate, amitriptyline, valproic acid, dihydroergotamine, and propranolol have been shown to have varying degrees of effectiveness; however, research on these medications was done primarily in adults in the outpatient setting and is less relevant to the acute management of PTH in children in the ED setting [24–30]. McBeath and Nanda [29] performed a case series of 34 adult inpatients with postconcussive syndrome treated with repeated doses of dihydroergotamine and metoclopramide, and 85% of patients experienced good-to-excellent relief of headache. Although this study was done on adult inpatients with postconcussive symptoms lasting up to 3 years, it demonstrated a similar rate of treatment success to our study.
In subgroup analysis of those who received individual or combination therapy with ketorolac, metoclopramide, or prochlorperazine, the response rate ranged from 80% to 93%. The group with the highest rate of treatment success (93%) was the group that only received metoclopramide or prochlorperazine; however, that was also the group with the smallest sample size (n = 30). The group that received a combination of ketorolac and either metoclopramide or prochlorperazine was our largest group (n = 132) and had a high rate of treatment success (89%). This is relevant because these medications have been shown to be more effective in the treatment of acute migraine headache in children when given in combination in the ED [13]. All patients in these 2 groups received a normal saline bolus, which could have contributed to the treatment response.
In our study, receipt of a head CT was less likely to be associated with treatment success. The factors that affected the physician’s decision to obtain a head CT may make this a higher risk group such as altered mental status, signs of a skull fracture, loss of consciousness, history of vomiting, persistent vomiting, severe mechanism of injury, severe headache, or parental influence [31]. Thus, we suspect that ordering a head CT was a surrogate marker of severity or physician concern, which may explain why these patients were less likely to respond to therapy.
Previous studies have cited that female sex and age play a role in the incidence of concussion and in predicting poor outcome; however, these factors were not associated with treatment success in our study [20,32,33]. Eisenberg et al [21] showed that children with previous concussions were at increased risk for prolonged symptoms; however, in our population, only 14% patients had a documented history of concussion, which limits our ability to evaluate this factor fully as a moderator or mediator of treatment response. Other factors such as race, history of migraines, number of days since initial mTBI, and pretreatment with oral analgesics were also not associated with treatment outcome.
Mihalik et al [34] showed that athletes with characteristics of post-traumatic migraine had more neurocognitive dysfunction compared to athletes without headaches or with nonmigrainous headaches after mTBI. Kontos et al [35] similarly showed that posttraumatic migraines were associated with neurocognitive impairment and made athletes 7 times more likely to take more than 21 days to recover. This further highlights the potential importance of treating PTHs acutely. Future prospective studies are needed to evaluate the association of treatment of PTH to neurocognitive recovery of postconcussive symptoms.
4.1. Limitations
This was a descriptive study without controls, and as a result, we cannot comment on the efficacy of this therapy. Some of the response may be related to placebo effect because of a lack of a control group. However, this study is the first step toward a larger prospective study evaluating the potential benefits of acute PTH treatment on longer term recovery after mTBI in children. This study was also limited by its retrospective design. Thus, we did not have follow-up data available and were unable to determine if medications had a long-term benefit. Some patients were missed and ineligible due to inadequate pain score documentation, which may have biased our results. In addition, 21% of patients with PTH had a history of migraine, which may have biased our results toward response to typical migraine therapy. Finally, we did not account for the potential treatment effect of IV fluids, which could have contributed to our results. The study was done at a single center, which limits its generalizability.
5. Conclusion
Our data suggest that IV migraine therapies reduce pain scores in children with PTHs within 14 days of an mTBI. Receipt of a head CT was associated with poor treatment response and was likely a surrogate marker of injury severity. Further prospective work is needed to determine efficacy, potential long-term benefit s, and injury-related and individual characteristics associated with better treatment response.
Footnotes
Funding: Funding from National Institutes of Health grant K23HD074683-01 supported, in part, Brad G. Kurowski’s time for participation in the study development and manuscript preparation.
Financial disclosure: The authors have indicated that they have no financial relationships relevant to this article to disclose.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Moreno MA. Youth sports and concussion risk. Arch Pediatr Adolesc Med. 2012;166(4):396. doi: 10.1001/archpediatrics.2012.79. [DOI] [PubMed] [Google Scholar]
- 2.Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W, Kraus J. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005;19(2):85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- 3.Blinman TA, Houseknecht E, Snyder C, Wiebe DJ, Nance ML. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J Pediatr Surg. 2009;44(6):1223–8. doi: 10.1016/j.jpedsurg.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Blume HK, Vavilala MS, Jaffe KM, Koepsell TD, Wang J, Tempkin N, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31–9. doi: 10.1542/peds.2011-1742. [DOI] [PubMed] [Google Scholar]
- 5.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in childhood migraines: clinical impact and comparison to other chronic illnesses. Pediatrics. 2003;112(1 Pt 1):e1–5. doi: 10.1542/peds.112.1.e1. [DOI] [PubMed] [Google Scholar]
- 6.Halstead ME, Walter KD Council on Sports M, Fitness American Academy of Pediatrics. . Clinical report—sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe TK, Bell KR, Walker WC, Schomer K. Systematic review of interventions for post-traumatic headache. PM R. 2012;4(2):129–40. doi: 10.1016/j.pmrj.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Lucas S. Headache management in concussion and mild traumatic brain injury. PM R. 2011;3(10 Suppl 2):S406–12. doi: 10.1016/j.pmrj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S. Characterization of headache after traumatic brain injury. Cephalalgia. 2012;32(8):600–6. doi: 10.1177/0333102412445224. [DOI] [PubMed] [Google Scholar]
- 10.Kuczynski A, Crawford S, Bodell L, Dewey D, Barlow KM. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: a prospective cohort. Dev Med Child Neurol. 2013;55(7):636–41. doi: 10.1111/dmcn.12152. [DOI] [PubMed] [Google Scholar]
- 11.Trottier ED, Bailey B, Lucas N, Lortie A. Prochlorperazine in children with migraine: a look at its effectiveness and rate of akathisia. Am J Emerg Med. 2012;30(3):456–63. doi: 10.1016/j.ajem.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Kabbouche MA, Vockell AL, LeCates SL, Powers SW, Hershey AD. Tolerability and effectiveness of prochlorperazine for intractable migraine in children. Pediatrics. 2001;107(4):E62. doi: 10.1542/peds.107.4.e62. [DOI] [PubMed] [Google Scholar]
- 13.Leung S, Bulloch B, Young C, Yonker M, Hostetler M. Effectiveness of standardized combination therapy for migraine treatment in the pediatric emergency department. Headache. 2013;53(3):197–491. doi: 10.1111/head.12042. [DOI] [PubMed] [Google Scholar]
- 14.Friedman BW, Esses D, Solorzano C, Dua N, Greenwald P, Radulescu R, et al. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Ann Emerg Med. 2008;52(4):399–406. doi: 10.1016/j.annemergmed.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 16.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143(3):223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine versus ketorolac. Ann Emerg Med. 2004;43(2):256–62. doi: 10.1016/s0196-0644(03)00716-9. [DOI] [PubMed] [Google Scholar]
- 18.Hamalainen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology. 1997;48(4):1100–3. doi: 10.1212/wnl.48.4.1100. [DOI] [PubMed] [Google Scholar]
- 19.Trottier ED, Bailey B, Dauphin-Pierre S, Gravel J. Clinical outcomes of children treated with intravenous prochlorperazine for migraine in a pediatric emergency department. J Emerg Med. 2010;39(2):166–73. doi: 10.1016/j.jemermed.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27(3):527–39. doi: 10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8–17. doi: 10.1542/peds.2013-0432. [DOI] [PubMed] [Google Scholar]
- 22.Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JPA, Curtis S, et al. Standard 6: age groups for pediatric trials. Pediatrics. 2012;129(Suppl 3):S153–60. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- 23.Lumba-Brown A, Harley J, Lucio S, Vaida F, Hilfiker M. Hypertonic saline as a therapy for pediatric concussive pain: a randomized controlled trial of symptom treatment in the emergency department. Pediatr Emerg Care. 2014;30(3):139–45. doi: 10.1097/PEC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache. 2011;51(6):932–44. doi: 10.1111/j.1526-4610.2011.01909.x. [DOI] [PubMed] [Google Scholar]
- 25.Lew HL, Lin PH, Fuh JL, Wang SJ, Clark DJ, Walker WC. Characteristics and treatment of headache after traumatic brain injury: a focused review. Am J Phys Med Rehabil. 2006;85(7):619–27. doi: 10.1097/01.phm.0000223235.09931.c0. [DOI] [PubMed] [Google Scholar]
- 26.Gawel MJ, Rothbart P, Jacobs H. Subcutaneous sumatriptan in the treatment of acute episodes of posttraumatic headache. Headache. 1993;33(2):96–7. doi: 10.1111/j.1526-4610.1993.hed3302096.x. [DOI] [PubMed] [Google Scholar]
- 27.Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache. 2000;40(9):736–9. doi: 10.1046/j.1526-4610.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 28.Weiss HD, Stern BJ, Goldberg J. Post-traumatic migraine: chronic migraine precipitated by minor head or neck trauma. Headache. 1991;31(7):451–6. doi: 10.1111/j.1526-4610.1991.hed3107451.x. [DOI] [PubMed] [Google Scholar]
- 29.McBeath JG, Nanda A. Use of dihydroergotamine in patients with postconcussion syndrome. Headache. 1994;34(3):148–51. doi: 10.1111/j.1526-4610.1994.hed3403148.x. [DOI] [PubMed] [Google Scholar]
- 30.Lay CL, Newman LC. Posttraumatic hemicrania continua. Headache. 1999;39(4):275–9. doi: 10.1046/j.1526-4610.1999.3904275.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Atabaki SM, Holubkov R, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 32.Covassin T, Swanik CB, Sachs ML. Sex differences and the incidence of concussions among collegiate athletes. J Athl Train. 2003;38(3):238–44. [PMC free article] [PubMed] [Google Scholar]
- 33.Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245–53. doi: 10.1016/j.pmrj.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihalik JP, Stump JE, Collins MW, Lovell MR, Field M, Maroon JC. Posttraumatic migraine characteristics in athletes following sports-related concussion. J Neurosurg. 2005;102(5):850–5. doi: 10.3171/jns.2005.102.5.0850. [DOI] [PubMed] [Google Scholar]
- 35.Kontos AP, Elbin RJ, Lau B, Simensky S, Freund B, French J, et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013;41(7):1497–504. doi: 10.1177/0363546513488751. [DOI] [PubMed] [Google Scholar]