Abstract
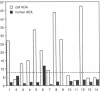
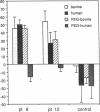
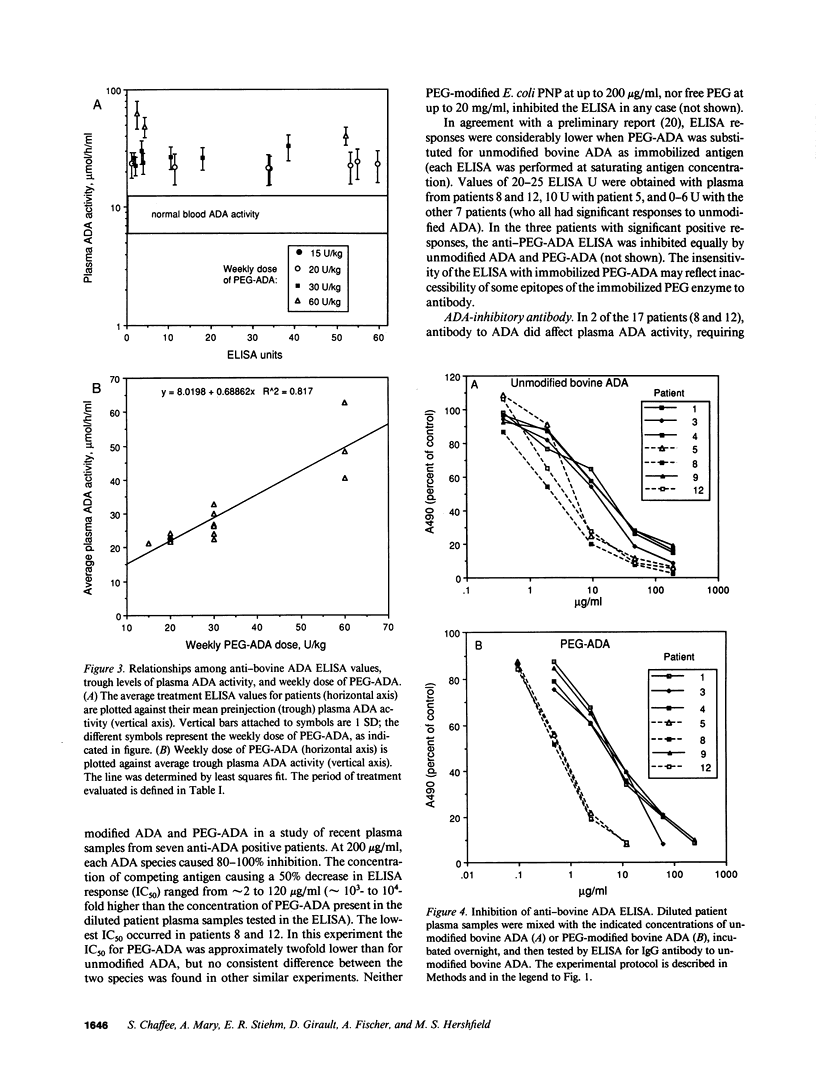
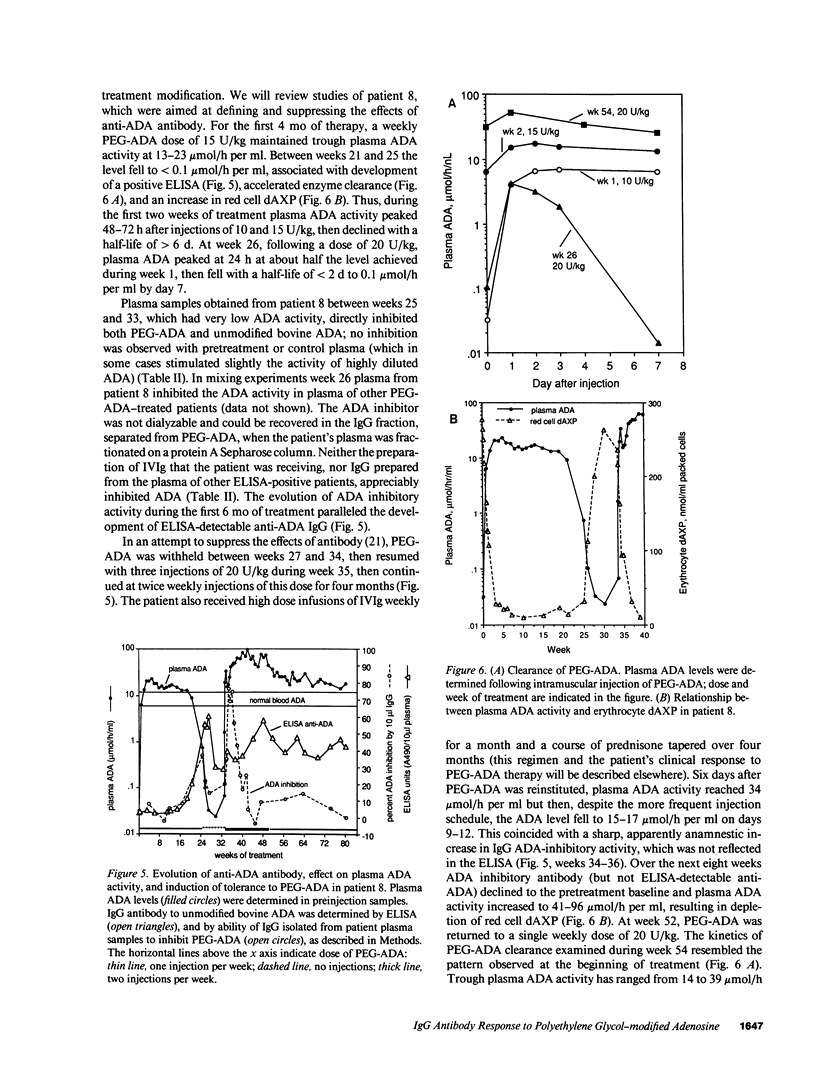
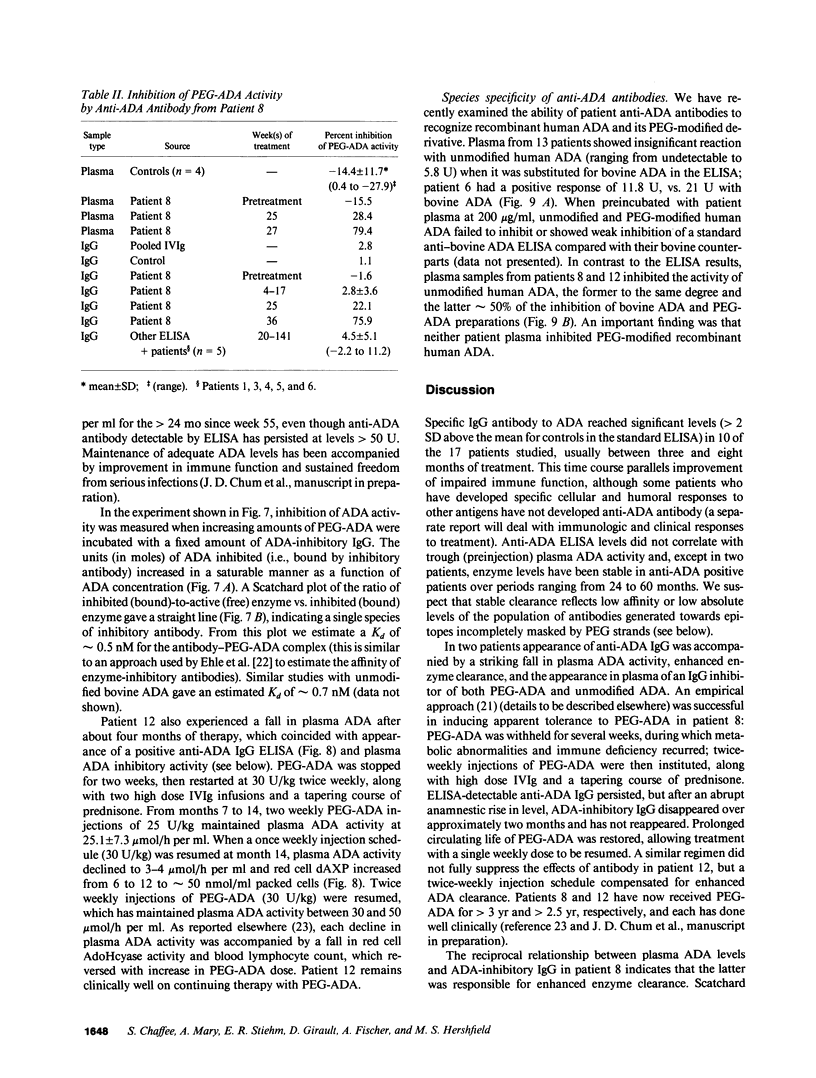
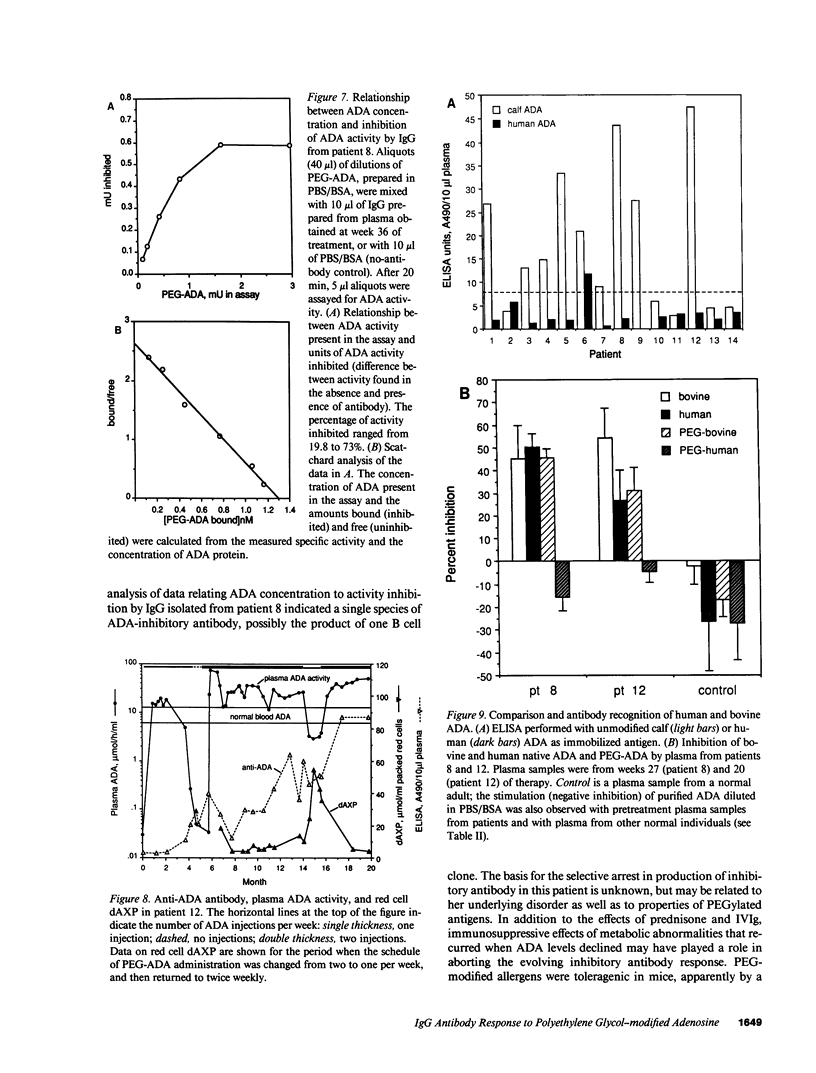
Polyethylene glycol (PEG)-modified bovine adenosine deaminase (ADA) is used for replacement therapy of severe combined immunodeficiency disease due to inherited ADA deficiency. We monitored IgG anti-ADA antibody in 17 patients treated by intramuscular injections of PEG-ADA for 1 to greater than 5.5 yr. ELISA-detectable anti-ADA IgG appeared in 10 patients, usually between the third and eighth months of treatment. Anti-ADA levels did not correlate with trough plasma ADA activity, which averaged 1.8-5 times normal blood (erythrocyte) ADA activity, depending on dose (15-60 U/kg per wk). ELISA-detectable anti-ADA antibodies were directed primarily at bovine-specific peptide (rather than PEG-containing) epitopes. Enhanced enzyme clearance, mediated by antibody that directly inhibited native and PEG-modified bovine ADA, and native, but not PEG-modified human ADA, occurred in two patients. In one, tolerance was induced; in the second, twice weekly injections of PEG-ADA compensated for accelerated clearance. We speculate that inhibitory antibodies recognize conserved, relatively PEG-free epitope(s) encompassing the active site, and that in human, but not bovine, ADA a PEG-attachment site "shields" the active site from immune recognition. We conclude that PEG-modification largely prevents the development of high affinity, or high levels of clearing antibodies to bovine ADA, and that PEG-modified human ADA should be further investigated as a possible treatment for ADA deficiency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., Kazo G. M., Verhoest C. R., Jr, Van Es T., Kafkewitz D., Nucci M. L., Viau A. T., Davis F. F. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984 Jun;7(2):175–186. [PubMed] [Google Scholar]
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Arredondo-Vega F. X., Kurtzberg J., Chaffee S., Santisteban I., Reisner E., Povey M. S., Hershfield M. S. Paradoxical expression of adenosine deaminase in T cells cultured from a patient with adenosine deaminase deficiency and combine immunodeficiency. J Clin Invest. 1990 Aug;86(2):444–452. doi: 10.1172/JCI114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Daddona P. E., Menapace D. P. Properties of a novel PEG derivative of calf adenosine deaminase. Adv Exp Med Biol. 1984;165(Pt A):47–52. doi: 10.1007/978-1-4684-4553-4_9. [DOI] [PubMed] [Google Scholar]
- Bory C., Boulieu R., Souillet G., Chantin C., Rolland M. O., Mathieu M., Hershfield M. Comparison of red cell transfusion and polyethylene glycol-modified adenosine deaminase therapy in an adenosine deaminase-deficient child: measurement of erythrocyte deoxyadenosine triphosphate as a useful tool. Pediatr Res. 1990 Aug;28(2):127–130. doi: 10.1203/00006450-199008000-00010. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Mitchell B. S., Meuwissen H. J., Davidson B. L., Wilson J. M., Koller C. A. Adenosine deaminase deficiency with normal immune function. An acidic enzyme mutation. J Clin Invest. 1983 Aug;72(2):483–492. doi: 10.1172/JCI110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Abuchowski A., Park Y. K., Davis F. F. Alteration of the circulating life and antigenic properties of bovine adenosine deaminase in mice by attachment of polyethylene glycol. Clin Exp Immunol. 1981 Dec;46(3):649–652. [PMC free article] [PubMed] [Google Scholar]
- Ehle H., Gödicke C., Horn A. A new graphical method for determining the affinity constants of monoclonal antibodies to enzymes. J Immunol Methods. 1989 Feb 8;117(1):17–23. doi: 10.1016/0022-1759(89)90113-0. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Buckley R. H., Greenberg M. L., Melton A. L., Schiff R., Hatem C., Kurtzberg J., Markert M. L., Kobayashi R. H., Kobayashi A. L. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987 Mar 5;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Chaffee S., Koro-Johnson L., Mary A., Smith A. A., Short S. A. Use of site-directed mutagenesis to enhance the epitope-shielding effect of covalent modification of proteins with polyethylene glycol. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7185–7189. doi: 10.1073/pnas.88.16.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. Y., Sehon A. H., Akerblom E. Suppression of reaginic antibodies with modified allergens. IV. Induction of suppressor T cells by conjugates of polyethylene glycol (PEG) and monomethoxy PEG with ovalbumin. Int Arch Allergy Appl Immunol. 1981;64(1):100–114. [PubMed] [Google Scholar]
- Levy Y., Hershfield M. S., Fernandez-Mejia C., Polmar S. H., Scudiery D., Berger M., Sorensen R. U. Adenosine deaminase deficiency with late onset of recurrent infections: response to treatment with polyethylene glycol-modified adenosine deaminase. J Pediatr. 1988 Aug;113(2):312–317. doi: 10.1016/s0022-3476(88)80271-3. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Schiff R. I., Buckley R. H. Adenosine deaminase and purine nucleoside phosphorylase deficiencies: evaluation of therapeutic interventions in eight patients. J Clin Immunol. 1987 Sep;7(5):389–399. doi: 10.1007/BF00917017. [DOI] [PubMed] [Google Scholar]
- Nilsson I. M., Berntorp E., Zettervall O. Induction of immune tolerance in patients with hemophilia and antibodies to factor VIII by combined treatment with intravenous IgG, cyclophosphamide, and factor VIII. N Engl J Med. 1988 Apr 14;318(15):947–950. doi: 10.1056/NEJM198804143181503. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Karvelas M. Soluble antigen abrogates the appearance of anti-protein IgG1-forming cell precursors during primary immunization. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1615–1619. doi: 10.1073/pnas.87.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polmar S. H., Stern R. C., Schwartz A. L., Wetzler E. M., Chase P. A., Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med. 1976 Dec 9;295(24):1337–1343. doi: 10.1056/NEJM197612092952402. [DOI] [PubMed] [Google Scholar]
- Stocks S. J., Jones A. J., Ramey C. W., Brooks D. E. A fluorometric assay of the degree of modification of protein primary amines with polyethylene glycol. Anal Biochem. 1986 Apr;154(1):232–234. doi: 10.1016/0003-2697(86)90520-8. [DOI] [PubMed] [Google Scholar]
- Wiginton D. A., Hutton J. J. Immunoreactive protein in adenosine deaminase deficient human lymphoblast cell lines. J Biol Chem. 1982 Mar 25;257(6):3211–3217. [PubMed] [Google Scholar]