Abstract
Secretion of inorganic mercury (Hg2+) from proximal tubular cells into the tubular lumen has been shown to involve the multidrug resistance-associated protein 2 (Mrp2). Considering similarities in localization and substrate specificity between Mrp2 and the breast cancer resistance protein (Bcrp), we hypothesize that Bcrp may also play a role in the proximal tubular secretion of mercuric species. In order to test this hypothesis, the uptake of Hg2+ was examined initially using inside-out membrane vesicles containing Bcrp. The results of these studies suggest that Bcrp may be capable of transporting certain conjugates of Hg2+. To further characterize the role of Bcrp in the handling of mercuric ions and in the induction of Hg2+-induced nephropathy, Sprague-Dawley and Bcrp knockout (bcrp−/−) rats were exposed intravenously to a non-nephrotoxic (0.5 μmol • kg−1), a moderately nephrotoxic (1.5 μmol • kg−1) or a significantly nephrotoxic (2.0 μmol • kg−1) dose of HgCl2. In general, the accumulation of Hg2+ was greater in organs of bcrp−/− rats than in Sprague-Dawley rats, suggesting that Bcrp may play a role in the export of Hg2+ from target cells. Within the kidney, cellular injury and necrosis was more severe in bcrp−/− rats than in controls. The pattern of necrosis, which was localized in the inner cortex and the outer stripe of the outer medulla was significantly different from that observed in Mrp2-deficient animals. These findings suggest that Bcrp may be involved in the cellular export of select mercuric species and that its role in this export may differ from that of Mrp2.
Keywords: Breast cancer resistance protein, Multidrug resistance-associated protein 2, mercury, proximal tubule, kidney
1.0 INTRODUCTION
Inorganic mercury (Hg2+) accumulates predominately in the kidneys, specifically in the epithelial cells lining the S1, S2 and S3 segments of proximal tubules (Rodier and Kates, 1988; Rodier et al., 1988; Zalups, 1991; Zalups, 2000). Within biological systems, mercuric ions are thought to bind preferentially to thiol-containing molecules to form thiol S-conjugates of Hg2+ (Zalups, 2000; Bridges and Zalups, 2010). Thiol-S-conjugates of Hg2+ have been shown to be taken up by transport mechanisms present in luminal and basolateral membranes of proximal tubular epithelial cells (Zalups, 2000; Bridges and Zalups, 2010).
Once mercuric ions gain access to the intracellular compartment of target cells, they tend to be retained within the intracellular compartment due to complex binding reactions of these ions with protein- and non-protein thiols (Zalups, 2000; Clarkson et al., 2007). Mercuric ions are powerful electrophiles and thus, retention of these ions within cells may lead to serious deleterious effects in target cells. Indeed, exposure to moderate (1.5 μmol • kg−1 in rats) levels of Hg2+ can lead to acute renal tubular changes, which can be characterized by loss of membrane integrity, atrophy and subsequent death of the epithelial cells lining the proximal tubule. In cases of mild to moderate intoxication, cellular injury and death occur primarily in S2 segments located at the cortico-medullary junction and in S3 segments in the outer stripe of the outer medulla (OSOM) (Zalups and Diamond, 1987; Zalups et al., 1991; Zalups, 1997; Zalups, 2000; Bridges and Zalups, 2010). In cases of severe nephropathy, cellular necrosis may be evident in other segments of the nephron, including S1 segments in the cortex.
A number of recent studies have identified specific mechanisms that are involved in the entry of mercuric species into proximal tubular epithelial cells (Zalups, 2000; Bridges and Zalups, 2010). However, little is known about the precise mechanisms involved in the export of mercuric ions from target cells. In vivo and in vitro studies have recently implicated the multidrug resistance-associated protein 2 (Mrp2), localized in the luminal membrane of proximal tubular cells (Schaub et al., 1999), in the export of certain mercuric species from within proximal tubular cells into the tubular lumen (Bridges et al., 2008a; Bridges et al., 2008b; Zalups and Bridges, 2009; Bridges and Zalups, 2010; Bridges et al., 2011). The results of these studies suggest that additional transport proteins may also be involved in the proximal tubular secretion of mercuric species into the tubular lumen. One potential candidate for this secretion is the breast cancer resistance protein (Bcrp; Abcg2). Like, Mrp2, Bcrp is localized in the apical membrane of proximal tubular epithelial cells (Huls et al., 2008) and it has been shown to be involved in the transport of a wide variety of drugs and xenobiotics (Leslie et al., 2005; Vlaming et al., 2009; Konig et al., 2013). Considering the similarities in localization and substrate specificity between Bcrp and Mrp2, we hypothesize that Bcrp may also play a role in the export of mercuric species from within proximal tubular epithelial cells. To test this hypothesis we 1) assessed the transport of mercuric species in inside-out membrane vesicles containing Bcrp; and 2) examined the disposition and nephrotoxicity of various doses of mercuric chloride (HgCl2) in control and Bcrp knockout rats.
2.0 METHODS
2.1 Animals
Male Bcrp (Abcg2) knockout rats (SD-Abcg2tm1sage; bcrp−/−) were obtained from Sage Labs (Huang et al., 2012; Zamek-Gliszczynski et al., 2012). Male Sprague-Dawley (SD) rats were used as controls and were obtained from Charles River Laboratories. Rats were housed in the Mercer University School of Medicine animal facility. Animals were provided a commercial laboratory diet (Teklad Global Soy Protein Free Extruded Rodent Diet, Harlan Laboratories) and water ad libitum throughout all aspects of the present study. All procedures involving animals were reviewed and approved by the Mercer University Institutional Animal Care and Use Committee. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
2.2 Intravenous Injections
SD and bcrp−/− rats, weighing 225–250 g, were injected intravenously (i.v.) with either a non-nephrotoxic (0.5 μmol • kg−1 • 2 mL−1 normal saline), a moderately nephrotoxic dose (1.5 μmol • kg−1 • 2 mL−1 normal saline) or a significantly nephrotoxic (2.0 μmol • kg−1 • 2 mL−1 normal saline) dose of HgCl2 according to our previously published protocol (Bridges et al., 2008a; Bridges et al., 2008b). HgCl2 is an inorganic salt to which humans and animals may be exposed. The injection solution contained radioactive mercury ([203Hg2+]) and was designed to deliver 1 μCi [203Hg2+] to each animal. [203Hg2+] was generated by neutron activation of mercuric oxide for four weeks at the University of Missouri Research Reactor (MURR) (Belanger et al., 2001; Bridges et al., 2008a).
At the time of injection, each animal was anesthetized with isoflurane and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. A 0.5-μmol, 1.5-μmol or 2.0-μmol • kg−1 dose of HgCl2 was administered into the vein. The wound was closed using two 9-mm stainless steel wound clips. Animals were then housed individually in metabolic cages. Forty-eight hours after injection with HgCl2, animals were sacrificed and organs and tissues were harvested.
2.3 Collection of Organs
At the time of euthanasia, animals were anesthetized with an intraperitoneal (i.p.) injection of ketamine (70 mg • kg−1) and xylazine (30 mg • kg−1). A 1-mL sample of blood was obtained from the inferior vena cava and set aside for determination of [203Hg2+] content. A separate sample of blood was placed in a Microtainer plasma separation tube in order to estimate content of [203Hg2+] in plasma and cellular fractions. The total volume of blood was estimated to be 6% of body weight (Lee and Blaufox, 1985).
The liver and kidneys were also removed from each rat. Each kidney was trimmed of fat and fascia, weighed, and cut in half along the mid-traverse plane. One-half of the right kidney was placed in fixative (40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH) as preparation for histological analyses. The remaining half was frozen in liquid nitrogen for future RNA analyses. One-half of the left kidney was utilized for estimation of [203Hg2+] content. A 3-mm traverse slice was obtained from the remaining half and was used for dissection of renal zones (cortex, outer stripe of the outer medulla (OSOM), inner stripe of the outer medulla, and inner medulla). Each sample was weighed and placed in a separate tube for estimation of [203Hg2+]. The liver was weighed and a 1-g sample was removed for determination of [203Hg2+] content.
Urine and feces were collected in 24-h periods throughout the duration of the experiment. At the end of each 24-h collection period, a 1-mL sample of urine was weighed and placed in a tube for estimation of [203Hg2+] content. All of the feces excreted during each 24-h collection period were counted for estimation of [203Hg2+] content. The content of [203Hg2+] in each sample was determined by counting in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Boston, MA) and the content of Hg2+ in each sample was estimated using standard computational methods.
2.4 Histological Analyses
Kidneys were fixed in 40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH for 48 hours at 4°C. Following fixation, kidneys were washed twice with normal saline and placed in 70% ethanol. Tissues were processed in a Tissue-Tek VIP processor using the following sequence: 95% ethanol for 30 min (twice); 100% ethanol for 30 min (twice); 100% xylene (twice). Tissue was subsequently embedded in POLY/Fin paraffin (Fisher) and 5 μm sections were cut using a Leitz 1512 microtome and were mounted on glass slides. Sections were stained with hematoxylin and eosin (H & E) and were viewed using an Olympus IX70 microscope. Images were captured with a Jenoptix Progress C12 digital camera.
2.5 Measurement of Creatinine and Blood Urea Nitrogen
Plasma creatinine and blood urea nitrogen (BUN) levels were assessed in order to estimate alterations in renal function. Following separation of plasma from cellular components of blood, an aliquot of plasma was stored at −20°C. For determination of plasma creatinine, 30 μL of plasma was utilized and the concentration of creatinine was assessed using the QuantiChrome creatinine assay (BioAssay). Similarly, using a 5 μL sample of plasma, the concentration of BUN was determined using the QuantiChrome urea assay (BioAssay).
2.6 Vesicular Transport Assays
Inside-out membrane vesicles made from Sf9 cells containing mouse Bcrp were purchased from Solvo Biotechnology. Control membrane vesicles made from normal Sf9 cells were also purchased from Solvo Biotechnology. Bcrp transport activity was validated by measuring the uptake of 100 nM [3-H]-estrone sulfate. Cysteine (Cys)-S-conjugates of Hg2+ were utilized for these experiments because there is substantial in vivo and in vitro evidence implicating this mercuric species in the luminal and basolateral uptake of Hg2+ by proximal tubular cells (Zalups, 2000; Bridges et al., 2004; Bridges and Zalups, 2010). Mercuric conjugates of 2,3-dimercapto-1-propane succinate (DMPS) were also examined since this species of Hg has been shown to be transported by the multidrug resistance-associated protein 2 (MRP2) as a means of eliminating mercuric ions from proximal tubular cells (Bridges et al., 2008a; Bridges et al., 2008b; Bridges et al., 2011). Vesicular transport assays were carried out using a rapid filtration method according to a published protocol (Van Aubel et al., 1999; El-Sheikh et al., 2007; Bridges et al., 2013). Briefly, DMPS- and Cys-S-conjugates of Hg2+ were formed by mixing 5 μM (5 nmol • mL−1) [203Hg] with 12.5 μM (12.5 nmol • mL−1) DMPS or Cys, respectively, in incubation buffer (250 mM sucrose, 10 mM Tris/HCl, pH 7.4) supplemented with 10 mM MgCl2, 10 mM creatine phosphate and 100 μg/ml creatine phosphokinase in the presence of 4 mM ATP or AMP. Incubation buffer containing mercuric conjugate or estrone sulfate was added to vesicle mixture (7.5 μg protein) and incubated for 30 seconds at 37°C. Following incubation, ice-cold buffer containing 1 mM DMPS (to remove bound Hg) was added and each sample was filtered through a Multiscreen plate (0.45 μm; Millipore, Billarica, MA). Filters were removed and radioactivity contained on filter was determined using liquid scintillation spectroscopy.
In order to assess ATP-dependent transport, the amount of estrone sulfate or [203Hg] associated with vesicles in the presence of AMP was subtracted from that in the presence of ATP.
2.7 Western Blot Analyses
Western blot analyses utilized kidney sections from three different male bcrp−/− and SD rats that were not exposed to HgCl2. Animals were anesthetized with ketamine (70 mg • kg−1) and xylazine (30 mg • kg−1) and both kidneys were removed. A three-mm slice was obtained from each kidney and the cortex, OSOM, inner stripe of the outer medulla and inner medulla were isolated. Each section was placed in an individual tube and frozen immediately in liquid nitrogen. At the time of protein extraction, tissue sections were pulverized in liquid nitrogen using a mortar and pestle. Following pulverization, RIPA buffer (Sigma, St. Louis, MO), protease inhibitor cocktail and phosphatase inhibitor cocktail were added to the powdered tissue. Samples were mixed and incubated on ice for 45 min. Samples were homogenized, centrifuged and the supernatant was collected for analysis. Protein concentrations were determined using Bradford’s method and the concentration of each sample was adjusted so that 20 μg of protein in Laemmli buffer with β-mercaptoethanol was loaded into each well of a 7.5% Tris-HCl gel (BioRad). The proteins were transferred to a PDVF membrane (BioRad, Hercules, CA) using a Criterion blotter. The membrane was incubated in blocking buffer (BioRad) for one h, followed by incubation with mouse, anti-rat Mrp2 antibody (1:500; Abcam); mouse, anti-rat Bcrp antibody (1:500; Abcam); or mouse, anti-rat β-actin antibody (1:1000; Abcam). The membrane was washed and subsequently incubated with goat, anti-mouse IgG (1:3000; BioRad) and StrepTactin-AP (1:5000, BioRad). After washing, the membrane was incubated in alkaline phosphatase substrate solution (BioRad) and exposed to X-ray film for one min. Band intensity was analyzed three times using Image J software.
2.8 Statistical Analyses
Means for the control and Bcrp-containing vesicles were assessed by the unpaired, Student’s t-test. For each group of vesicles, a mean was generated from three replicate means for each triplicate set of data. A p-value of ≤ 0.05 was considered statistically significant.
Data for each rat experiment were analyzed first with the Kolmogorov-Smirnov test for normality and then with Leven’s test for homogeneity of variances. Data were then analyzed using a 3 x 2 two-way analysis of variance (ANOVA) to assess differences among the means. When statistically significant F-values were obtained with ANOVA, the data were analyzed using Tukey’s post hoc multiple comparison test. A p-value of ≤ 0.05 was considered statistically significant. Each group of animals contained four rats and each experiment was conducted two times.
3.0 RESULTS
3.1 Uptake of Mercuric Conjugates into Inside-out Membrane Vesicles
In order to confirm normal functionality of inside-out membrane vesicles expressing Bcrp, the uptake of [3H]-estrone sulfate was measured in both control and Bcrp-containing vesicles. The ATP-dependent uptake of estrone sulfate was significantly greater in Bcrp-containing vesicles than in control vesicles (Figure 1A).
Figure 1.
The uptake of 100 nM estrone sulfate (A), 5 μM Cys-S-conjugate of Hg2+ (B), and 5 μM DMPS-S-conjugate of Hg2+ (C) was measured in control and Bcrp-expressing inside-out membrane vesicles prepared from Sf9 cells. *, significantly different (p < 0.05) from the corresponding mean for control vesicles treated with the same compound.
When the uptake of Cys-S-conjugates of Hg2+ was measured in control and Bcrp-containing vesicles, uptake was significantly greater in Bcrp-containing vesicles than in controls (Figure 1B). Similarly, when the uptake of DMPS-S-conjugates of Hg2+ was measured in control and Bcrp-containing vesicles, the uptake of this conjugate was greater in Bcrp-containing vesicles than in controls (Figure 1C).
3.2 Disposition of Mercuric Ions in Renal Tissue and Urine
When rats were exposed to 0.5, 1.5 or 2.0 μmol • kg−1 HgCl2, the burden of Hg2+ in the total renal mass (nmol • g−1) was significantly greater in bcrp−/− rats at each dose than in corresponding SD controls (Figure 2). Similarly, the amount of Hg2+ in the renal cortex after each dose was significantly greater in bcrp−/− rats than in corresponding SD rats (Figure 3A). Interestingly, in rats exposed to 0.5 or 1.5 μmol • kg−1 HgCl2, the amount of Hg2+ in the OSOM was not significantly different between SD and bcrp−/− rats (Figure 3B). However, when rats were exposed to 2.0 μmol • kg−1 HgCl2, the amount of Hg2+ in the OSOM was significantly greater in bcrp−/− rats than in SD controls (Figure 3B).
Figure 2.

Amount of Hg2+ in the total renal mass (nmol • g−1) in SD and bcrp−/− rats. Rats were injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2 and kidneys were harvested for determination of Hg2+ content 48 hours later. Data represent mean ± SE of four rats. *, significantly different (p < 0.05) from the corresponding mean for the SD rats exposed to the same dose. +, significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to the 0.5-μmol • kg−1 dose of HgCl2.
Figure 3.
Amount of Hg2+ (nmol • g−1) in the cortex (A) and the outer stripe of the outer medulla (OSOM) (B) in SD and bcrp−/− rats. Rats were injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2 and kidneys were harvested for determination of Hg2+ content 48 hours later. Data represent mean ± SE of four rats. *, significantly different (p < 0.05) from the corresponding mean for the SD rats exposed to the same dose. +, significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to the 0.5-μmol • kg−1 dose of HgCl2.
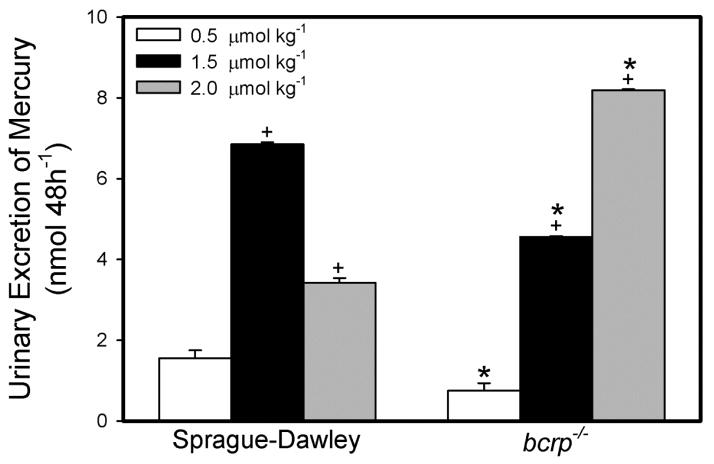
Urine volumes are shown in Table 1. In rats exposed to 0.5 or 1.5 μmol • kg−1 HgCl2, the urinary excretion of mercuric ions was significantly lower in bcrp−/− rats than that of corresponding SD rats (Figure 4). In contrast, when rats were exposed to 2.0 μmol • kg−1 HgCl2, the urinary excretion of Hg2+ was significantly greater in bcrp−/− rats than in controls.
Table 1.
Plasma creatinine and blood urea nitrogen (BUN) levels in Sprague-Dawley and bcrp−/− rats exposed to various doses of HgCl2.
| Strain | Dose | Creatinine (mg/dL) | BUN (mg/dL) |
|---|---|---|---|
| Sprague-Dawley | 0 | 0.34 ± 0.03 | 12.20 ± 0.4 |
| bcrp−/− | 0 | 0.32 ± 0.01 | 7.88 ± 0.1 |
| Sprague-Dawley | 0.5 μmol • kg−1 | 0.33 ± 0.01 | 12.91 ± 0.5 |
| bcrp−/− | 0.5 μmol • kg−1 | 0.40 ± 0.03 | 8.05 ± 0.1 |
| Sprague-Dawley | 1.5 μmol • kg−1 | 1.19 ± 0.64+ | 6.40 ± 1.4 |
| bcrp−/− | 1.5 μmol • kg−1 | 2.53 ± 0.97+ | 41.04 ± 1.5+* |
| Sprague-Dawley | 2.0 μmol • kg−1 | 2.05 ± 0.84+ | 24.08 ± 7.0+ |
| bcrp−/− | 2.0 μmol • kg−1 | 3.62 ± 0.8+* | 31.0 ± 5.4+* |
significantly different from corresponding Sprague-Dawley rats exposed to same dose;
significantly different from rats of same strain rats not exposed to HgCl2.
Figure 4.
Amount of Hg2+ (nmol • 48h−1) excreted in urine of SD and bcrp−/− rats. Rats were injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2 and urine was collected in two 24-h time periods following injection of HgCl2. Data represent mean ± SE of four rats. *, significantly different (p < 0.05) from the corresponding mean for the SD rats exposed to the same dose. +, significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to the 0.5-μmol • kg−1 dose of HgCl2.
3.3 Disposition of Hg2+ in Blood, Liver and Feces
The hematologic burden of Hg2+ in blood was significantly greater in bcrp−/− rats exposed to 1.5 or 2.0 μmol • kg−1 HgCl2 than in corresponding SD rats (Figure 5). The amount of Hg2+ in blood following exposure to 0.5 μmol • kg−1 HgCl2 was not significantly different between SD and bcrp−/− rats.
Figure 5.

Amount of Hg2+ (nmol) in the total blood volume of SD and bcrp−/− rats. Rats were injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2 and were euthanized 48 h later. Blood was collected for determination of Hg2+ content. Data represent mean ± SE of four rats. *, significantly different (p < 0.05) from the corresponding mean for the SD rats exposed to the same dose. +, significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to the 0.5-μmol • kg−1 dose of HgCl2.
The hepatic burden of Hg2+ was significantly greater in bcrp−/− rats following exposure to 1.5 and 2.0 μmol • kg−1 HgCl2 than in corresponding SD rats (Figure 6A). There was no significant difference in the hepatic burden of Hg2+ between SD and bcrp−/− rats exposed to 0.5 μmol • kg−1 HgCl2.
Figure 6.
Amount of Hg2+ in the liver (A) (nmol • g−1) and feces (B) (nmol • 48h−1) of SD and bcrp−/− rats. Rats were injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2 and were euthanized 48 h later, at which time the liver was harvested. Feces were collected in two 24-h time periods. Data represent mean ± SE of four rats. *, significantly different (p < 0.05) from the corresponding mean for the SD rats exposed to the same dose. +, significantly different (p < 0.05) from the corresponding mean for rats of the same strain exposed to the 0.5-μmol • kg−1 dose HgCl2.
The amount of Hg2+ excreted in the feces was significantly greater in bcrp−/− rats exposed to the 1.5 or 2.0-μmol • kg−1 dose of HgCl2 than in corresponding groups of SD rats (Figure 6B). There was no significant difference in fecal elimination of Hg2+ between SD and bcrp−/− rats exposed to the 0.5-μmol • kg−1 dose of HgCl2.
3.4 Histological Analyses of Renal Pathology
No pathological changes were observed in the cortex (data not shown) or the OSOM of SD (Figure 7A) or bcrp−/− (Figure 7B) rats following exposure to 0.5 μmol • kg−1 HgCl2. In contrast, when SD rats were exposed to 1.5 μmol • kg−1 HgCl2, small focal areas of cellular injury, characterized by pyknotic nuclei and eosinophilic cytoplasm, were observed throughout the cortex (data not shown) and OSOM (Figure 7C). In corresponding bcrp−/− rats, cellular injury and death in the OSOM was more widespread and more severe than that of SD rats (Figure 7D). Necrosis was not observed in the cortex of these animals (data not shown). When SD rats were exposed to 2.0 μmol • kg−1 HgCl2, cellular injury and death were more severe than that of corresponding rats exposed to lower doses of HgCl2 (Figure 7E). Areas of cellular necrosis were severe and were widespread through the cortex (data not shown) and OSOM (Figure 7E). In bcrp−/− rats exposed to the 2.0-μmol • kg−1 dose of Hg (Figure 7F), cellular necrosis was more severe than that of corresponding SD rats. In addition, the injury and necrosis observed in the OSOM (Figure 7F) was much more severe than that of the cortex (data not shown).
Figure 7.
Histological analyses of kidneys from SD and bcrp−/− rats injected (i.v.) with 0.5, 1.5, or 2.0 μmol • kg−1 HgCl2. No pathological changes were observed in the outer stripe of the outer medulla (OSOM) of SD (A) or bcrp−/− (B) rats exposed to 0.5 μmol • kg−1 HgCl2. When SD rats were exposed to 1.5 μmol • kg−1 HgCl2, focal areas of cellular injury and death (arrows) were observed in the OSOM (C). In bcrp−/− rats exposed to 1.5 μmol • kg−1 HgCl2, cellular necrosis (arrows) was widespread throughout the OSOM and inner cortex (D). In SD rats exposed to 2.0 μmol • kg−1 HgCl2, cellular necrosis (arrows) was localized to the OSOM and inner cortex and was more severe than that in kidneys of corresponding rats exposed to the 1.5-μmol • kg−1 dose mercury (E). In bcrp−/− rats exposed to 2.0 μmol • kg−1 HgCl2, cellular necrosis (arrows) was more severe than that of corresponding rats exposed to the 1.5-μmol • kg−1 dose mercury.
3.5 Analyses of Serum Creatinine and Blood Urea Nitrogen
Levels of serum creatinine increased significantly in SD and bcrp−/− rats following exposure to the 1.5- or the 2.0-μmol • kg−1 dose of HgCl2, compared with the 0.5-μmol dose (Table 2). In rats exposed to 2.0 μmol • kg−1 HgCl2, plasma creatinine was significantly greater in bcrp−/− rats than in SD rats. Similarly, BUN levels increased significantly in SD rats exposed to 2.0 μmol • kg−1 HgCl2 and in Bcrp−/− rats exposed to 1.5 or 2.0 μmol • kg−1 HgCl2 (Table 1). BUN levels in bcrp−/− rats exposed to the 1.5- or 2.0-μmol • kg−1 dose of HgCl2 were significantly greater than that of corresponding SD rats.
3.6 Western Blot Analyses of Bcrp and Mrp2 in Renal Zones
Representative Western blots are shown in Figure 8. Western blot analyses demonstrated that the expression of Bcrp in SD rats (not exposed to HgCl2) is localized primarily in the cortex and OSOM (Figure 8A). Bcrp protein was not detected in the inner stripe of the outer medulla or in the inner medulla. Expression of Bcrp was normalized against β-actin levels.
Figure 8.

Representative Western blot analyses of Bcrp in SD rats (A) showing that this protein is localized exclusively in the cortex and the outer stripe of the outer medulla (OSOM). The protein levels of Mrp2 (B) were also examined in SD and bcrp−/− rats. Blot results are representative of three different rats. Lane 1: cortex; Lane 2: OSOM; Lane 3: inner stripe of the outer medulla; Lane 4: inner medulla
The expression of Mrp2 was compared in SD and bcrp−/− rats. This protein was detected in the cortex and OSOM of both stains of rats. The expression of Mrp2 was not significantly different between SD and bcrp−/− rats (Figure 8B).
4.0 DISCUSSION
Recent studies provide evidence that Mrp2 plays an important role in the proximal tubular secretion of mercuric ions (Bridges et al., 2008a; Bridges et al., 2008b; Zalups et al., 2014). Given the similarities in function and substrate specificity between Mrp2 and Bcrp (Leslie et al., 2005; Noguchi et al., 2014), we hypothesized that Bcrp may also play a role in the export of mercuric ions from within proximal tubular cells.
Initially, we chose to utilize inside-out membrane vesicles containing Bcrp as a means to test directly whether conjugates of Hg2+ are transportable substrates of this carrier. We examined the transport of Cys- and DMPS-S-conjugates of Hg2+ since these conjugates have been shown previously to be transported by Mrp2 (Bridges et al., 2008b; Bridges et al., 2008a). Furthermore, the Cys-S-conjugate of Hg2+ appears to be the form of Hg2+ that is taken up most readily at the apical membrane of proximal tubular cells (Bridges and Zalups, 2010). Both of the conjugates tested in the current study were transported into inside-out membrane vesicles containing Bcrp suggesting that these two species of Hg2+ may be transportable substrates of Bcrp in vivo.
We then examined the disposition and toxicity of Hg2+ in bcrp−/− and SD rats by exposing rats to 0.5 μmol, 1.5 μmol or 2.0 μmol • kg−1 HgCl2. Following administration of each dose of HgCl2, the renal burden of mercury was significantly greater in bcrp−/− rats than in corresponding SD rats, suggesting that the absence of Bcrp leads to a retention of Hg2+ in the kidneys. The increased renal accumulation of Hg2+ in bcrp−/− rats correlates well with the diminished urinary excretion of Hg2+ in these rats. Interestingly, in rats exposed to 2.0 μmol • kg−1 HgCl2, more Hg2+ was excreted in urine by bcrp−/− rats than that in urine of corresponding SD rats. This finding is most likely related to the observation that the severity of cellular necrosis in kidneys of bcrp−/− rats was greater than that in kidneys of SD rats. Extensive destruction of tubular cells, like that in bcrp−/− rats, can lead to the release of cellular contents, including Hg2+, into the tubular lumen and urine. This pattern of renal Hg2+ accumulation and excretion is similar to that observed previously in Mrp2-deficient animals (Bridges et al., 2008a; Bridges et al., 2008b; Zalups et al., 2014).
When the distribution of Hg2+ within each of the four zones of the kidney was examined, we found that Hg2+ was localized primarily in the cortex and OSOM. The amount of Hg2+ detected in the cortex of bcrp−/− rats was greater than that of SD rats, suggesting that the absence of Bcrp leads to a decrease in the export of Hg2+ and increased retention in S1 and S2 proximal tubular segments. In contrast, the accumulation of Hg2+ in the OSOM was similar in SD and bcrp−/− rats exposed to 0.5 or 1.5 μmol • kg−1 HgCl2. Since some proteins have been shown to be upregulated in bcrp−/− rats (Zamek-Gliszczynski et al., 2013), we initially postulated that the expression of Mrp2 may also be increased in order to compensate for the loss of Bcrp activity. Surprisingly, our Western blot data suggest that Mrp2 protein is not greater in bcrp−/− rats. Another possible explanation for the similar accumulation of Hg2+ in the OSOM of SD and bcrp−/− rats is that the activity, but not the number, of Mrp2 transporters is increased in bcrp−/− rats in order to compensate for the absence of Bcrp. Indeed, Mrp2 activity has been shown to be enhanced (without an increase in protein) by the presence of certain substrates (El-Sheikh et al., 2013; Guyot et al., 2014). A third possible explanation is that additional, yet unknown, transport mechanisms for Hg2+ are present in S3 segments. It is possible that these mechanisms are involved in the export of mercuric species from within proximal tubules located in the OSOM and that this export is able to account for the absence of Bcrp activity.
The current histological analyses show that in kidneys of bcrp−/− rats exposed to the 1.5- or 2.0-μmol • kg−1 dose of HgCl2, cellular necrosis occurs in S2 and S3 segments of proximal tubules located in the inner cortex and the OSOM, which is the typical pattern of Hg+-induced renal injury in normal rats (Diamond and Zalups, 1998; Zalups, 2000; Zalups et al., 2014). Necrosis was more severe in bcrp−/− rats than in SD rats but the localization was similar in both strains of rats. The severity of necrosis correlated well with the current analyses of plasma creatinine and BUN. Interestingly, the localization of tubular necrosis in the current study differs from that of our previous study in which mrp2−/− mice and TR− rats were exposed to nephrotoxic doses of HgCl2 (Zalups et al., 2014). In our previous study, the absence of Mrp2 altered the pattern of nephropathy so that the majority of cellular necrosis was present in the S1 segments of proximal tubules located in the outer cortex (Zalups et al., 2014). Since Mrp2 is localized primarily in S1 segments of proximal tubules, it was hypothesized that the absence of this transporter led to enhanced retention of mercuric ions within cells of S1 and early S2 segments. This retention not only resulted in cellular necrosis in these segments but also protected terminal S2 and S3 segments in that mercuric ions were unable to be secreted from S1 segments and thus, Hg2+ was unable to cause injury to downstream segments of the proximal tubule (Zalups et al., 2014).
Exposure of bcrp−/− rats to nephrotoxic doses of HgCl2 resulted in necrosis in the inner cortex and OSOM rather than in the outer cortex like in Mrp2-deficient animals. We suggest that these differences in the pattern of nephropathy may relate to differences in 1) differences in the distribution of Bcrp and Mrp2 along the proximal tubule, 2) axial heterogeneity in the synthesis and secretion of GSH along the proximal tubule, and/or 3) the ability of Bcrp and Mrp2 to transport mercuric species. Previous and current Western blot analyses suggest that the amount of Mrp2 protein in the cortex (S1 and S2 segments) is significantly greater than that in the OSOM (Zalups et al., 2014). In contrast, the current Western blot analyses indicate that the level of Bcrp protein is similar in cortex and OSOM. Considering that Mrp2 is localized primarily in the cortex and that it has been shown to transport mercuric species, we suggest that it plays a major role in the secretion of mercuric ions from within cells of S1 and S2 proximal tubules. Mrp2 has been shown to utilize GSH as a co-factor for the transport of certain substrates (Leslie et al., 2001; Leslie et al., 2005); therefore, it is possible that GSH-S-conjugates of Hg2+ formed within proximal tubular cells can be secreted into the lumen via Mrp2. It is important to note that the synthesis and secretion of GSH have been shown to be greater in S1 segments than in S2 and S3 segments (Parks et al., 1998). Thus, it is possible that Hg2+ within cells of S1 segments binds to GSH to form a GSH-S-conjugate, which can then be transported into the tubular lumen via Mrp2. Currently, there is no evidence to suggest that Bcrp utilizes GSH as a co-factor for transport; therefore, we suggest that the affinity of Bcrp for GSH-S-conjugates of Hg2+ may be lower than that of Mrp2. If this is indeed the case, Mrp2 may play a predominant role in the tubular secretion of Hg2+ (as a conjugate of GSH) from within S1 segments of proximal tubules while Bcrp may play a more minor role. Consequently, the absence of Mrp2 would greatly reduce the cellular secretion of mercuric ions and enhance the nephropathy in S1 segments while the absence of Bcrp may have less of an effect on this process. Indeed, the absence of Mrp2 led to a 2.5-fold increase in the cortical burden of Hg (Zalups et al., 2014) while the absence of Bcrp led to only a 50% increase, suggesting that Mrp2 may play a greater role than Bcrp in the secretion of mercuric ions from cells of S1 proximal tubular segments.
In the OSOM (S3 segments) in bcrp−/− rats, the accumulation of Hg2+ and the cellular necrosis was somewhat confounding. In bcrp−/− rats, necrosis was present throughout the inner cortex and OSOM. This pattern was in contrast to that in mrp2−/− mice and TR− rats where no necrosis was observed in the OSOM (Zalups et al., 2014). We suggest that this pattern of nephropathy may be because Mrp2 (in S1 and S2 segments) remains functional in bcrp−/− rats and continues to transport mercuric conjugates out of cells lining S1/early S2 segments into the lumen. Once in the lumen, mercuric conjugates are delivered to and taken up by cells of the late S2 and S3 segments, a process that may subsequently lead to cellular necrosis in these segments. Interestingly, necrosis was more severe in bcrp−/− rats than in SD rats. This finding was surprising considering that the amount of Hg2+ in the OSOM was similar in these two strains of rats. Therefore, we suggest that S3 segments of the proximal tubule, particularly in bcrp−/− rats, may be more sensitive to the effects of Hg2+ than segments in other zones of the kidney. Indeed, under certain conditions, S3 segments of proximal tubules have been shown to be more sensitive to heavy metal injury than other segments (Hultman and Enestrom, 1986; Zhang et al., 2008).
When the hematologic burden of Hg2+ was examined, the amount of Hg2+ in the total blood volume of bcrp−/− rats after the 1.5 μmol- and 2.0-μmol • kg−1 doses was significantly greater than that of corresponding SD rats. This difference may be due, in part, to a “saturation” effect in target organs. In other words, once the burden of Hg in a particular organ reaches a certain level, the organ appears to be incapable of taking up additional Hg. This has been shown previously when rats were exposed to higher doses of Hg (Zalups, 1997; Zalups et al., 2014). This “saturation” may be due to 1) saturation of transport mechanisms involved in the uptake of Hg or 2) intoxication of cells and consequent inactivity of transport mechanisms.
In liver, the burden of Hg2+ was significantly greater in bcrp−/− rats exposed to 1.5 or 2.0 μmol • kg−1 HgCl2 than in corresponding SD rats. This finding is not surprising considering that Bcrp is localized in the canalicular membrane of hepatocytes and is thought to participate in the export of drugs and metabolites from within hepatocytes into bile for elimination (Maliepaard et al., 2001). Thus, the absence of Bcrp in hepatocytes of bcrp−/− rats may be related directly to the enhanced accumulation of mercuric ions in these cells. Interestingly, there were no differences in the hepatic burden of Hg2+ between SD and bcrp−/− rats following the 0.5-μmol • kg−1 dose of HgCl2. The reason for this finding is currently unclear, but it may be due to the compensatory activity of another transport protein.
Surprisingly, fecal elimination of mercuric ions was greater in bcrp−/− rats than in corresponding SD rats. This finding may be related to the hematologic burden of Hg2+, which was greater in bcrp−/− rats than in SD rats. A higher hematologic burden of Hg2+ may lead to increased exposure of enterocytes to Hg2+. Previous studies have shown that mercuric ions can be secreted into the lumen of the intestine for elimination in the feces (Zalups, 1998); therefore, it is possible that the increased levels of Hg2+ in blood may lead to increased uptake by enterocytes and eventual secretion and/or leak into the lumen of the intestines.
Multiple mechanisms have been identified for the uptake of Hg2+ into proximal tubular cells. Therefore, it is logical that multiple mechanisms may also be involved in the proximal tubular elimination of Hg2+. The current study provides the first evidence that Bcrp may play a role in the elimination of Hg2+ from target cells. It appears that additional mechanisms, including Mrp2, may also play important roles in the proximal tubular elimination of Hg2+. Defining and characterizing these mechanisms will provide potential therapeutic targets for the treatment of Hg2+ intoxication.
Highlights.
Bcrp may mediate transport of mercury out of proximal tubular cells
Hg-induced nephropathy was more severe in Bcrp knockout rats
Bcrp and Mrp2 may differ in their ability to transport Hg
Acknowledgments
This work was supported by the National Institutes of Health (National Institute of Environmental Health Sciences) grant awarded to Dr. Bridges (ES019991).
Abbreviations
- Bcrp
breast cancer resistance protein
- Mrp2
multidrug-associated resistance protein 2
- DMPS
2,3 dimercapto-1-propane succinate
- Hg2+
inorganic mercury
- OSOM
outer stripe of the outer medulla
- Cys
cysteine
- SD
Sprague-Dawley
- bcrp−/−
Bcrp knockout
- mrp2−/−
Mrp2 knockout
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 REFERENCES
- Belanger M, Westin A, Barfuss DW. Some health physics aspects of working with 203Hg in university research. Health Phys. 2001;80:S28–30. [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, et al. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol. 2004;15:663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, van den Heuvel JJ, et al. Glutathione status and the renal elimination of inorganic mercury in the Mrp2(−/−) mouse. PloS one. 2013;8:e73559. doi: 10.1371/journal.pone.0073559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008a;105:211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008b;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the handling of mercuric ions in rats exposed acutely to inorganic and organic species of mercury. Toxicol Appl Pharmacol. 2011;251:50–58. doi: 10.1016/j.taap.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. Journal of toxicology and environmental health Part B, Critical reviews. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- Diamond GL, Zalups RK. Understanding renal toxicity of heavy metals. Toxicol Pathol. 1998;26:92–103. doi: 10.1177/019262339802600111. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, Greupink R, Wortelboer HM, et al. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Translational research: the journal of laboratory and clinical medicine. 2013;162:398–409. doi: 10.1016/j.trsl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, et al. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320:229–235. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- Guyot C, Hofstetter L, Stieger B. Differential effects of membrane cholesterol content on the transport activity of multidrug resistance-associated protein 2 (ABCC2) and of the bile salt export pump (ABCB11) Mol Pharmacol. 2014;85:909–920. doi: 10.1124/mol.114.092262. [DOI] [PubMed] [Google Scholar]
- Huang L, Be X, Tchaparian EH, et al. Deletion of Abcg2 has differential effects on excretion and pharmacokinetics of probe substrates in rats. J Pharmacol Exp Ther. 2012;343:316–324. doi: 10.1124/jpet.112.197046. [DOI] [PubMed] [Google Scholar]
- Huls M, Brown CD, Windass AS, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- Hultman P, Enestrom S. Localization of mercury in the kidney during experimental acute tubular necrosis studied by the cytochemical Silver Amplification method. Br J Exp Pathol. 1986;67:493–503. [PMC free article] [PubMed] [Google Scholar]
- Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmacogenomics and personalized medicine. 2014;7:53–64. doi: 10.2147/PGPM.S38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LD, Zalups RK, Barfuss DW. Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit. Am J Physiol. 1998;274:F924–931. doi: 10.1152/ajprenal.1998.274.5.F924. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Kates B. Histological localization of methylmercury in mouse brain and kidney by emulsion autoradiography of 203Hg. Toxicol Appl Pharmacol. 1988;92:224–234. doi: 10.1016/0041-008x(88)90382-1. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Kates B, Simons R. Mercury localization in mouse kidney over time: autoradiography versus silver staining. Toxicol Appl Pharmacol. 1988;92:235–245. doi: 10.1016/0041-008x(88)90383-3. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, Konig J, et al. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J Am Soc Nephrol. 1999;10:1159–1169. doi: 10.1681/ASN.V1061159. [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Koenderink JB, Peters JG, et al. Mechanisms and interaction of vinblastine and reduced glutathione transport in membrane vesicles by the rabbit multidrug resistance protein Mrp2 expressed in insect cells. Mol Pharmacol. 1999;56:714–719. [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Advanced drug delivery reviews. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Autometallographic localization of inorganic mercury in the kidneys of rats: effect of unilateral nephrectomy and compensatory renal growth. Exp Mol Pathol. 1991;54:10–21. doi: 10.1016/0014-4800(91)90039-z. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Reductions in renal mass and the nephropathy induced by mercury. Toxicol Appl Pharmacol. 1997;143:366–379. doi: 10.1006/taap.1996.8084. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Intestinal handling of mercury in the rat: implications of intestinal secretion of inorganic mercury following biliary ligation or cannulation. J Toxicol Environ Health A. 1998;53:615–636. doi: 10.1080/009841098159079. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235:10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Diamond GL. Mercuric chloride-induced nephrotoxicity in the rat following unilateral nephrectomy and compensatory renal growth. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53:336–346. doi: 10.1007/BF02890261. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Gelein RM, Cernichiari E. DMPS as a rescue agent for the nephropathy induced by mercuric chloride. J Pharmacol Exp Ther. 1991;256:1–10. [PubMed] [Google Scholar]
- Zalups RK, Joshee L, Bridges CC. Novel hg2+-induced nephropathy in rats and mice lacking mrp2: evidence of axial heterogeneity in the handling of hg2+ along the proximal tubule. Toxicol Sci. 2014;142:250–260. doi: 10.1093/toxsci/kfu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Bedwell DW, Bao JQ, et al. Characterization of SAGE Mdr1a (P-gp), Bcrp, and Mrp2 knockout rats using loperamide, paclitaxel, sulfasalazine, and carboxydichlorofluorescein pharmacokinetics. Drug Metab Dispos. 2012;40:1825–1833. doi: 10.1124/dmd.112.046508. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Goldstein KM, Paulman A, et al. Minor compensatory changes in SAGE Mdr1a (P-gp), Bcrp, and Mrp2 knockout rats do not detract from their utility in the study of transporter-mediated pharmacokinetics. Drug Metab Dispos. 2013;41:1174–1178. doi: 10.1124/dmd.113.051409. [DOI] [PubMed] [Google Scholar]
- Zhang J, Brown RP, Shaw M, et al. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicol Pathol. 2008;36:397–409. doi: 10.1177/0192623308315832. [DOI] [PMC free article] [PubMed] [Google Scholar]