Abstract
Albumin fusion/conjugation (albumination) has been an effective method to prolong in vivo half-life of therapeutic proteins. However, its broader application to proteins with complex folding pathway or multi-subunit is restricted by incorrect folding, poor expression, heterogeneity, and loss of native activity of the proteins linked to albumin. We hypothesized that the site-specific conjugation of albumin to a permissive site of a target protein will expand the utilities of albumin as a therapeutic activity extender to proteins with a complex structure. We show here the genetic incorporation of a non-natural amino acid (NNAA) followed by chemoselective albumin conjugation to prolong therapeutic activity in vivo. Urate oxidase (Uox), a therapeutic enzyme for treatment of hyperuricemia, is a homotetramer with multiple surface lysines, limiting conventional approaches for albumination. Incorporation of p-azido-l-phenylalanine into two predetermined positions of Uox allowed site-specific linkage of dibenzocyclooctyne-derivatized human serum albumin (HSA) through strain-promoted azide-alkyne cycloaddition (SPAAC). The bio-orthogonality of SPAAC resulted in the production of a chemically well-defined conjugate, Uox-HSA, with a retained enzymatic activity. Uox-HSA had a half-life of 8.8 h in mice, while wild-type Uox had a half-life of 1.3 h. The AUC increased 5.5-fold (1657 vs. 303 mU/mL × h). These results clearly demonstrated that site-specific albumination led to the prolonged enzymatic activity of Uox in vivo. Site-specific albumination enabled by NNAA incorporation and orthogonal chemistry demonstrates its promise for the development of long-acting protein therapeutics with high potency and safety.
Keywords: Non-natural amino acid, Site-specific albumination, Therapeutic protein, Urate oxidase, Strain-promoted azide-alkyne cycloaddition
1. Introduction
The past three decades have witnessed the clinical success of therapeutic proteins for treatment of numerous diseases, and the momentum continues overriding even the growth of overall pharmaceutical sectors [1]. One of major considerations in protein therapeutics development is to increase the circulation time to avoid frequent injections. Since therapeutic proteins administered to patients are continuously removed, engineering efforts have been made to shield proteins from glomerular filtration, pinocytosis, and immune response. Employing human serum albumin (HSA) as a drug carrier is one of the effective modifications for prolonging in vivo half-life of a drug. HSA has unusually long circulation time (over two weeks) attributed by electrostatic repulsion in kidneys and FcRn-mediated recycling in endothelium [2–5]. Furthermore, its long serum half-life can be used to increase serum half-lives of other proteins through genetic fusion or chemical conjugation, termed together as albumination. Peptides and small-sized proteins have been successfully albuminated, thereby displaying prolonged circulation and enhanced pharmacodynamics [6–11]. However, there are still challenges to fuse albumin with a therapeutic protein with multi-subunit and complex folded structure due to poor expression and misfolding resulting in a significant therapeutic activity loss [12–14]. The chemical conjugation of albumin to a therapeutic protein generates heterogeneous mixture of the conjugates making the downstream process very difficult, limiting its application to small peptides with a very restricted number of reactive functional groups available for albumin conjugation [5]. Such long-standing hurdles that the conventional methods of albumination have suffered may be overcome by site-specific protein conjugation. A flexible choice of incorporation sites and a site-directed reaction would allow essentially any therapeutic protein to be linked to HSA regardless of folding pathway, multimerization, and abundance of reactive natural amino acids.
The expanded genetic code has made a breakthrough in protein conjugation, allowing the incorporation of non-natural amino acids (NNAAs), in particular those having bio-orthogonal reactivity, into any position of a target protein site-specifically in diverse expression hosts including Escherichia coli, yeast, and CHO cells [15–21]. Upon incorporation, the reactive NNAA serves as a chemical handle to which any molecule of interest with a cognate functional group can be attached without cross-reaction with other natural amino acids [22,23]. Of noteworthy significance is its emerging application to protein therapeutics, e.g., site-specific PEGylation and antibody-drug conjugate [24–28]. It has been shown that combined use of NNAA incorporation and chemoselective chemistry, i.e., site-specific protein conjugation, produces a highly homogenous end product with a minimal loss of native functions, streamlining the manufacturing process and adding greater clinical benefits. Although click chemistry was proven a powerful tool to modify proteins containing an NNAA [29,30], to our knowledge, it was not reported that the combined use of NNAA incorporation and click chemistry can be used to site-specifically conjugate albumin to a therapeutic protein with multi-subunit. Here we demonstrate that albumination through site-specific incorporation of p-azido-l-phenylalanine and bio-orthogonal strain-promoted azidealkyne cycloaddition (SPAAC) (SI Fig. 1E) [31,32] can readily improve the duration of in vivo activity of urate oxidase (Uox)—a therapeutic enzyme with multi-subunit.
Uox originating from Aspergillus flavus is a 135 kDa homotetramer with a large central tunnel with both N- and C-termini in close proximity, and has four active sites located at the interfaces between subunits [33]. Uox catalyzes degradation of a poorly soluble uric acid into a soluble allantoin, thus lowering serum uric acid level, and is used to treat patients suffering from hyperuricemia [34–36]. It suffers rapid clearance upon injection narrowing its applicability to short-term treatment of tumor lysis syndrome [37,38]. Although PEGylation of Uox through random coupling to lysine residues achieved a significant extension of serum half-life [39–42], non-specific and excessive modification raises concerns of heterogeneity, immune responses against PEGs [43], and reduced efficacy [44,45]. Therefore, in order to circumvent these concerns, developing alternative strategies to prolong in vivo activity of Uox is required. Alternative to PEG conjugation, the genetic fusion of HSA to a therapeutic protein is a popular method to generate a long-acting bio-better. However, close proximity between N- or C-termini of each subunit and the multimeric nature of Uox likely prevent a stable formation of a native three-dimensional structure upon fusion to HSA. We therefore investigate whether site-specific albumination could be a novel approach to construct a Uox-HSA conjugate with the prolonged activity. Considering that a tetrameric Uox has a size of 135 kDa exceeding the renal filtration cutoff, FcRn-mediated recycling in endothelium was expected to mainly contribute to the prolonged activity in vivo. Uox variants developed for therapeutic applications have a non-human origin and have varying degrees of immune responses [46]. Prolonged activity of Uox by HSA conjugation is expected to reduce immune response of Uox due to less frequent injections with efficacy comparable to the conventional Uox therapy. Although Uox obtained from A. flavus was used in this study, the use of a recently found ‘human-like’ Uox [46] would further lessen a potential immunogenic concern in the treatment of gout and tumor lysis syndrome. Broadly, this strategy should make it possible to empower long-lived property of HSA to ‘hard-to-fuse’ therapeutic proteins that form multi-subunit complex or have termini important to their native structure/function. An additional advantage lies in the modulation of protein conjugate topology such that the relative orientation between a cargo protein and HSA can be controlled for optimal performance towards their respective biological targets.
2. Materials and methods
2.1. Materials
p-Azido-l-phenylalanine (AzF) was obtained from Chem-Impex International (Wood Dale, IL) and dissolved in 0.2 M NaOH to make 100 mM stock solution. Ni-NTA agarose and pQE80 plasmid were obtained from Qiagen (Valencia, CA). Vivaspin centrifugal concentrators with a MWCO of 50 kDa were purchased from Sartorius Corporation (Bohemia, NY). ZipTip with C18 resin was purchased from Millipore Corporation (Billerica, MA). Sequencing grade modified trypsin was obtained from Promega Corporation (Madison, WI). DBCO-PEG4-carboxyrhodamine, DBCO-PEG4-MAL, and DBCO-PEG4-DBCO were purchased from Bioconjugate Technology Company (Scottsdale, AZ). 15-Azidopentadecanoic acid was obtained from Life Technologies (Gaithersburg, MD). PD-10 desalting columns, HiTrap SP HP cation exchange column, and Superdex 200 10/300 GL size exclusion column were obtained from GE Health care (Piscataway, NJ). UNO Q1 anion exchange column and Biologic DuoFlow chromatography system were obtained from Bio-Rad (Hercules, CA). All chemicals were obtained from Sigma-Aldrich Corporation (St. Louis, MO) unless otherwise stated.
2.2. Plasmid construction and bacterial strains
A plasmid pEVOL-pAzF [47] encoding an AzF-specific engineered pair of tyrosyl-tRNA synthetase/amber suppressor tRNA derived from Methanococcus jannaschii (Plasmid ID: 31186) was obtained from Addgene (Cambridge, MA), and used without modification. To construct a bacterial expression vector for C-terminally His×6-tagged recombinant urate oxidase (Uox) originating from A. flavus, its coding sequence was amplified from pCG62-Uox [36], a kind gift from Dr. Weber (University of Freiburg, Germany), and cloned into pQE80 to give pQE80-Uox. Site-directed mutagenic PCR was performed with pQE80-Uox as a template to replace tryptophan codons at positions 160 and 174 with amber codons (UAG), yielding pQE80-Uox-W160.174amb. All DNA cloning in this study were performed by the restriction-free cloning technique [48]. E. coli TOP10 was transformed with pQE80-Uox for expression of the wild-type Uox (Uox-WT), affording TOP10 [Uox]. As an expression host for AzF-incorporated Uox (Uox-W160.174AzF), genomically engineered E. coli C321.ΔA.exp [49] was obtained from Addgene (ID: 49018), and co-transformed with pEVOL-pAzF and pQE80-UoxW160.174amb, affording C321.ΔA.exp [Uox-W160.174amb].
2.3. Site-specific incorporation of AzFs into Uox and purification
The saturated culture of C321.ΔA.exp [Uox-W160.174amb] was inoculated into fresh 2× YT medium containing 100 µg/mL ampicillin and 35 µg/mL chloramphenicol at 1:100 (v/v) dilution, and was subjected to vigorous shaking (220 rpm) at 37 °C. When the OD600 of 0.5 was reached, AzF solution was added to a final concentration of 1 mM. After 10 min, protein expression was induced by 1 mM IPTG and 0.2% (w/v) l-(+)-arabinose. Cells were harvested after 5 h, and pelleted by centrifugation at 5000 rpm for 10 min before storage at−20 °C. To extract and purify Uox-W160.174AzF, cell pellets were resuspended with the lysis buffer consisting of 50 mM sodium phosphate (pH 7.5), 0.3 M NaCl, 10 mM imidazole, 1 mg/mL lysozyme, DNase, RNase, and protease inhibitor cocktail, and mixed by rotation at 37 °C for 1 h followed by at 4 °C for 2 h. After centrifugation at 11,000 rpm for 30 min, the clear supernatant was recovered, mixed with Ni-NTA agarose for 1 h, and then washed with the washing buffer consisting of 50 mM sodium phosphate (pH 7.5), 0.3 M NaCl, and 20 mM imidazole on a gravity-flow column to remove impurities. Proteins were eluted by the elution buffer consisting of 50 mM sodium phosphate (pH 7.5), 0.3 M NaCl, and 250 mM imidazole, and then buffer-exchanged to a storage buffer consisting of 50 mM HEPES (pH 8.2) and 0.2 M NaCl by a PD-10 column. Expression and purification of Uox-WT were performed similarly except that TOP10 [Uox] was used as an expression host without adding AzF and l-(+)-arabinose.
2.4. Spectrometric quantification of Uox-WT and Uox-W160.174AzF
The molar absorbance of AzF at 280 nm was measured by NanoDrop Spectrometer (Thermo Scientific, Wilmington, DE), and found to be 2620 M−1 cm−1 (data not shown). The molar absorption coefficients, ε280 (M−1 cm−1), of Uox-WT and Uox-W160.174AzF were calculated using the following equation [50],
ε280 = (5500 × nTrp) + (1490 × nTyr) + (2620 × nAz F)
where the molar absorbances of Trp, Tyr, and AzF are multiplied by the number of each residue (nresidue), and then combined. ε280 of Uox-WT and Uox-W160.174AzF were determined to be 53,400 M−1 cm−1 and 47,640 M−1 cm−1, respectively. The concentrations were measured by the Beer–Lambert Law [51].
2.5. MALDI-TOF mass spectrometry
Proteins in the storage buffer at 0.5 mg/mL were digested with trypsin at 37 °C overnight, and then desalted on a ZipTip C18 according to the manufacturer's protocol. Purified tryptic digests mixed with DHB matrix (20 mg/mL of 2,5-dihydroxybenzoic acid and 2 mg/mL of l-(−)-fucose dissolved in 10% ethanol) at 1:1 (v/v) were subjected to mass characterization by Microflex MALDI-TOF M/S (Bruker Corporation, Billerica, MA).
2.6. Labeling of Uox-W160.174AzF by SPAAC
Uox-WT and Uox-W160.174AzF at 30 µM in the storage buffer were separately reacted with DBCO-PEG4-carboxyrhodamine at 100 µM at RT for 2 h, and then loaded onto SDS-PAGE to measure in-gel fluorescence in a BioSpectrum imaging system (UVP, Upland, CA). Upon illumination at λex = 480 nm, the emitted light above 510 nm was captured. Conjugation of DBCO-PEG4-palmitic acid was performed by reacting Uox-W160.174AzF at 30 µM in the storage buffer with DBCO-PEG4-DBCO at 150 µM at RT for 6 h, affording Uox-PEG4-DBCO. After desalting on a PD-10, five equivalents of 15-azidopentadecanoic acid was added to Uox-PEG4-DBCO, and then reacted at RT for 2 h to give Uox-PEG4-palmitic acid.
2.7. Circular dichroism (CD) and data analysis
The secondary structures of Uox-WT and Uox-W160.174AzF in 10 mM sodium phosphate (pH 7.0) were evaluated using a Jasco 710 spectropolarimeter with 1-mmpath length quartz cuvette at room temperature at a protein concentration of 0.03 mg/mL. The background-subtracted sample spectra were then deconvoluted to obtain numerical estimations of secondary structure content using the DichroWeb online CD analysis software server [52,53], employing the SELCON3 analysis program along with ‘Set #4’ reference sets [54,55].
2.8. Generation of Uox-HSA conjugate
Lyophilized human serum albumin (HSA) was dissolved in PBS, and purified on an anion exchange column, UNO Q1, to remove high-molecular-weight impurities. The purified HSA at 50 µM was conjugated to DBCO-PEG4-MAL at 200 µM by thiol–maleimide coupling in PBS buffered at pH 7.0 for 2 h, and then buffer-exchanged to 20 mM sodium phosphate (pH 6.0), yielding HSA-PEG4-DBCO. To synthesize Uox-HSA conjugate by SPAAC, Uox-W160.174AzF was mixed with HSA-PEG4-DBCO at 1:1 molar ratio in 20 mM sodium phosphate (pH 6.0), and then concentrated to a final protein concentration of 10 mg/mL. After 7 h incubation at room temperature, the mixture was subjected to the two-step column chromatography consisting of a cation exchange one removing unreacted HSA-PEG4-DBCO and an anion exchange one removing unreacted Uox-W160.174AzF to obtain a purified Uox-HSA conjugate. The cation exchange chromatography was run on a HiTrap SP-HP equilibrated in 20 mM sodium phosphate (pH 6.0) with NaCl gradient elution. The eluate containing Uox-HSA conjugate and residual Uox-W160.174AzF was desalted and buffer-exchanged to 20 mM bis– tris (pH 6.5) on a centrifugal filter with MWCO of 50 kDa, and then loaded onto the anion exchanger, UNO Q1, equilibrated with 20 mM bis–tris (pH 6.5). Pure Uox-HSA conjugate was eluted by applying NaCl gradient. Proteins were analyzed by SDS-PAGE in tris/glycine buffer system after reduction by DTT or subjected to size-exclusion chromatography using Superdex 200 10/300 GL column, equilibrated with PBS (pH 7.4).
2.9. Enzymatic activity assay
The spectrophotometric method was used to determine the kinetic constants of Uox-WT and Uox-HSA [46]. After determining concentrations by BCA protein assay (Thermo Scientific, Wilmington, DE) and the densitometric analysis of subunit composition, Uox-WT or Uox-HSA at 60 nM was reacted with uric acid at various concentrations in 200 µL of the assay buffer consisting of 50 mM sodium borate (pH 9.5) and 0.2 M NaCl. Reduction in absorbance at 293 nm attributed by conversion of uric acid to 5-hydroxyisourate was monitored in triplicate at 25 °C in a standard 96-well plate on the Synergy™ four multimode microplate reader (BioTek, Winooski, VT). The consumption rate of uric acid (nmol/min) was obtained by dividing the rate of OD change (min−1) by a molar absorptivity of uric acid (12,300 M−1 cm−1) [56] and a path length (0.56 cm), and then multiplying an assay volume (2.0 × 105 nL). Average consumption rates at each substrate concentration were fitted to a typical Michaelis–Menten curve to yield Vmax and Km. In order to evaluate the enzymatic activity in blood, 10 µL of serum was mixed with 190 µL of the assay buffer containing 100 µM of uric acid, and then monitored as described above. The enzymatic activity was obtained in an arbitrary unit (mU/mL) by dividing the consumption rate of uric acid by the volume of serum (10−2 mL) used in the assay, where one unit (mU) is defined as the amount of an enzyme used to catalyze the oxidation of 1 nmol of uric acid per minute at 25 °C.
2.10. In vivo study of residual Uox activity in serum
The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Virginia. Enzymatic activities of Uox-WT and Uox-HSA in vivo were investigated by injecting 5.7 nmol (based on a monomeric Uox) of each protein in 200 µL PBS into the tail vein of young female C57BL/6 mice (n = 4). Female C57BL/6 mice were chosen in this study, because they have been successfully used for the PK studies including one in our laboratory [28, 57, 58]. The blood was sampled at 0.5, 1, 2, and 4 h post-injection for Uox-WT, and at 0.5, 1, 2, 4, 8, 12 and 24 h post-injection for Uox-HSA.
3. Results
3.1. Site-specific double incorporation of AzFs into permissive sites of Uox
In order to take advantage of an unusually long serum half-life of HSA, we chose to conjugate the Uox variant containing AzFs (SI Fig. 1A) with HSA using a hetero-bifunctional hydrophilic linker, DBCO-PEG4-MAL (SI Fig. 1B), to ensure chemoselectivity and native activity of Uox and HSA. Cysteine at position 34 (C34) of HSA can be readily utilized for thiol-maleimide cross-linking since C34 is the only free cysteine and located away from FcRn-binding domain (Fig. 1) [59,60]. DBCO can be chemoselectively conjugated to an azide functional group via SPAAC. AzF is a non-natural amino acid containing an azide group.
Fig. 1.
Schematic representation of Uox-HSA conjugate. C34 (yellow sphere) of HSA is located remote from the FcRn-binding domain (colored orange), and linked to DBCO-PEG4-MAL through thiol–maleimide coupling to yield HSA-PEG4-DBCO. Uox is a homotetramer each of which is colored differently. Strain-promoted azide-alkyne cycloaddition (SPAAC) mediates site-specific albumination between Uox-W160.174AzF and HSA-PEG4-DBCO. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Uox can be made reactive towards DBCO by genetically incorporating AzFs into permissive sites of Uox. Predetermined sites in Uox not critical for native activity could be targeted for chemically well-defined conjugation with HSA. To determine mutation sites which are least likely to perturb the native structure and function of Uox upon substitution by AzFs, amino acids bearing an aromatic group, Phe, Trp, and Tyr, were chosen as potential targets for their structural similarity with AzF. Next, ten Phes, seven Trps, and ten Tyrs in the primary structure of Uox were screened for their solvent accessibility by ASA-View server [61] (SI Fig. 2A). A residue with higher solvent accessibility is preferred for AzF incorporation since subsequent conjugation with DBCO moiety would be made effective by increased chance of collisions. Four sites (W160, W174, F258, and W264) showed relatively high solvent accessibility. Two conjugation sites–Trps at positions 160 (W160) and 174 (W174) with high solvent accessibility of 0.46 and 0.62–were selected for double AzF incorporation (SI Fig. 2B). F258 and W264, despite their high solvent accessibility, were excluded since they are located in subunit interfaces and so the conjugation at those sites will likely lead to substantial perturbations of the Uox subunit assembly. Importantly, W160 and W174 have not been reported to play a role in structural/functional integrity of Uox. In the preliminary experiments, we first tested single AzF incorporation atW160, which led to the conjugation efficiency below 10% (data not shown). Therefore, we chose to incorporate two AzFs into Uox, eventually providing eight reactive sites per tetrameric Uox, in order to enhance conjugation efficiency between two macromolecules—Uox and HSA. It has been reported that C321.ΔA.exp strain can incorporate an NNAA at multiple sites without compromising protein production yield [49]. Uox-W160.174AzF was expressed from C321.ΔA.exp [Uox-W160.174amb] in the presence of 1 mM AzF, and then purified by His-tag affinity chromatography with a yield of 8 mg/L of culture medium.
Fidelity of double AzF incorporation was analyzed by tryptic digestion of Uox-W160.174AzF, along with Uox-WT as a control, followed by MALDI-TOF mass spectrometry. For Uox-WT, two tryptic digests, X and Y, containing W160 and W174, respectively, were found at 1342.8 m/z (theoretical m/z=1342.7) and 706.3 m/z (theoretical m/z = 706.3) (Fig. 2A, top). The replacement of tryptophan (204.2 Da) with AzF (206.2 Da) is expected to yield m/z change of +2, i.e., 1344.7 m/z for X-Az and 708.3 m/z for Y-Az. However, major shifts were detected at 1318.4m/z and 682.1 m/z, respectively (Fig. 2A, bottom).
Fig. 2.
Validation of site-specific incorporation of AzFs into Uox. (A) MALDI-TOF M/S analyses of trypsin-digested Uox-WT (top) and Uox-W160.174AzF (bottom). Peptide Y (residues 172–176; ETWDR) contains the 174th Trp which is substituted by AzF in Peptide Y-Az. Peptide X (residues 154–164, STNSQFWGFLR) contains the 160th Trp which is substituted by AzF in Peptide X-Az. (B) Protein gel images of Uox-W160.174AzF and Uox-WT treated with a fluorescent dye, DBCO-PEG4-carboxyrhodamine. The gel was subjected to UV (390 nm) irradiation to excite the fluorophore (fluorescence panel), and then stained with Coomassie blue (Coomassie panel) to visualize proteins. (C) CD spectra of Uox-WT (solid) and Uox-W160.174AzF (dotted).
It was previously reported that an aryl and an aliphatic azide is unusually susceptible to fragmentation via expulsion of N2 during matrix-assisted laser desorption ionization leading to the generation of amine group [62–65], which is evidenced by the major shifts corresponding to a substitution of Trp by amino-phenylalanine (AmF, 180.20 Da). To further verify that the mass discrepancy had resulted from fragmentation during MALDI-TOF analysis, not from metabolic conversion of AzF during cell culture, Uox-W160.174AzF was reacted with a DBCO-functionalized polymer, DBCO-PEG4-palmitic acid (1138.4 Da, SI Fig. 1D). New m/z signals corresponding to conjugates formed by SPAAC between DBCO-PEG4-palmitic acid and X-Az or Y-Az were observed at 2482.5 (theoretical = 2483.0) and 1846.6 (theoretical = 1846.7), respectively (SI Fig. 3). Furthermore, no or substantially reduced intensity of original m/z values were detectable, indicating the high SPAAC reactivity of two AzFs incorporated. The orthogonal reactivity of SPAAC was visualized by dye-labeling. DBCO-PEG4-carboxyrhodamine (880.9 Da, SI Fig. 1C) was reacted with Uox-WT and Uox-W160.174AzF, separately, and then analyzed by in-gel fluorescence and migration. In contrast to undetectable fluorescence of Uox-WT, Uox-W160.174AzF exhibited high fluorescence upon excitation and slower migration resulting from dye conjugation (Fig. 2B). In addition, double bands in Fluorescence panel as well as triple bands in Coomassie panel demonstrate double incorporation and progressive labeling of both azide groups.
Circular dichroism spectroscopy (CD) has been successfully used to investigate secondary structural changes in a target protein [53,66]. Therefore, in order to determine whether there are any structural changes in Uox upon AzF incorporation, CD was employed to characterize and compare the secondary structures of Uox-WT and Uox-W160.174AzF. The CD spectrum of Uox-W160.174AzF showed no significant deviation from that of Uox-WT (Fig. 2C). The secondary structure contents analyzed from the CD spectra also supported that the structural perturbation of Uox by AzF double incorporation was negligible (Table 1).
Table 1.
Secondary structure contents of Uox-WT and Uox-W160.174AzF.
| α-Helix | β-Sheet | β-Turn | Unordered | |
|---|---|---|---|---|
| Uox-WT | 0.557 | 0.093 | 0.124 | 0.237 |
| Uox-W160.174AzF | 0.549 | 0.097 | 0.126 | 0.227 |
3.2. Synthesis and characterization of Uox-HSA
To append DBCO functionality to HSA, a bifunctional linker, DBCO-PEG4-MAL, was attached to C34 through Michael addition between a thiol and a maleimide, affording HSA-PEG4-DBCO. Site-specific conjugation of Uox to HSA through SPAAC was performed by mixing Uox-W160.174AzF with HSA-PEG4-DBCO, and confirmed by a new band at 150 kDa (Fig. 3, lane 3). After removing residual HSA-PEG4-DBCO by cation exchange chromatography (SI Fig. 4A), the partially purified Uox-HSA solution was subjected to anion exchange chromatography and then the purified Uox-HSA conjugate was obtained (SI Fig. 4B). The absence of HSA band in the purified Uox-HSA sample indicates that any residual unreacted HSA molecules were effectively removed during the purification process (Fig. 3, lane 4). The band of Uox monomer in the purified Uox-HSA sample can be explained by the disruption of non-covalent subunit interaction of the tetrameric Uox-HSA in the presence of denaturing SDS.
Fig. 3.
Coomassie blue-stained SDS-PAGE gel: lane 1, Uox-W160.174AzF; lane 2, HSA-PEG4-DBCO; lane 3, reaction mixture; and lane 4, column-purified Uox-HSA.
The molar ratio of an unreacted Uox monomer to a monomeric Uox conjugated to HSA was calculated on the basis of the Coomassie-stained band intensities, and found to be approximately 2.5 (SI Fig. 5A). In order to investigate the subunit stoichiometry in a native tetrameric form, Uox-HSA was subjected to size exclusion chromatography. In comparison to the unmodified Uox-W160.174AzF tetramer which eluted as a single symmetric peak, Uox-HSA displayed a major peak at an earlier elution volume followed by a smaller peak corresponding to the unmodified tetramer (SI Fig. 5B). The major peak is likely to represent Uox tetramer carrying one HSA while a small poorly resolved peak observed at the elution front appears to contain Uox tetramer carrying mostly two or more HSAs. This is supported by a simple random combination analysis of the subunit stoichiometry of Uox-HSA based upon the molar ratio determined by densitometry, in which Uox tetramer having one HSA has been found to be the most probable tetrameric assembly of subunits, provided that HSA-conjugated Uox monomers has the same subunit interaction with unconjugated Uox monomers (SI Table 1).
The effect of HSA conjugation on enzymatic activity was evaluated by Michaelis–Menten kinetics (Fig. 4). The catalytic rates of both Uox-HSA and Uox-WT were determined at varying concentrations of uric acid. Uox-HSA had a Vmax comparable to that of Uox-WT (Table 2). Although Uox-HSA had a lower Km for uric acid than that of Uox-WT (Table 2), the difference was not substantial due to relatively large standard errors of regression. These kinetic analysis results clearly indicated that the enzymatic activity of Uox was fully retained upon HSA conjugation.
Fig. 4.
Michaelis–Menten plots. The uric acid-degrading assay was performed at various substrate concentrations with a fixed enzyme concentration of 60 nM. Means (n = 3) ± standard deviations of Uox-WT or Uox-HSA were fitted to a curve to calculate kinetic parameters.
Table 2.
Kinetic parameters.
| Vmax (nmol/min) |
Km (µM) |
|
|---|---|---|
| Uox-WT | 1.74 ± 0.18 | 164.6 ± 35.2 |
| Uox-HSA | 1.76 ± 0.16 | 125.5 ± 25.6 |
Values ± standard errors of regression.
3.3. In vivo study
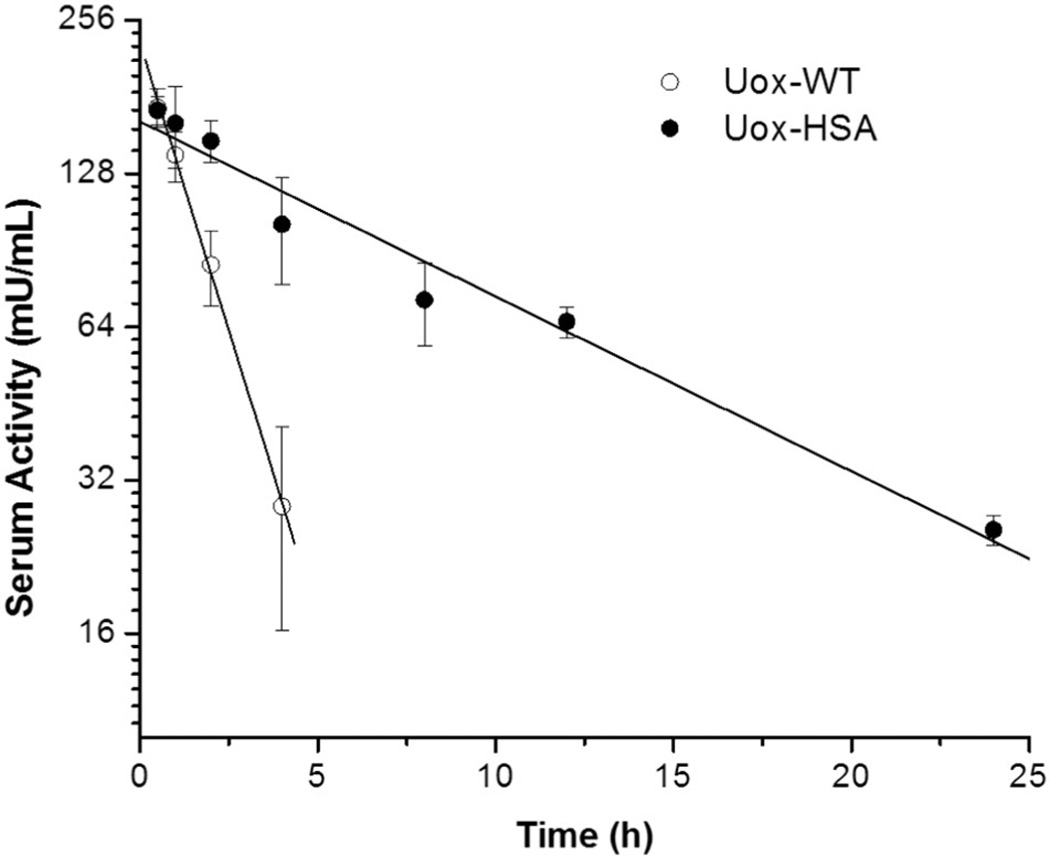
In order to evaluate pharmacological benefits from site-specific albumination, a single dose of either Uox-WT or Uox-HSA was intravenously administered to mice (n = 4), and their enzymatic activities in serum samples taken at different time points were measured by the uric acid-degradation assay. Assuming the one-compartment distribution with first-order elimination [28,67], the logarithmic residual activity versus time post-injection was fitted to amono-exponential decay to calculate a serum activity half-life (t1/2) and area under the curve (AUC) (Fig. 5). In contrast to rapid elimination of Uox-WT with a t1/2 of 1.3 h, Uox-HSA exhibited a prolonged activity with a t1/2 of 8.8 h (Table 3).
Fig. 5.
Residual enzymatic activity in serum of intravenously injected Uox-WT and Uox-HSA in mice (n = 4). Uric acid-degrading activities of Uox-WT and Uox-HSA were measured from blood samples drawn at different time points: 0.5, 1, 2, and 4 h for Uox-WT; 0.5, 1, 2, 4, 8, 12, and 24 h for Uox-HSA. The catalytic reaction rate per mL of serum on a logarithmic scale over time was plotted to give a linear fit. Each data point represents the mean (n = 4) ± standard deviations.
Table 3.
In vivo study results.
From 0.5 to 4 h.
From 0.5 to 24 h.
4. Discussion
Site-specific albumination of a therapeutic protein with complex structures, i.e. multi-subunit, was successfully demonstrated by the combination of site-specific incorporation of AzF and SPAAC. The high fidelity reassignment of a UAG codon to encode AzF was achieved by exploiting a genomically engineered E. coli equipped with an engineered pair of tyrosyl-tRNA synthetase/amber suppressor tRNA [47,68]. The absence of release factor 1 (RF1) and genomic UAG codons in the expression host rendered the pair strictly targeted to amber mutations of Uox-encoding gene without unwanted truncation by RF1, allowing stable expression of Uox containing AzFs at two distinct sites.
A careful selection of NNAA incorporation sites lies at the core of successful site-specific albumination. First, incorporation should be made at a surface-exposed position for high-yielding conjugation. It has been reported that higher solvent-accessibility of a NNAA generally ensures higher conjugation efficiency [69–71]. However, it should be noted that NNAA substitution for an original amino acid with a fairly different structure and charge may undermine the accessibility-efficiency correlation [72]. Therefore, AzF incorporation sites, W160 and W174, were chosen out of amino acids bearing an aromatic ring, i.e., Phe, Trp, and Tyr, as well as high solvent accessibility. Judging from mass spectra of Uox conjugated to DBCO-PEG4-palmitic acid (SI Fig. 3) and the SDS-PAGE analysis upon dye conjugation (Fig. 2B), our scheme seems to be suitable for selecting incorporation site with high conjugation efficiency. Second, the incorporation site should not be critically involved in native function of a target protein. Combining the literature search and three-dimensional structure analysis, the best incorporation sites can be predicted from candidates screened by the solvent accessibility.
The prolonged activity of Uox-HSA relative to Uox-WT demonstrates the benefits of site-specific albumination. Random PEGylation onto multiple lysine residues of Uox resulted in severe heterogeneity in the conjugates and reduced activity, though the serum half-life was significantly extended [73]. In particular, PEGylation extended the serum activity half-life of Uox obtained from Candida utilis from 1 h to 8 h in mice [74]. Although Uox from A. flavus was used in this study, single HSA conjugation to Uox led to the prolonged activity comparable to PEGylation.
Emerging concerns over immunogenicity of the methoxy terminus of PEG should not be overlooked [75,76]. Tetra(ethylene glycol) (PEG4) used here as a backbone of the heterobifunctional linker, DBCO-PEG4-MAL, has no exposed terminus upon conjugation, and its short length greatly minimizes the immunogenic potential [75]. Moreover, if PEG in the linker causes any immune response issue, the linker can be easily modified not to include PEG without altering the overall site-specific albumination scheme. Therefore, site-specific albumination is a promising platform for developing long acting versions of large and complex proteins. The elimination kinetics of Uox-HSA activity observed in the mice study resulted in 6.8-fold increase in serum activity half-life, in comparison to Uox-WT. Furthermore, it is very likely that the effect of FcRn-mediated recycling of Uox-HSA would be more pronounced in higher animal models since the murine FcRn is known to exhibit binding affinity towards HSA substantially lower than that between human FcRn and HSA [77]. Introduction of HSA variants with higher FcRn affinity in site-specific albumination might contribute to a much longer extension of activity in vivo [78].
5. Conclusions
We show here the site-specific conjugation of HSA to Uox with multi-subunit is a promising approach to prolonging in vivo efficacy, which is challenging to achieve by conventional bioconjugation or genetic fusion techniques. Genetic incorporation of NNAAs into permissive positions of Uox followed by albumination through bio-orthogonal chemistry generated a well-defined conjugate with retained enzymatic activity and longer duration in vivo. The platform may find diverse applications for delivery of therapeutic proteins.
Supplementary Material
Acknowledgments
We appreciate Dr. Nicholas E. Sherman and Dr. Peter Krueger at the University of Virginia for their assistance with mass spectrometry analysis and animal study. This work was mainly supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (2014R1A2A1A11050322). This work was also supported by the Bio Imaging Research Center at GIST.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jconrel.2015.04.004.
References
- 1.Aggarwal RS. What's fueling the biotech engine—2012 to 2013. Nat. Biotechnol. 2014;32(1):32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- 2.Kontos S, Hubbell JA. Drug development: longer-lived proteins. Chem. Soc. Rev. 2012;41(7):2686–2695. doi: 10.1039/c2cs15289d. [DOI] [PubMed] [Google Scholar]
- 3.Elsadek B, Kratz F. Impact of albumin on drug delivery—new applications on the horizon. J. Control. Release. 2012;157(1):4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 4.Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. J. Control. Release. 2012;161(2):429–445. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta. 2013;1830(12):5526–5534. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Duttaroy A, Kanakaraj P, Osborn BL, Schneider H, Pickeral OK, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R, Crossan D, Bock J, Kaufman TE, Reavey P, Carey-Barber M, Krishnan SR, Garcia A, Murphy K, Siskind JK, McLean MA, Cheng S, Ruben S, Birse CE, Blondel O. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54(1):251–258. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 7.Ikuta S, Chuang VT, Ishima Y, Nakajou K, Furukawa M, Watanabe H, Maruyama T, Otagiri M. Albumin fusion of thioredoxin—the production and evaluation of its biological activity for potential therapeutic applications. J. Control. Release. 2010;147(1):17–23. doi: 10.1016/j.jconrel.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Joung CH, Shin JY, Koo JK, Lim JJ, Wang JS, Lee SJ, Tan HK, Kim SL, Lim SM. Production and characterization of long-acting recombinant human albumin–EPO fusion protein expressed in CHO cell. Protein Expr. Purif. 2009;68(2):137–145. doi: 10.1016/j.pep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Osborn BL, Sekut L, Corcoran M, Poortman C, Sturm B, Chen G, Mather D, Lin HL, Parry TJ. Albutropin: a growth hormone–albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur. J. Pharmacol. 2002;456(1–3):149–158. doi: 10.1016/s0014-2999(02)02644-4. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–1886. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian GM, Fiscella M, Lamouse-Smith A, Zeuzem S, McHutchison JG. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat. Biotechnol. 2007;25(12):1411–1419. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 12.Cordes AA, Platt CW, Carpenter JF, Randolph TW. Selective domain stabilization as a strategy to reduce fusion protein aggregation. J. Pharm. Sci. 2012;101(4):1400–1409. doi: 10.1002/jps.23049. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt SR. Fusion Protein Technologies for Biopharmaceuticals: Applications and Challenges. New Jersey: Wiley; 2013. p. 95. [Google Scholar]
- 14.Zhao HL, Xue C, Wang Y, Sun B, Yao XQ, Liu ZM. Elimination of the free sulfhydryl group in the human serum albumin (HSA) moiety of human interferonalpha2b and HSA fusion protein increases its stability against mechanical and thermal stresses. Eur. J. Pharm. Biopharm. 2009;72(2):405–411. doi: 10.1016/j.ejpb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Kwon I, Wang P, Tirrell DA. Design of a bacterial host for site-specific incorporation of p-bromophenylalanine into recombinant proteins. J. Am. Chem. Soc. 2006;128(36):11778–11783. doi: 10.1021/ja0626281. [DOI] [PubMed] [Google Scholar]
- 16.Plass T, Milles S, Koehler C, Schultz C, Lemke EA. Genetically encoded copper-free click chemistry. Angew. Chem. Int. Ed. Engl. 2011;50(17):3878–3881. doi: 10.1002/anie.201008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seitchik JL, Peeler JC, Taylor MT, Blackman ML, Rhoads TW, Cooley RB, Refakis C, Fox JM, Mehl RA. Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. J. Am. Chem. Soc. 2012;134(6):2898–2901. doi: 10.1021/ja2109745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Brock A, Herberich B, Schultz PG. Expanding the genetic code of Escherichia coli. Science. 2001;292(5516):5498–500. doi: 10.1126/science.1060077. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003;125(11):3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 20.Chen PR, Groff D, Guo J, Ou W, Cellitti S, Geierstanger BH, Schultz PG. A facile system for encoding unnatural amino acids in mammalian cells. Angew. Chem. Int. Ed. Engl. 2009;48(22):4052–4055. doi: 10.1002/anie.200900683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock SM, Uprety R, Deiters A, Chin JW. Expanding the genetic code of yeast for incorporation of diverse unnatural amino acids via a pyrrolysyl-tRNA synthetase/ tRNA pair. J. Am. Chem. Soc. 2010;132(42):14819–14824. doi: 10.1021/ja104609m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King M, Wagner A. Developments in the field of bioorthogonal bond forming reactions-past and present trends. Bioconjug. Chem. 2014;25(5):825–839. doi: 10.1021/bc500028d. [DOI] [PubMed] [Google Scholar]
- 23.Spicer CD, Davis BG. Selective chemical protein modification. Nat. Commun. 2014;5:4740. doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 24.Cho H, Daniel T, Buechler YJ, Litzinger DC, Maio Z, Putnam AM, Kraynov VS, Sim BC, Bussell S, Javahishvili T, Kaphle S, Viramontes G, Ong M, Chu S, Becky GC, Lieu R, Knudsen N, Castiglioni P, Norman TC, Axelrod DW, Hoffman AR, Schultz PG, DiMarchi RD, Kimmel BE. Optimized clinical performance of growth hormone with an expanded genetic code. Proc. Natl. Acad. Sci. U. S. A. 2011;108(22):9060–9065. doi: 10.1073/pnas.1100387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg. Med. Chem. Lett. 2004;14(23):5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 26.Sun SB, Schultz PG, Kim CH. Therapeutic applications of an expanded genetic code. Chembiochem. 2014;15(12):1721–1729. doi: 10.1002/cbic.201402154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, Phuong T, Barnett R, Hehli B, Song F, DeGuzman MJ, Ensari S, Pinkstaff JK, Sullivan LM, Biroc SL, Cho H, Schultz PG, DiJoseph J, Dougher M, Ma D, Dushin R, Leal M, Tchistiakova L, Feyfant E, Gerber HP, Sapra P. A general approach to site-specific antibody drug conjugates. Proc. Natl. Acad. Sci. U. S. A. 2014;111(5):1766–1771. doi: 10.1073/pnas.1321237111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim SI, Mizuta Y, Takasu A, Hahn YS, Kim YH, Kwon I. Site-specific fatty acid-conjugation to prolong protein half-life in vivo. J. Control. Release. 2013;170(2):219–225. doi: 10.1016/j.jconrel.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41(14):2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Q, Saha S, Lee LA, Barnhill H, Oxsher J, Dreher T, Wang Q. Chemoselective modification of turnip yellow mosaic virus by Cu(I) catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction and its application in cell binding. Bioconjug. Chem. 2011;22(1):58–66. doi: 10.1021/bc100351n. [DOI] [PubMed] [Google Scholar]
- 31.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004;126(46):15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 32.Ning X, Guo J, Wolfert MA, Boons GJ. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem. Int. Ed. Engl. 2008;47(12):2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabison L, Chiadmi M, El Hajji M, Castro B, Colloc'h N, Prange T. Near-atomic resolution structures of urate oxidase complexed with its substrate and analogues: the protonation state of the ligand. Acta Crystallogr. D Biol. Crystallogr. 2010;66(Pt 6):714–724. doi: 10.1107/S090744491001142X. [DOI] [PubMed] [Google Scholar]
- 34.Bessmertny O, Robitaille LM, Cairo MS. Rasburicase: a new approach for preventing and/or treating tumor lysis syndrome. Curr. Pharm. Des. 2005;11(32):4177–4185. doi: 10.2174/138161205774913291. [DOI] [PubMed] [Google Scholar]
- 35.Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, Goldman SC, Jeha SC, Shanholtz CB, Leonard JP, McCubrey JA. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January 2002. Leukemia. 2003;17(3):499–514. doi: 10.1038/sj.leu.2402847. [DOI] [PubMed] [Google Scholar]
- 36.Geraths C, Daoud-El Baba M, Charpin-El Hamri G, Weber W. A biohybrid hydrogel for the urate-responsive release of urate oxidase. J. Control. Release. 2013;171(1):57–62. doi: 10.1016/j.jconrel.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Jeha S, Pui CH. Recombinant urate oxidase (rasburicase) in the prophylaxis and treatment of tumor lysis syndrome. Contrib. Nephrol. 2005;147:69–79. doi: 10.1159/000082545. [DOI] [PubMed] [Google Scholar]
- 38.Sood AR, Burry LD, Cheng DK. Clarifying the role of rasburicase in tumor lysis syndrome. Pharmacotherapy. 2007;27(1):111–121. doi: 10.1592/phco.27.1.111. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase. Nat. Rev. Drug Discov. 2011;10(1):17–18. doi: 10.1038/nrd3349. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Fan K, Luo H, Ma X, Liu R, Yang L, Hu C, Chen Z, Min Z, Wei D. Characterization, efficacy, pharmacokinetics, and biodistribution of 5 kDa mPEG modified tetrameric canine uricase variant. Int. J. Pharm. 2012;430(1–2):307–317. doi: 10.1016/j.ijpharm.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 41.Bomalaski JS, Holtsberg FW, Ensor CM, Clark MA. Uricase formulated with polyethylene glycol (uricase-PEG 20): biochemical rationale and preclinical studies. J. Rheumatol. 2002;29(9):1942–1949. [PubMed] [Google Scholar]
- 42.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008;97(10):4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 43.McDonnell T, Ioannou Y, Rahman A. PEGylated drugs in rheumatology—why develop them and do they work? Rheumatology (Oxford) 2014;53(3):391–396. doi: 10.1093/rheumatology/ket278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas Dda S, Spencer PJ, Vassao RC, Abrahao-Neto J. Biochemical and biopharmaceutical properties of PEGylated uricase. Int. J. Pharm. 2010;387(1–2):215–222. doi: 10.1016/j.ijpharm.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 45.Tian H, Guo Y, Gao X, Yao W. PEGylation enhancement of pH stability of uricase via inhibitive tetramer dissociation. J. Pharm. Pharmacol. 2012;65(1):53–63. doi: 10.1111/j.2042-7158.2012.01575.x. [DOI] [PubMed] [Google Scholar]
- 46.Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, Ortlund EA, Johnson RJ, Gaucher EA. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. U. S. A. 2014;111(10):3763–3768. doi: 10.1073/pnas.1320393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chin JW, Santoro SW, Martin AB, King DS, Wang L, Schultz PG. Addition of p-azido-l-phenylalanine to the genetic code of Escherichia coli. J. Am. Chem. Soc. 2002;124(31):9026–9027. doi: 10.1021/ja027007w. [DOI] [PubMed] [Google Scholar]
- 48.Bond SR, Naus CC. RF-Cloning.org: an online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 2012;40:W209–W213. doi: 10.1093/nar/gks396. (Web Server issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lajoie MJ, Rovner AJ, Goodman DB, Aerni HR, Haimovich AD, Kuznetsov G, Mercer JA, Wang HH, Carr PA, Mosberg JA, Rohland N, Schultz PG, Jacobson JM, Rinehart J, Church GM, Isaacs FJ. Genomically recoded organisms expand biological functions. Science. 2013;342(6156):357–360. doi: 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4(11):2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimsley GR, Pace CN. In: Current Protocols in Protein Science. Unit 3.1. Taylor GP, editor. New Jersey: Wiley; 2004. [DOI] [PubMed] [Google Scholar]
- 52.Whitmore L, Wallace BA. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008;89(5):392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 53.Irwin JA, Wong HE, Kwon I. Different fates of Alzheimer's disease amyloid-beta fibrils remodeled by biocompatible small molecules. Biomacromolecules. 2013;14(1):264–274. doi: 10.1021/bm3016994. [DOI] [PubMed] [Google Scholar]
- 54.Sreerama N, Venyaminov SY, Woody RW. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 1999;8(2):370–380. doi: 10.1110/ps.8.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreerama N, Woody RW. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 1993;209(1):32–44. doi: 10.1006/abio.1993.1079. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Fan K, Ma X, Wei D. Impact of large aggregated uricases and PEG diol on accelerated blood clearance of PEGylated canine uricase. PLoS ONE. 2012;7(6):e39659. doi: 10.1371/journal.pone.0039659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahren B, Baldwin RM, Havel PJ. Pharmacokinetics of human leptin in mice and rhesus monkeys. Int. J. Obes. Relat. Metab. Disord. 2000;24(12):1579–1585. doi: 10.1038/sj.ijo.0801447. [DOI] [PubMed] [Google Scholar]
- 58.Sanders JM, Knudsen GA, Birnbaum LS. The fate of beta-hexabromocyclododecane in female C57BL/6 mice. Toxicol. Sci. 2013;134(2):251–257. doi: 10.1093/toxsci/kft121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon M, Frey R, Zangemeister-Wittke U, Pluckthun A. Orthogonal assembly of a designed ankyrin repeat protein-cytotoxin conjugate with a clickable serum albumin module for half-life extension. Bioconjug. Chem. 2013;24(11):1955–1966. doi: 10.1021/bc4004102. [DOI] [PubMed] [Google Scholar]
- 60.Andersen JT, Dalhus B, Cameron J, Daba MB, Plumridge A, Evans L, Brennan SO, Gunnarsen KS, Bjoras M, Sleep D, Sandlie I. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat. Commun. 2012;3:610. doi: 10.1038/ncomms1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad S, Gromiha M, Fawareh H, Sarai A. ASAView: database and tool for solvent accessibility representation in proteins. BMC Bioinf. 2004;5:51. doi: 10.1186/1471-2105-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guillaneuf Y, Dufils PE, Autissier L, Rollet M, Gigmes D, Bertin D. Radical chain end chemical transformation of SG1-based polystyrenes. Macromolecules. 2010;43(1):91–100. [Google Scholar]
- 63.Li Y, Hoskins JN, Sreerama SG, Grayson SM. MALDI-TOF mass spectral characterization of polymers containing an azide group: evidence of metastable ions. Macromolecules. 2010;43(14):6225–6228. doi: 10.1021/ma100599n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutz JF, Borner HG, Weichenhan K. Combining atom transfer radical polymerization and click chemistry: a versatile method for the preparation of end-functional polymers. Macromol. Rapid Commun. 2005;26(7):514–518. [Google Scholar]
- 65.Raynaud J, Absalon C, Gnanou Y, Taton D. N-heterocyclic carbene-induced zwitterionic ring-opening polymerization of ethylene oxide and direct synthesis of alpha, omega-difunctionalized poly(ethylene oxide)s and poly(ethylene oxide)-b-poly (epsilon-caprolactone) block copolymers. J. Am. Chem. Soc. 2009;131(9):3201–3209. doi: 10.1021/ja809246f. [DOI] [PubMed] [Google Scholar]
- 66.Gregoire S, Zhang S, Costanzo J, Wilson K, Fernandez EJ, Kwon I. Cis-suppression to arrest protein aggregation in mammalian cells. Biotechnol. Bioeng. 2014;111(3):462–474. doi: 10.1002/bit.25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiPiro JT. Concepts in Clinical Pharmacokinetics. fifth ed. Bethesda: American Society of Health System Pharmacists; 2010. [Google Scholar]
- 68.Lajoie MJ, Kosuri S, Mosberg JA, Gregg CJ, Zhang D, Church GM. Probing the limits of genetic recoding in essential genes. Science. 2013;3424(6156):361–363. doi: 10.1126/science.1241460. [DOI] [PubMed] [Google Scholar]
- 69.Banerjee PS, Ostapchuk P, Hearing P, Carrico IS. Unnatural amino acid incorporation onto adenoviral (Ad) coat proteins facilitates chemoselective modification and retargeting of Ad type 5 vectors. J. Virol. 2011;85(15):7546–7554. doi: 10.1128/JVI.00118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleissner MR, Brustad EM, Kalai T, Altenbach C, Cascio D, Peters FB, Hideg K, Peuker S, Schultz PG, Hubbell WL. Site-directed spin labeling of a genetically encoded unnatural amino acid. Proc. Natl. Acad. Sci. U. S. A. 2009;106(51):21637–21642. doi: 10.1073/pnas.0912009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim SI, Mizuta Y, Takasu A, Kim YH, Kwon I. Site-specific bioconjugation of a murine dihydrofolate reductase enzyme by copper(I)-catalyzed azide-alkyne cycloaddition with retained activity. PLoS ONE. 2014;9(6):e98403. doi: 10.1371/journal.pone.0098403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddington SC, Tippmann EM, Jones DD. Residue choice defines efficiency and influence of bioorthogonal protein modification via genetically encoded strain promoted Click chemistry. Chem. Commun. (Camb.) 2012;48(67):8419–8421. doi: 10.1039/c2cc31887c. [DOI] [PubMed] [Google Scholar]
- 73.Sherman MR, Saifer MG, Perez-Ruiz F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv. Drug Deliv. Rev. 2008;60(1):59–68. doi: 10.1016/j.addr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Fujita T, Yasuda Y, Takakura Y, Hashida M, Sezaki H. Tissue distribution of 111Inlabeled uricase conjugated with charged dextrans and polyethylene glycol. J. Pharmacobiodyn. 1991;14(11):623–629. doi: 10.1248/bpb1978.14.623. [DOI] [PubMed] [Google Scholar]
- 75.Saifer MG, Williams LD, Sobczyk MA, Michaels SJ, Sherman MR. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol. Immunol. 2014;57(2):236–246. doi: 10.1016/j.molimm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MG. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug. Chem. 2012;23(3):485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J. Biol. Chem. 2010;285(7):4826–4836. doi: 10.1074/jbc.M109.081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt MM, Townson SA, Andreucci AJ, King BM, Schirmer EB, Murillo AJ, Dombrowski C, Tisdale AW, Lowden PA, Masci AL, Kovalchin JT, Erbe DV, Wittrup KD, Furfine ES, Barnes TM. Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure. 2013;21(11):1966–1978. doi: 10.1016/j.str.2013.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.