Abstract
Problem
TLR4 mediates host responses to pathogens through a mechanism that involves protein myeloid differentiation-2 (MD-2) and its soluble form sMD-2. The role of sMD2 in intra-amniotic inflammation induced preterm birth has not been previously explored.
Method of study
Human amniotic fluid (AF) sMD-2 was studied by Western blotting in 152 AF samples of patients who had an amniocentesis to rule-out infection (yes infection, n=50; no infection, n=50) or women with normal pregnancy outcome (2nd trimester genetic karyotyping, n=26; 3rd trimester lung maturity testing, n=26). Histologic localization and mRNA expression of MD2 in fetal membranes were studied by immunohistochemistry and RT-PCR. The ability of fetal membrane to release sMD-2 and inflammatory cytokines was studied in-vitro.
Results
Human AF contains three sMD-2 proteoforms whose levels of expression were lower at term. Intra-amniotic infection up-regulated sMD-2. MD-2 mRNA and immunohistochemistry findings concurred. In vitro, LPS and monensin increased while cycloheximide decreased sMD-2 production. Recombinant sMD-2 modulated TNF-α and IL-6 levels in a dose and time-dependent fashion.
Conclusion
sMD2 proteoforms are constitutively present in human AF. The intensity of the intra-amniotic inflammatory response to bacteria or perhaps to other TLR4 ligands may be facilitated through synthesis and release of sMD2 by the amniochorion.
Keywords: pregnancy, inflammation, fetus, early-onset neonatal sepsis, infection, bacteria, fetal membranes
Introduction
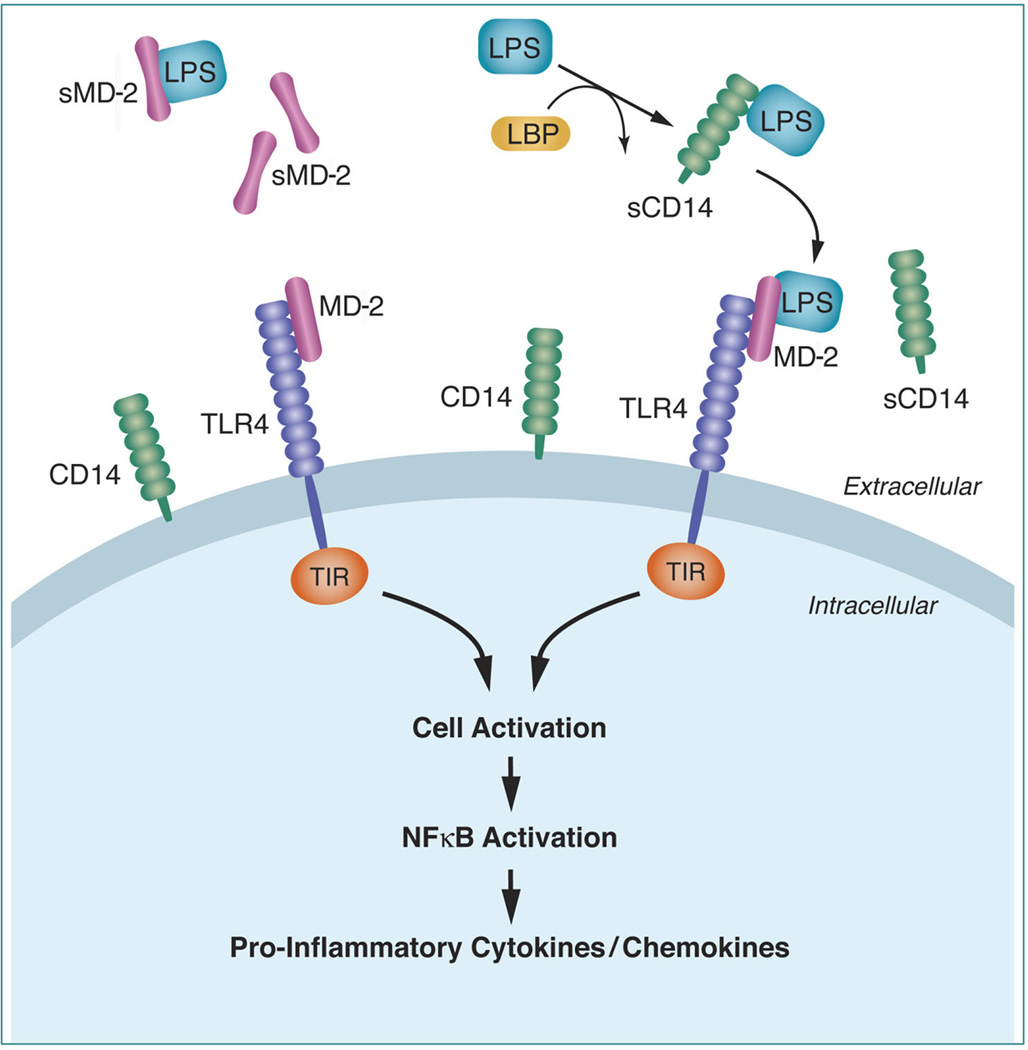
A breakthrough in understanding the molecular mechanisms responsible for host sensing of Gram-negative bacteria was the discovery of TLR4 as the membrane bound receptor that triggers LPS signaling [1,2]. TLR4 is a type I transmembrane receptor with an extracellular domain, and an intra-cytoplasmic Toll-IL-1 resistance (TIR) domain. TIR contains adaptor molecules involved in triggering synthesis of pro-inflammatory cytokines (i.e. TNF-α, IL-1, IL-6), through NF-kB mediated mechanisms (Fig. 1) [3]. However, activation of intra-cellular signalling pathways occurs only following endotoxin recognition by TLR4. This process is intricate because the extracellular domain of the TLR4 receptor involves an LPS-recognition-complex, comprised of LPS binding protein (LBP), CD14 and the adaptor glycoprotein myeloid differentiation-2 (MD-2) [4]. Given that MD-2 lacks a transmembrane domain, it is attached via physical association to the extracellular domain of TLR4 [2]. In a multistep mechanism, LBP transfers endotoxin to the membrane-anchored protein CD14, a molecule that lacks intrinsic signalling capabilities [5]. A soluble proteolytic CD14 fragment (sCD14) enhances the LPS response of cells that do not normally express membrane bound CD14 [6]. Following LPS binding, CD14 brings endotoxin in close proximity to the TLR4-MD-2 receptor unit (4). Targeted point mutagenesis experiments, and studies in genetically engineered animals provide support for the assertion that MD-2 is critical for ligand recognition of LPS [7,8,9]. Yet, while a large body of evidence confirms MD-2 plays a key inflammatory role by recognizing LPS and forming a heterodimer with TLR4, limited amount of data are available on the cellular source of MD-2 and function of the soluble (non-TLR4-anchored) form of MD-2 (sMD-2) [8]. Experimental data in vitro suggests that when present in excess, sMD-2 might function to block LPS responses [10].
Figure 1. Schematic representation of myeloid differentiation-2 (MD-2) and TLR4 interactions in inflammation.
Abbreviations: soluble myeloid differentiation-2 (sMD-2); LPS-binding protein (LBS); Toll-IL-1 resistance (TIR) domain.
At the maternal-fetal interface, control of innate immunity is partially achieved through TLRs with TLR4 serving as a guardian against Gram-negative bacteria attempting to reach the amniotic cavity [11]. Amniotic fluid (AF) seems to be an active participant because it contains factors that modulate immune responses, of which LBP and sCD14 have already been reported [12]. Herein, we tested the hypothesis that sMD-2 is present in human AF and has a functional role in modulating the intra-amniotic inflammatory response to bacteria.
Materials and Methods
Patient population and amniotic fluid samples
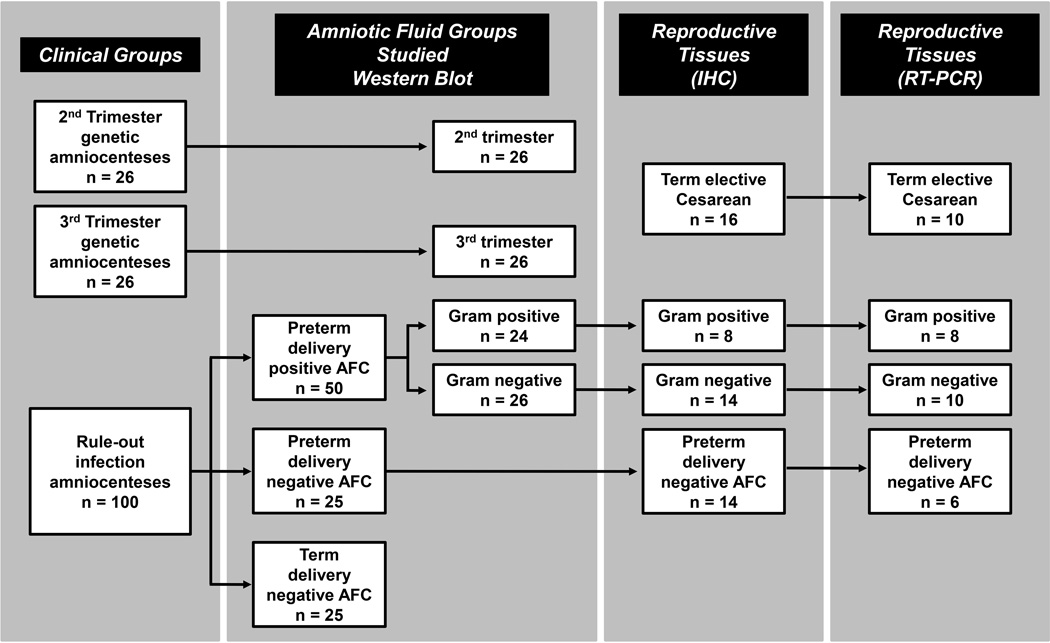
A flowchart of the samples analyzed in this study is presented in the Fig. 2. We investigated AF samples from 152 women with singletons pregnancies. Samples were retrieved by trans-abdominal amniocentesis for the purpose of 2nd trimester genetic karyotyping (gestational age [GA], median [range]: 19 [15–23] weeks, n=26); 3rd trimester fetal lung maturity testing (GA: 37 [35–38] weeks, n=26) or to rule-out AF infection in women who presented with symptoms of preterm birth (GA: 28 [23–33] weeks, n=100). Of the last group, AF microbial cultures results turned positive in 50 cases [Gram-negative bacteria (n=26); Gram-positive bacteria (n=24)]. Although AF infection was excluded in 50 of the remaining cases, 25 women delivered preterm, while the rest (n=25) delivered a healthy fetus at term (≥37 weeks GA). This study was approved by the Human Investigation Committee of Yale University. All patients provided written informed consent. The clinical characteristics of the participating subjects are presented in Supplemental Table 1.
Figure 2. Flowchart of enrolled women and samples analyzed in this study.
Abbreviations: AFC amniotic fluid culture; IHC immunohistochemistry.
GA was established based on recognized clinical and ultrasonographic criteria [13]. Indications for 2nd trimester fetal karyotype testing included: personal decision and/or maternal age over 35, maternal serum screening test suggestive of increased risk for aneuploidy, and ultrasound markers associated with aneuploidy (i.e. choroid plexus cyst, intra-cardiac echogenic foci). The karyotype testing of all fetuses returned normal results. Breech presentation or prior uterine scars were considered clinical indications for fetal lung maturity testing prior to delivery by scheduled Cesarean-section. Maternal medical complications such as chronic hypertension, preeclampsia, diabetes, thyroid disease, cholestasis, connective-tissue-disease, viral infections (i.e. HIV, hepatitis B or C), anhydramnios, and fetal intra-uterine growth restriction (ultrasonographic estimated fetal weight <10th percentile for GA) or structural anomalies were considered as exclusion criteria. Persistent regular uterine contractions, advanced cervical dilatation (≥3 centimeters) or effacement at <37 weeks GA, were considered symptoms of preterm labor [14]. Preterm premature rupture of the membranes was confirmed either by “pooling” on speculum examination, positive “nitrazine” and “ferning” tests, or by a positive amnio-dye test. Clinical chorioamnionitis was established in the presence of maternal fever (>37.8°C), leukocytosis (≥15,000 cells/mm3), uterine tenderness, foul smelling AF or visualization of pus at the time of the speculum exam, maternal or fetal tachycardia [15].
The clinical characteristics and the results of the AF analysis for group presenting with preterm birth symptoms are presented in Supplemental Table 2. The clinical laboratory assessed AF glucose concentration, lactate dehydrogenase activity and the white blood cell count [16,17]. Concurrently, the AF was examined for the presence of infection using Gram staining and culturing methods for aerobic and anaerobic bacteria, Ureaplasma, and Mycoplasma species. The microbiological cultures results are presented in Supplemental Tables 3 and 4. For research purposes, excess AF was centrifuged at 3,000 g and 4°C for 20 min., aliquoted and stored at −80°C.
sMD-2 detection by Western blot
Gel electrophoresis was carried out on 4–20% SDS-PAGE gels. Ten microliters of AF was diluted 1:2 v/v with Laemmli sample buffer containing 5% v/v β-mercaptoethanol (BME) (Bio-Rad, La Jolla, CA) and reduced by boiling for 5 min. After electrophoretic transfer, the PVDF membranes (Bio-Rad) were blocked with 5% milk, and incubated overnight at 4°C with polyclonal rabbit anti-MD-2 primary antibody (1:1,000, ProSci Inc., Poway, CA, Cat. No: 3289). This MD-2 antibody was raised against a peptide corresponding to amino acids near the middle region of human MD-2. Blots were subjected to Enhanced Chemiluminescence using horseradish peroxidase conjugated anti-rabbit IgG (1:15,000, Amersham Biosystems, Piscataway, NJ) as secondary antibody. Optical density of each band was quantified using ImageJ v.1.33 software (NIH, http:\\rsb.info.nih.gov). Inter-gel comparison was achieved by expressing the optical density of each band relative (%) to that of an AF sample pool which was loaded on each gel. Antibody specificity was confirmed by omitting the primary antibody and by pre-adsorbing the primary antibody with neutralizing peptide from the same manufacturer.
sMD-2 is an unusually cysteine-rich glycoprotein known to form oligomers with varying molecular weights [8]. Representative samples of AF were ran under varying conditions (non-reduced, reduced with 50mM dithiothreitol (DTT), reduced and alkylated (50mM DTT and 25mM iodoacetamide) to block freed cysteine and prevent formation of artifactual oligomers. The banding pattern was compared to that obtained with the BME reduction described above. N-linked de-glycosylation was carried out using N-glycosidase F, á-2–3,6,8,9-neuraminidase, and endo-á-N-acetyl-galactosaminidase. This was done under reducing conditions with BME according to the manufacturer’s instructions (Calbiochem-EMD Chemicals Inc., Gibbstown, NJ). Electrophoresis was employed to monitor sMD-2 banding patterns in AF.
Real-time quantitative RT-PCR
mRNA expression levels of MD-2 and TLR4 were investigated in amniochorion tissues retrieved from cases where MD-2 expression level was evaluated by immunostaining (Fig. 2). At delivery, tissues were immediately frozen in liquid nitrogen and kept at −80°C. RNA was extracted and reverse transcribed into cDNA with random hexamer primers using standard procedures. Quantitative RT-PCR was performed using TaqMan® (Applied Biosystems, Foster City, CA) chemistry in 20 µL reactions composed of 10 µL mastermix (TaqMan® Fast Universal PCR 2x Master Mix), 8 µL water, 1µL cDNA template normalized and 1 µL PCR probe set (TaqMan® Gene Expression Assays-on-Demand). The following probes were used from Applied Biosystems: Hs00209771_m1 (MD-2 transcript variant 1); and Hs01026733_m1 (MD-2 transcript variants 1 and 2) [18]; Hs00152939_m1 (TLR4). As endogenous controls, we used Taqman® probes hs99999907_m1 (beta-2 microglobulin) and hs00265497_m1 (ribosomal protein L30). Their combination was validated using pools of cDNA amplified in the TaqMan® Human Endogenous Control Plate (Applied Biosystems). Selection of the two endogenous control genes was based on low cycle threshold (Ct) values that were not different among the cDNA reference pools. For each target, amplification was performed in duplicate reactions in a two-step cycle (denaturation, 95°C for 15 seconds, annealing/extension at 62°C for 60 seconds) for 40 cycles. Data output was analysed by using StepOne Software (v2.1, Applied Biosystems). The geometric mean of the endogenous control RNAs was used to normalize the data obtained for each target by using the delta Ct calculation (dCt: Ct of the target - Ct geometric mean of endogenous controls). A dCt of 0 indicates a ratio of 1 between the target and housekeeping genes. This ratio may be used as an indication of relative abundance between different targets among different tissues. Calculation of delta-delta Ct (ddCt: dCt of individual sample - dCt of same target in reference pool) adds an additional normalization within targets. Thus, ddCt improves estimation of relative mRNA abundance among different biological groups [19,20].
Immunohistochemistry
Immunostaining for MD-2 expression was performed on formalin-fixed amniochorion of women who delivered preterm in the presence [Gram-negative bacteria, n=14; Gram-positive bacteria, n=8]) or absence (n=14) of intra-amniotic infection and/or histological chorioamnionitis. In addition, we investigated tissues of healthy women delivered by elective Cesarean-section at term (n=16).
Five µm paraffin sections were deparaffinized in xylene and rehydrated with graded ethanol to potassium-phosphate-buffered saline solution, pH 7.2. Following antigen retrieval with citrate buffer, the sections were pretreated with 1% hydrogen peroxide for 15 min. followed by overnight incubation at 4°C with a rabbit polyclonal anti-human MD-2 antibody (1:1,000, ProSci). Detection was performed with biotinylated donkey anti-rabbit IgG (1:600, Jackson ImmunoReserach, West Grove, PA) followed by avidin-biotin staining and NovaRed as chromogen (Vectastain Elite ABC, Vector Labs, Burlingame, CA). Specificity of staining was confirmed by substituting the primary antibody with rabbit IgG. Staining intensity of chromogen deposits in the amnion and choriodecidua was evaluated by the HSCORE method as previously described [21].
Fetal membranes explant culture system
To evaluate the ability of the fetal membranes to release sMD-2, we employed an amniochorion explant system (term Cesarean-section, GA: 38–40 weeks, n=6). Fresh fetal membranes were retrieved at the time of surgery from healthy non-laboring women with normal placentation and appropriately grown fetuses. Sterile technique was used to harvest amniochorion samples away from the site of rupture. Membranes were cut into pieces of similar weight, and washed thoroughly with ice-cold saline. Using a 24-well plate experimental set-up we cultured ~100 mg tissue/well (wet weight) in 1.5 mL RPMI 1640 medium (Gibco, Grand Island, NY) containing 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco). Tissue cultures were maintained at 37°C in a humidified gas mixture of 5% CO2-95% air. To mimic inflammation, tissues were incubated in the presence of UltraPure LPS (100 ng/mL, InVivoGen, San Diego, CA). After 1, 6, 18 and 24 hours of incubation supernatants were collected and analyzed for presence and pattern of sMD-2 immunoreactivity by Western blot. Monensin (10 µg/mL) and Cycloheximide (20µM/mL) were used as disruptors of assembly/trafficking and secretion and protein synthesis, respectively. The Monensin and Cycloheximide doses were previously tested and determined to be biologically effective in a similar experimental setup [22]. Parallel wells were treated with the equivalent dose of vehicle (dimethylsulfoxide [DMSO]). All drugs and chemicals were from Sigma Chemical Co. (St Louis, MO) unless specified.
To investigate the possible biological function of sMD-2, the amniochorion was challenged with recombinant MD-2 (rMD-2, expressed in human HEK293 cells, 0.1, 1 or 10 ng/mL, OriGene, Rockville, MD) in the absence or presence of LPS (100 ng/mL). LPS content of rMD-2 was undetectable as measured by the Limulus Amebocyte Lysate (LAL) method (Lonza Biologics, Inc. Portsmouth NH). Explant media was collected at 6 and 18 hours and assayed for levels of TNF-α and IL-6.
The incubated tissue was homogenized in 1 mL cell extraction buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail [Roche, Indianapolis, IN]). Homogenates were spun at 1,000 g at 4°C for 15 min and protein content assessed using bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). To correct for variations in tissue amounts per well, explant medium concentration of each analyte was normalized to total protein in tissue extract. For each condition, values were derived by averaging normalized values from duplicate wells either without (untreated) or with the various treatments. Data was interpreted as % from untreated level for each experiment. Tissue viability during in-vitro incubations was assessed by the release of the intracellular enzyme LDH into the incubation medium using the LDH Liqui-UV® Assay (Stanbio, Boerne, TX). The inter-assay and intra-assay coefficients of variation were <5%.
TNF-α and IL-6 immunoassays
ELISA systems (eBioscience, San Diego, CA) were used to quantify TNF-α and IL-6 in AF and explant medium in accordance with manufacturer instructions. Replicates were measured and averaged. The minimal detectable concentrations were 4 and 2 pg/mL for TNF-α and IL-6, respectively. The inter- and intra-assay coefficients of variation were <10%.
Statistical analysis
Comparisons between groups were performed using Student t tests, Mann-Whitney tests, one- or two-way ANOVA followed Student-Newman-Keuls tests (parametric) or Kruskal-Wallis ANOVA on ranks followed by Dunn’s tests (non-parametric), to adjust for multiple comparisons as appropriate. Proportions were compared with Chi-square or Fischer exact tests. Time and dose-dependent changes in in-vitro experiments were examined using one- and two-way repeated measures ANOVA. MedCalc (Mariakerke, Belgium) and SigmaPlot statistical softwares (RockWare, Golden, CO) were used for data analysis.
Results
Characterization of the human amniotic fluid sMD-2 isoforms
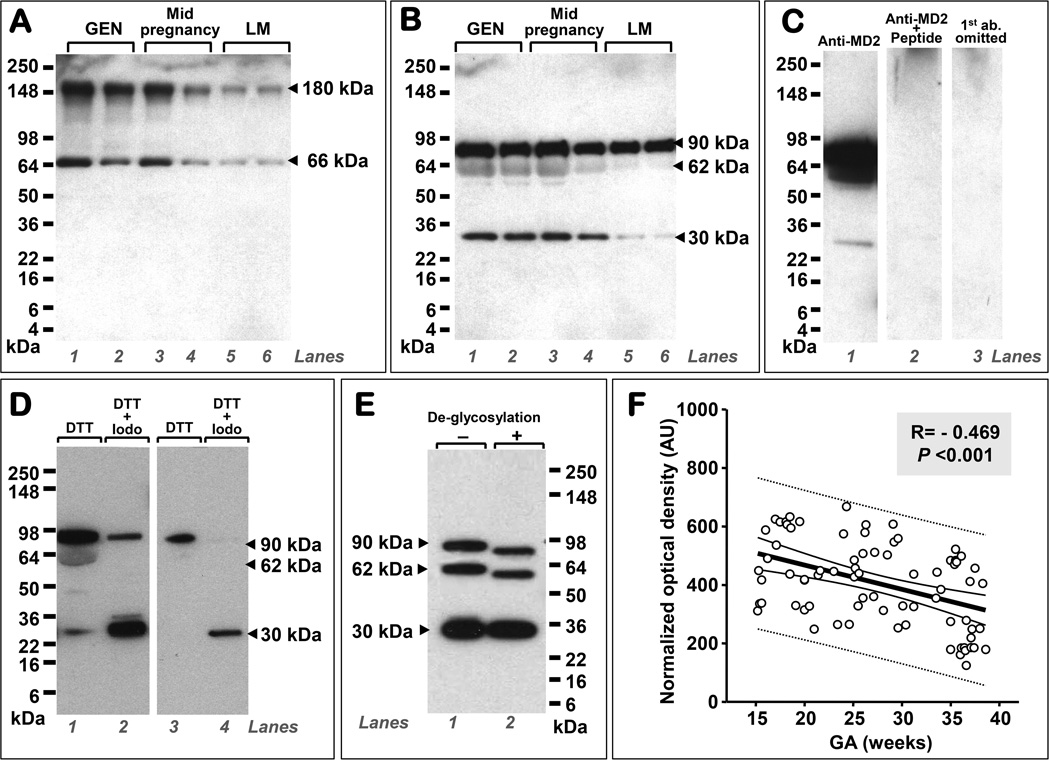
As shown in Fig. 3A, by Western blotting, we first determined that the immunoreactivity of human AF sMD-2 in non-reducing conditions was characterized by two bands located at ~180 and ~66 kDa, respectively. Under reducing conditions, three bands were identified at 90, 62, and 30 kDa (Fig. 3B). Specificity of the bands for sMD-2 was proven by competition with blocking peptide (Fig. 3C). To assess whether AF sMD-2 has a tendency to create multimers, we performed reduction+alkylation experiments (Fig. 3D). In these conditions, the molecular forms of sMD-2 migrating at 90 and 62 kDa (lane 1) diminished in intensity (lane 2) with the concurrent increase in intensity of the 30 kDa isoform. This shift was even more apparent if less AF protein was subjected to reduction+alkylation (lanes 3 and 4). Post-translational modification of the human AF sMD-2 was demonstrated through deglycosylation experiments (Fig. 3E) which produced a shift in the electrophoretic mobility of the 90 and 62 kDa bands but not of the 30 kDa band suggesting this smaller form was unglycosylated.
Figure 3. Human amniotic fluid (AF) contains soluble myeloid differentiation-2 (sMD-2) monomeric and multimeric proteoforms, is glycosylated, and its levels are gestational age (GA) regulated.
The figure is a composite of Western blot data demonstrating that the immunoreactivity of human AF sMD-2 in non-reducing conditions is characterized by two bands located at ~180 and ~66 kDa, respectively. The polyclonal rabbit anti-MD-2 primary Ab raised against a peptide corresponding to amino acids near the middle region of human MD-2 detected the two MD-2 forms in samples of AF retrieved by amniocentesis during the second trimester for genetic (GEN, n-26) purposes (A, lane 1 and 2), second trimester for symptoms of preterm labor in patients that delivered at term (n=50, A, lanes 3 and 4), and third trimester in patients who were tested for fetal lung maturity (LM, n=26) (A, lanes 5 and 6). Under reducing conditions, the same Ab detected three specific bands at 90, 62, and 30 kDa (B). Representative Western blot gels of AF retrieved during the second trimester for genetic (GEN) purposes (B, lanes 1 and 2), second trimester in patients with symptoms of preterm labor, negative microbial cultures who delivered at term (B, lanes 3 and 4), and third trimester for lung maturity (LM) testing (B, lanes 5 and 6). Each lane represents a sample from a different woman. Specificity of the sMD-2 bands was confirmed (C). Shown is a representative Western blot using the anti-MD-2 Ab in a second trimester AF genetic sample (C, lane 1), by pre-adsorbing the primary antibody with neutralizing peptide (C, lane 2) and by omitting the primary antibody (C, lane 3). Reduction+alkylation experiments performed using second trimester genetic amniocentesis fluid showed that the 90 and 62 kDa bands hold MD-2 multimers (D). As shown the molecular forms of sMD-2 migrating at 90 and 62 kDa (D, lane 1) diminished in intensity (D, lane 2) with the concurrent increase in intensity of the 30 kDa isoform (D, lane 2). This shift was more apparent if less AF protein was subjected to reduction+alkylation (D, lanes 3 and 4). Deglycosylation experiments produced a shift in the electrophoretic mobility of the 90 and 62 kDa bands (E, lane 1 vs. lane 2) but not of the 30 kDa band. Densitometric image analysis of the 90, 62, and 30 kDa polypeptides demonstrated that AF sMD-2 levels decrease with increasing gestational age (GA) (F). The thick black line represents the linear regression line, the thin black lines mark the confidence interval and the dotted lines show the prediction interval. Abbreviations: dithiothreitol (DTT), Iodo (iodoacetamine), AU: arbitrary densitometric units.
Gestational regulation of human amniotic fluid sMD-2 isoforms
In this analysis we included AF retrieved during the 2nd, 3rd trimester, and of women that presented mid-pregnancy with symptoms of preterm labor, had negative AF cultures, and ultimately delivered a healthy term baby. The densitometric analysis of the total AF sMD-2 immunoreactivity demonstrated that the lowest sMD-2 levels were seen in the 3rd trimester as a result of decreased intensity of all bands observed in either non-reducing (Fig. 3A) or reducing conditions (Fig. 3B). A significant inverse correlation between total sMD-2 immunoreactivity and GA was identified (r=-0.469, P<0.001) (Fig. 3F).
Amniotic fluid sMD-2 levels in pregnancies complicated by intra-amniotic infection
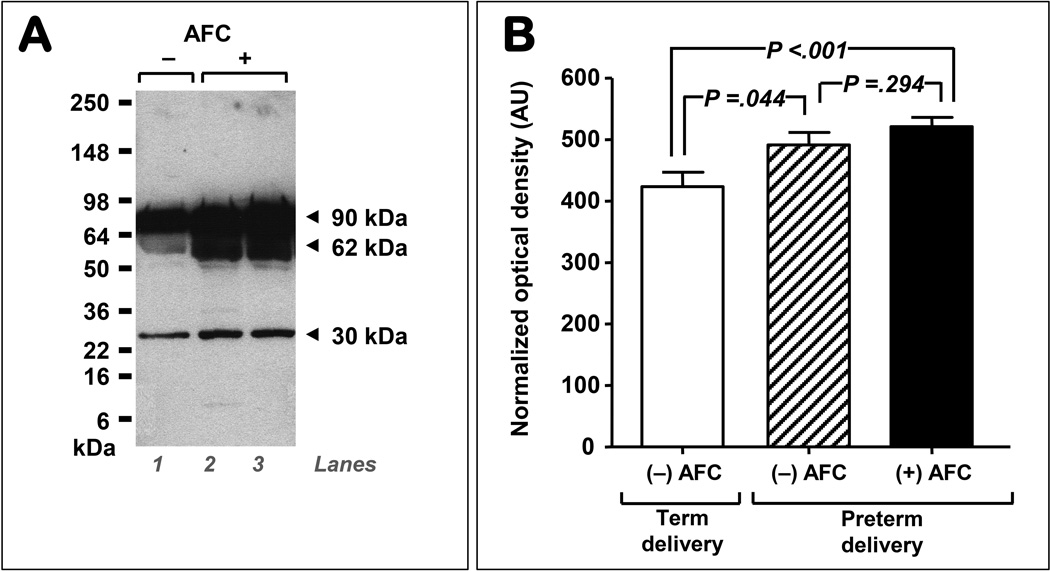
Fig. 4A displays a representative Western blot illustrating that in comparison to preterm control AF (lane 1: negative culture & term delivery), women with preterm intra-amniotic infection (lanes 2–3, positive culture & preterm delivery) had significantly increased immunoreactivity of all three AF sMD-2 bands. Densitometric analysis of the total AF sMD-2 immunoreactivity is presented in Fig. 4B. Compared with women who delivered at term and tested negative for bacteria, a significant increase in sMD-2 immunoreactivity was identified in intra-amniotic infection (P<0.001). Interestingly, the group of women with idiopathic spontaneous preterm birth also had elevated AF sMD-2 levels.
Figure 4. Levels of amniotic fluid (AF) myeloid differentiation-2 (sMD-2) isoforms (90, 62 and 30 kDa) are influenced by infections status.
A representative sMD-2 Western blot data (4–20% SDS-PAGE reducing gel) of AF retrieved from women that delivered preterm in the setting of negative AF culture (AFC) (A, lane 1), and preterm delivery with positive AF microbial cultures (lanes 2 and 3). Each lane represents a sample from a different woman. Quantification of the 90, 62 and 30 kDa optical density of the sMD-2 AF bands of women who had a preterm amniocentesis, negative AFC and delivered at term (n=25), and women who had negative AFC and delivered preterm (n=25), and women who had positive AFC and delivered preterm (n=50). Our analysis showed that AF sMD-2 levels are increased in infection (black bar) but also in women with idiopathic preterm birth (negative microbial cultures, hashed bar). Error bars show standard error.
Across the 4 groups of women presenting with symptoms of preterm birth (n=100), AF sMD-2 immunoreactivity correlated significantly with the levels of AF pro-inflammatory cytokines IL-6 (r=0.324, P=0.004) and TNF-α (r=0.267, P=0.034). The levels of AF sMD-2 did not differ significantly with the type of bacteria (Gram positive vs. Gram negative, P=0.123).
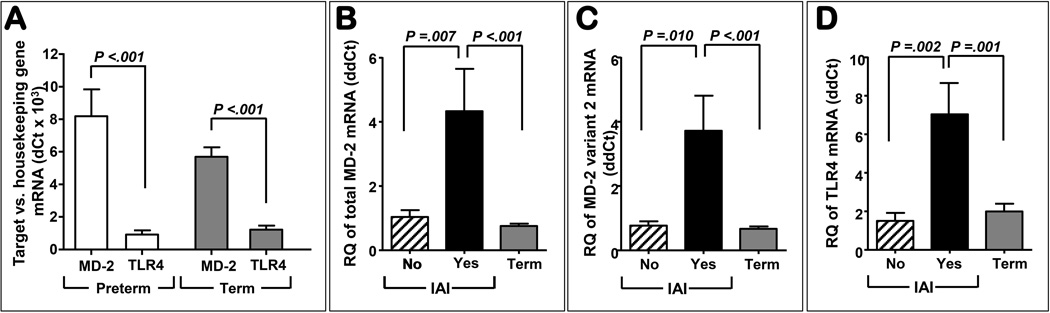
Transcriptional changes in TLR4 and MD-2 in the amniochorion
Expression of total MD-2 mRNA was more abundant than TLR4 both preterm and term (P<0.001) (Fig. 5A). Absent infection there was no difference in MD-2 expression levels between term and preterm amniochorion (Fig. 5B, P=0.747). In intra-amniotic infection the level of amniochorion MD-2 mRNA was significantly increased compared to idiopathic preterm birth (P=0.007) and term tissues (P<0.001). Both total MD-2 (Fig. 5B) and variant 2 (coding for sMD2, Fig 5C) showed a similar increase with infection, but not with GA. In Fig. 5D we show that the expression of TLR4 mRNA followed a similar pattern of expression with that of the MD-2. Both Gram-negative and Gram-positive infection upregulated MD-2 as well as TLR4 expression at similar levels (MD-2: P=0.778; TLR4: P=0.157). There was a significant correlation between MD-2 and TLR4 mRNA expression in women with intra-amniotic infection (r=0.737, P=0.002) which was not observed in the other groups (r=-0.230, P=0.410).
Figure 5. Amniochorion mRNA expression levels of myeloid differentiation-2 (MD-2) and TLR4 during gestation and in infection induced preterm birth.
Real-time quantitative PCR results showed that expression of total MD-2 mRNA was more abundant than TLR4 both preterm (idiopathic preterm, n=6) and term (n=10) (A). Absent intra-amniotic infection (IAI) there was no difference in either total MD-2 while IAI (n=8 Gram positive, n=10 Gram negative) was characterized by increased MD-2 mRNA expression compared to idiopathic preterm birth and term tissues (B). A similar trend was observed for variant 2 (C). Expression of TLR4 followed a similar pattern of expression with that of the MD-2 (D). Error bars show standard error.
Immunolocalization of MD-2 and sMD-2 in human fetal membranes
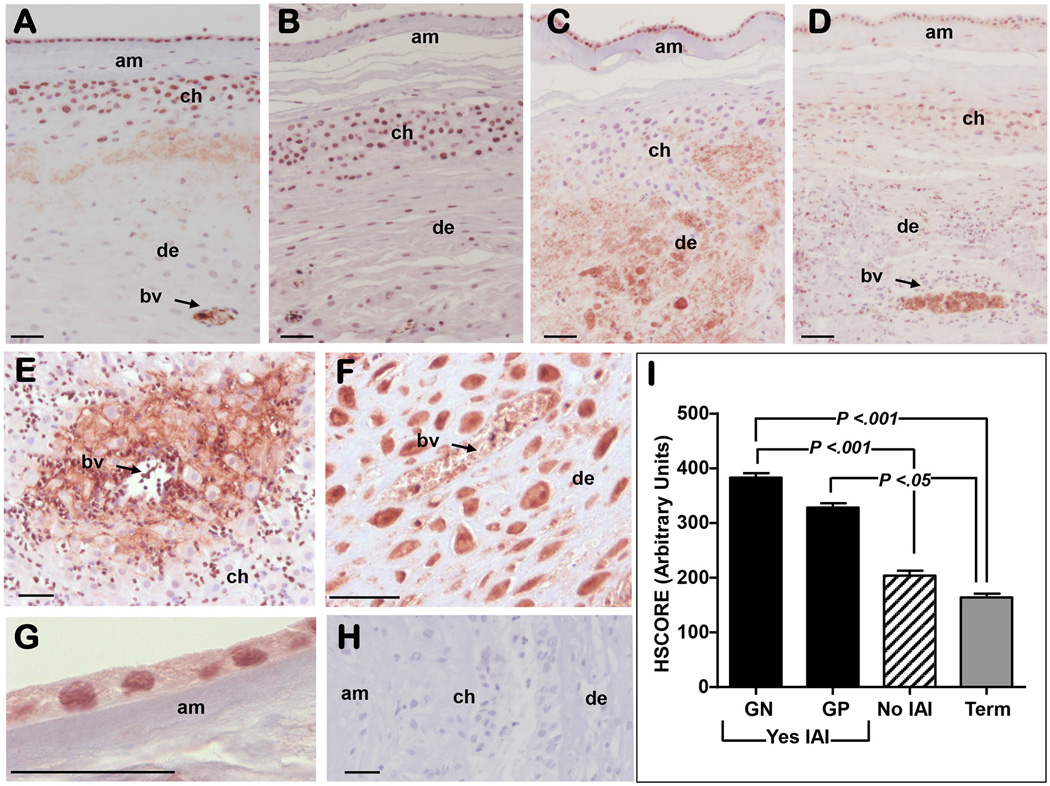
Fig. 6A illustrates that in women with idiopathic preterm birth, immunoreactive MD-2 was localized in both amnion epithelium and choriodecidua. Blood vessels in the decidua showed strong intraluminal staining consistent with the detection of sMD-2. A similar staining pattern was observed in term tissues (Fig. 6B). Histological scoring did not reveal a significant difference in amniochorion immunostaining between term and preterm tissues absent infection which concurred with the mRNA MD-2 data. Intra-amniotic infection with both Gram-negative (Fig. 6C) and Gram-positive bacteria (Fig. 6D) was associated with significant increase in MD-2 staining (P<0.001) compared to term and idiopathic preterm tissues. In the choriodecidua of women with intra-amniotic infection, MD-2 staining was notable both in inflammatory cells (Fig. 6E) and in the resident decidual cells (Fig. 6E&6F). Areas surrounding blood vessels showed prominent pericellular (Fig. 6E) and intra-cellular (Fig. 6F) staining. Within the amnion (Fig. 6G), infection increased MD-2 staining intensity both in the perinuclear and cytoplasmic region of the amnion epithelial cells. Specificity of staining was confirmed by substituting the MD-2 antibody with non-immune IgG Fig. 6H. Semiquantitative analysis of global amnion and choriodecidua staining intensity is presented in Fig. 6I.
Figure 6. Immunoreactivity of myeloid differentiation-2 (MD-2) in the amniochorion.
This figure includes a representative image of MD-2 immunoreactivity in a woman with idiopathic preterm birth (negative AF cultures and no histological chorioamnionitis) demonstrating that MD-2 was localized in both amnion (am), chorion (ch), and decidua (de) (A). Blood vessel displayed intraluminal staining, implying presence of sMD-2. For comparison we show representative images of MD-2 immunoreactivity in tissues retrieved from a woman at term (physiologic pregnancy) (B), a woman who had an intra-amniotic infection (IAI) with Gram negative (GN) bacteria (C), and a woman with IAI with Gram positive (GP) microbes (D). IAI was associated with a significant increase in MD-2 staining compared to term and idiopathic preterm tissues. In the ch of women with IAI, MD-2 staining was notable both in inflammatory cells (E) surrounding the bv and in the resident de cells (E&F). Infection increased MD-2 staining intensity both in the perinuclear and cytoplasmic region of the am epithelial cells (G). Specificity of staining was confirmed by using non-immune IgG (H). The length of the scale bar on all micrographs represents 50 µm. (I) Semiquantitative analysis of global MD-2 staining in the am and de reveal a significant increase in the MD-2 immunostaining in the setting of IAI. Error bars show standard error.
sMD-2 release from the amnionchorion under basal and LPS-stimulated conditions
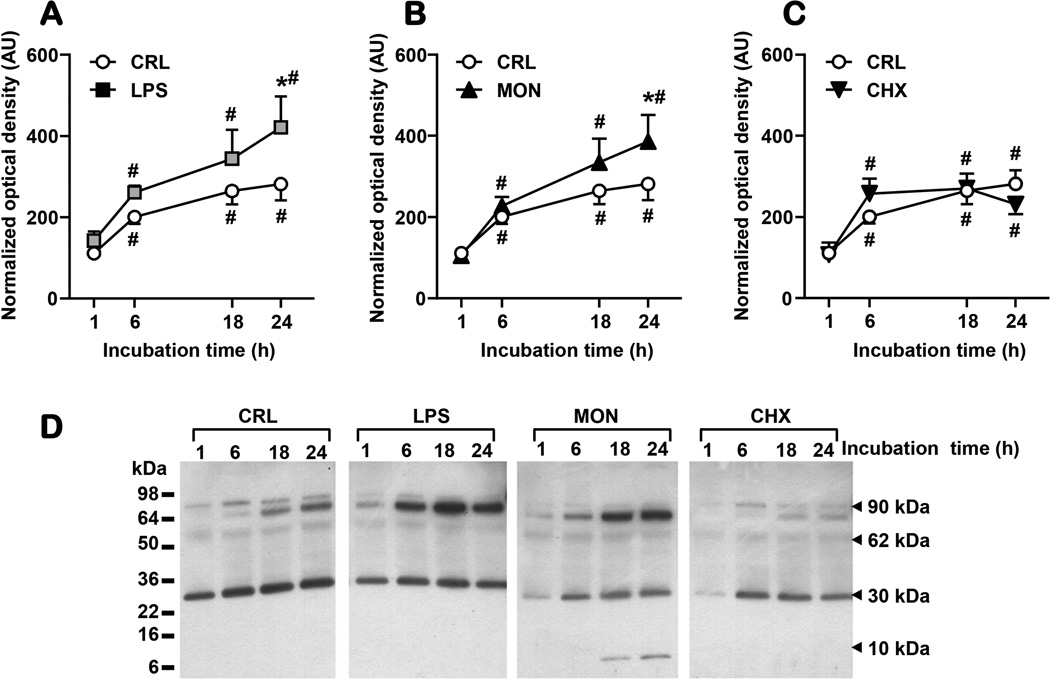
Fetal membranes release sMD-2 spontaneously in the explant medium (Fig. 7A). At 6 hours, LPS induced a significant increase in sMD-2 immunoreactivity which maintained thereafter. Incubation with monensin also increased the release of sMD-2 in explant medium in a time-dependent fashion (Fig. 7B). Conversely, addition of cycloheximide blunted the accumulation of sMD-2 in the medium (Fig. 7C). Representative gels for all treatments are displayed in Fig. 7D. As shown, all treatments had a larger impact on the 90kDa isoform as compared to the 30 kDa band. An additional small molecular weight band (~6kDa) was noted at 18 and 24 hours in the presence of monensin.
Figure 7. In an amniochorion explant system secretion of soluble myeloid differentiation-2 (sMD-2) is influenced by LPS, monensin (MON) and cycloheximide (CHX) treatment.
The presence of the sMD-2 (90, 62, and 30 kDa forms) in the explant media was significantly increased after 6 h of LPS (100 ng/mL) stimulation (A). A sustained response was seen after 18h and 24h of treatment. MON (10 µg/mL) had a similar stimulatory effect (B), while CHX (20µM/mL) decreased the amount of sMD-2 in the explant media (C). Representative Western blot gels for each treatment are shown (D). Data are presented as mean and standard error and analysed by 2-way repeated ANOVA. * P<0.05 vs. CRL; # P<0.05 vs. 1h time point.
Effect of recombinant MD (rMD-2) under basal and LPS-stimulated conditions
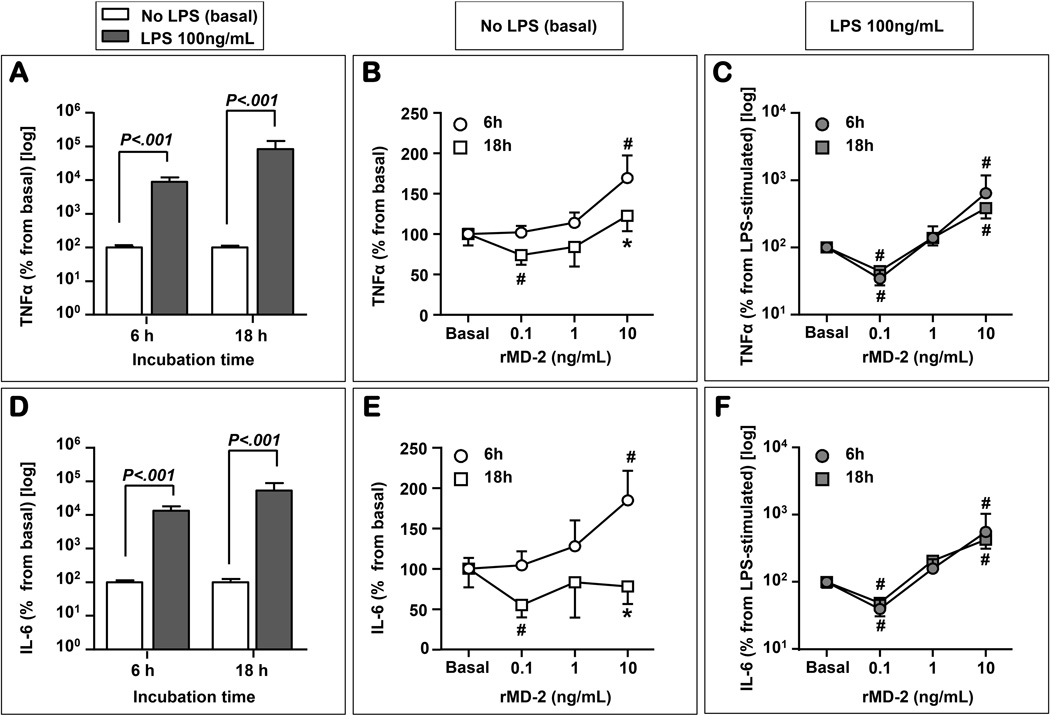
LPS significantly increased levels of TNF-α at both 6 and 18 hours (2-way repeated measures ANOVA: P<0.001 for effect of LPS and P=0.404 for time of incubation, Fig. 8A). In the absence of LPS, rMD-2 impacted on the release of TNF-α in a both dose- and time-dependent fashion (Fig. 8B). At 6 hours, the highest dose of rMD-2 resulted in increased TNF-α over the basal level (P=0.024), while at 18 hours this effect was no longer noted. In contrast, the lowest dose of rMD-2 had an inhibitory effect (P=0.048) which was not observed at 6 hours. In the presence of LPS (Fig. 8C) the inhibitory effect of the lowest dose and the stimulatory effect of the highest dose where significant at both 6 and 18 hours, respectively (P<0.05 for both). Levels of IL-6 followed a similar pattern to TNF-α (Fig. 8D–F). To determine how the inhibitory or stimulatory cytokine response is related to the sMD-2 proteoforms we perform Western blots of the explant media for each treatment. A representative Western blot is presented as Fig. 9. We found that the stimulatory effect was present in the setting of a higher intensity of the 90 kDa sMD-2 band.
Figure 8. TNF-α and IL-6 release is stimulated by LPS and influenced by recombinant soluble myeloid differentiation-2 (rMD-2) treatment.
LPS (100 ng/mL) treatment significantly stimulated the production of TNF-α after 6h and 18h of stimulation (A). In a time and dose dependent manner, and in the absence of LPS, rMD-2 impacted on the release of TNF-α (B). At 6 hours, the 10 ng/mL dose of rMD-2 resulted in a significant increase in the TNF-α level. This effect was no longer seen at 18 hours. Conversely, the 0.1 ng/mL rMD-2 had an inhibitory effect 18h after treatment. In the presence of LPS, the inhibitory effect of the 0.1 ng/mL and the stimulatory effect of the 10 ng/mL rMD-2 dose were seen at both 6h and 18h, respectively (C). LPS treatment significantly stimulated the production of IL-6 after 6h and 18h of stimulation (D). At 6 h, in the absence of LPS, the 10 ng/mL dose of rMD-2 significantly increasing the IL-6 levels (E). In contrast, the 0.1 ng/mL rMD-2 had an inhibitory effect 18h after treatment. In the presence of LPS, at both 6h and 18 h, the 0.1 ng/mL of rMD-2 inhibit the release of IL-6 (F), while the 10 ng/mL dose displayed a stimulatory effect (F). * P<0.05 vs. 6h time point; # P<0.05 vs. basal (no rMD-2).
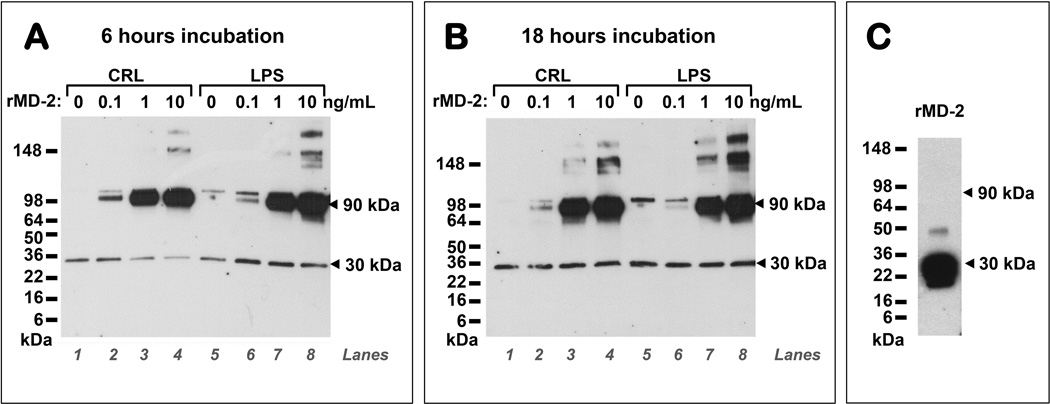
Figure 9. sMD-2 proteoforms released by amniochorion explants after challenge with recombinant MD-2 (rMD-2).
Fetal membrane explants were incubated with rMD-2 (0.1, 1 and 10 ng/mL) for (A) 6 hours or (B) 18 hours in the absence [control - (CRL), lanes 1–4] or presence of LPS (1µg/mL, lanes 5–8). sMD-2 proteoforms were detected by Western blotting. The stimulatory cytokine effect of the 10 ng/mL dose of rMD2 was associated with the assembly of sMD-2 into higher molecular weight proteoforms with predominance of the 90 kDa form which was otherwise absent in the rMD-2 preparation (C).
Discussion
In this study, we sought to provide knowledge about the presence of sMD-2 in human AF and translate this information into a functional output with specific focus on physiologic pregnancy and intra-amniotic infection induced preterm birth. We described for the first time that human AF sMD-2 contains three immunoreactive forms located at 90, 62, and 30 kDa. Specificity of the sMD-2 was confirmed by using a competitive peptide. Although the calculated molecular weight of MD-2 is ~18.4 kDa, the secreted form is expected to have a larger molecular weight [8]. This observation is consistent with the reported glycosylation of sMD-2and ability of to self-associate into larger order oligomeric and polymeric forms [8]. Our assertion is also supported by sequence analysis experiments which demonstrated that MD-2 has at least two glycosylation sites [23]. There is also evidence that N-linked oligosaccharides are added to the nascent MD-2 polypeptide, to allow for proper protein folding and trafficking of the mature glycosylated MD-2 (~30 kDa) to the cell surface [8]. Along this line, our study confirmed glycosylation of the AF sMD-2. This observation is key because the glycosylation process is critical for interaction of MD-2 with various ligands and with TLR4 and TLR2 conferring ability of various cells to be active participants during an inflammatory process [8,23,24]. Specifically, removal of the glycation residues exposes activation epitopes that enhance the binding interaction of sMD-2 and augment the functional response to LPS [24]. Adding to the complex biologic activity of MD-2 is its tendency to aggregate. MD-2 can exist in monomeric and/or multimeric forms [8,25]. The reduction-alkylation experiments demonstrated that the 90 and 62 kDa molecular forms of sMD-2 diminished with the concurrent increase in the intensity of the 30 kDa isoform. These results imply that the 90 and 62 kDa bands contain polymeric forms of MD-2, in contrast to the 30 kDa form which is a unit that cannot be further reduced. The precise functional relevance of the multi- vs. monomeric forms of sMD-2 continues to be subject of debate. Previous studies found that only monomeric MD-2 binds the TLR4’s extracellular domain and mediates LPS signalling [25], whereas others report that TLR4 can associate with MD-2 oligomers of all sizes [8].
Using immunohistochemistry and qRT-PCR we provided evidence that the amnion and choriodecidua are sources of human AF sMD-2. In addition to reproductive tissues, the present study does not exclude the possible contribution of the fetus. Previous studies showed that when MD-2 and TLR4 are expressed within the same cell, they first associate in the endoplasmic reticulum and Golgi complex [8]. The evidence that only a fraction of MD-2 is bound to membrane TLR4 argues that MD-2 is synthesized in excess and following saturation of the receptor, secreted into the extracellular space as sMD-2. To address the question if a similar mechanism could be responsible for the presence of sMD-2 in the AF, we used an amniochorion explant system. We showed that the abundance of sMD-2 was diminished by the protein synthesis inhibitor cycloheximide but increased in the presence of monesin. Monensin is a carboxylic ionophore that disrupts glycoprotein processing in the Golgi thereby reducing the density of cell surface receptors including TLRs [26]. An increase in the extracellular availability of MD-2 due to lack of intracellular TLRs processing and/or an increased release of incompletely assembled or mis-assembled sMD-2 may explain our in-vitro data. Further experiments will be necessary for confirmation.
Based on AF samples of pregnancies with normal outcome we determined that compared to preterm gestation, sMD-2 levels are decreased toward term. The mechanism responsible for GA regulation of sMD-2 in human AF remains unknown. The activity of the MAPKs that include ERK1/2, JNK, and the transcription factor Elk-1 has been reported to be involved in regulating expression of MD-2 gene [27]. The main effectors of these molecular pathways were identified in the fetal membranes [28]. Available data support the view that the intensity of the MAPK pathway activation is more pronounced early gestation [28]. Hence, we postulate that the inverse relationship of AF sMD-2 with GA is related to enhancement of MD-2 gene expression earlier in gestation, with a progressive decrease toward term. Remarkable is that in physiologic pregnancies the levels of AF sCD14 and LPS-binding protein, that are part of the TLR4 signaling mechanism, are also lower at term suggestive of parallel pattern of expression with sMD-2 [29].
Our findings indicate that, in vivo, both TLR4 and MD-2 transcripts are significantly upregulated in reproductive tissues under infectious conditions. As also shown, MD-2 immunostaining in the amnion and choriodecidua was augmented in pregnancies complicated by infection. This is not surprising because MD-2 is an obligatory molecule for the LPS response and plays a key Toll “gatekeeper” function in endotoxin downstream signaling. Because bacterial LPS activate MAPK and NF-kB pathways, we propose that these two mechanisms may participate in upregulation of the MD-2 gene during microbial invasion of the AF [28]. Interestingly, in infection-induced preterm birth, AF sMD-2 levels were elevated by both Gram-negative and Gram-positive bacteria. In addition to TLR4, MD-2 also binds to TLR2 facilitating a TLR2 mediated inflammatory response to both Gram-negative and Gram-positive bacteria [30]. Collectively, our data supports the concept that sMD-2 plays a critical role in controlling the functional activity of both TLR4 and TLR2.
In vitro experiments advise that the functional role of sMD-2 is heterogeneous [31]. Some cellular lines expressing TLR4 (i.e. Chinese Hamster Ovary) are hypo-responsive, while other (i.e. U373-astrocytoma cells, whole human blood) are hyper-responsive to LPS, in the presence of sMD-2 [31]. Level of TLR4 and sMD-2 expression, cellular specificity and glycosylation status dictate the functional capacity of sMD-2 [31]. Our data provide new knowledge about the role of AF sMD-2 on amending an inflammatory response to infection. We demonstrated a dose and time dependent pro-inflammatory cytokine response when fetal membranes were incubated with rMD-2 in the presence or absence of exogenously added LPS. At low concentration, sMD-2 dampened the endotoxin-induced inflammatory response, while higher doses displayed a stimulatory effect. Our Western blot data showed that the inhibitory cytokine response was associated with a lower intensity of the 90 and 62 kDa bands, raising the possibility that sMD-2 proteoforms may have a different functional roles depending on their monomeric versus multimeric conformation. As previously shown, sMD-2 can potentially inhibit the macrophage’s LPS-induced IL-8 production [32]. However, this effect was demonstrated to be major when sMD-2 and recombinant soluble TLR4 (C-terminal end of TLR4) were added together. Because the sTLR4/sMD-2 complex binds LPS [32], the down-regulatory mechanism could be the consequence of a competitive LPS binding effect between the soluble complex and membrane bound TLR4/MD-2 complex. Because in animal models of sepsis, the combination sTLR4-sMD-2 reduced neutrophil inflammation and TNF-α production it was proposed that this complex can be used therapeutically [33]. If this is the responsible mechanism in humans it remains to be further explored as none of the TLR4 antibodies that are commercially available so far were able to confirm presence of sTLR4 in human AF.
The stimulatory effect of the high dose of rMD-2 on cytokine release from the amniochorion explants in the absence of LPS together with the observation of higher sMD-2 immunoreactivity in cases of idiopathic preterm birth supports MD-2's role as regulator of innate immune responses of AF cavity. In this study, we ruled out LPS contamination of rMD-2 by using a peptide expressed in a human cell system and testing it with the LAL assay prior to its use in explant experiments. However, because bacterial colonization of placental tissue is a universal phenomenon that occurs frequently in absence of a positive AF culture [34], we can argue that higher levels of AF sMD-2 may modulate intra-amniotic innate immune responses to endogenous bacteria. Additionally, recent studies identified MD-2 as binding partner for several endogenous polypeptide ligands recognized by TLR4. Surfactant proteins (SPA, SPD), manose binding lectin and serum amyloid A3 where all shown to interact directly with MD-2 [35,36,37]. The endogenous ligands that may possibly interact with MD-2 on fetal membranes as part of a mechanism leading to preterm birth in women with negative AF cultures remain to be identified.
Due to its essential function as TLR-4 associated molecule, MD-2 has emerged as a promising target to quell inflammatory disorders [38]. Studies aimed at testing current or developing new anti-MD-2 therapies that can specifically modulate the intra-amniotic inflammatory response may have a place as adjuvants to antibiotic therapy to prevent infection-induced preterm birth.
Conclusion
We found that sMD-2 proteoforms are constitutively present in human AF and their levels are regulated with GA and infection status. We showed that the machinery responsible for synthesis and secretion of sMD-2 in the AF is located in the amnion and choriodecidua. Furthermore, we provided evidence that sMD-2 could be an important regulator of the intensity of the inflammatory response to infection, raising the possibility of novel therapeutic strategies to prevent inflammation-induced fetal and tissue damage.
Supplementary Material
Acknowledgements
This study was funded by The Society for Maternal-Fetal Medicine/American Association of Obstetricians and Gynecologists Scholarship Award (ATD) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) RO1 HD 047321-01 (IAB), R01 HD062007-01(CSB & IAB). We are indebted to the nurses, residents, and Maternal-Fetal Medicine physicians and fellows at Yale New Haven Hospital, Department of Obstetrics, Gynecology, and Reproductive Sciences, and to all patients who participated in the study.
Abbreviations
- MD-2
myeloid differentiation-2
- sMD-2
soluble myeloid differentiation-2
- rMD-2
recombinant MD-2
- LBP
LPS binding protein
- AF
amniotic fluid
- GA
gestational age
- BME
β-mercaptoethanol
- BCA
bicinchoninic acid
- PVDF
polyvinyl difluoride
- DTT
dithiothreitol
- LAL
Limulus Amebocyte Lysate
- DMSO
dimethylsulfoxide
- ARDS
adult respiratory distress syndrome
Footnotes
Conflict of interest statement
The authors have no financial conflicts of interest.
Author contributions
A.T.D. and I.A.B. designed the study, performed the experiments, collected, analyzed and interpreted the clinical and experimental data and drafted the manuscript. C.S.B participated in study design, supervised the clinical enrolment of the patients, collected, analyzed, interpreted the clinical and experimental data and participated with A.T.D and I.A.B in writing of the manuscript. G.Z. performed part of the ELISA experiments and participated in writing of the report. L.L.S. assisted with the amniochorion explant experiments and together with E.O., S.S.A-R., and M.O.B. participated with aspects of the study design, performance of the experiments and participated in writing of the report. All listed authors have reviewed and approved the submitted version of the manuscript.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Miyake K. Endotoxin recognition molecules, Toll-like receptor 4-MD-2. Sem Immunol. 2004;16:11–16. doi: 10.1016/j.smim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–477. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD-2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 6.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visintin A, Latz E, Monks BG, Espevik T, Golenbock DT. Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J Biol Chem. 2003;278:48313–48320. doi: 10.1074/jbc.M306802200. [DOI] [PubMed] [Google Scholar]
- 8.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci USA. 2001;98:12156–12161. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 10.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 11.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos T, Martin TR, Ruzinski JT, Leturcq DJ, Hillier SL, Patton DL, Eschenbach DA. Lipopolysaccharide binding protein and soluble CD14 receptor protein in amniotic fluid and cord blood in patients at term. Am J Obstet Gynecol. 1997;177:1230–1237. doi: 10.1016/s0002-9378(97)70044-9. [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists, ACOG Practice Bulletin. No. 101: Ultrasonography in pregnancy. Obstet Gynecol. 2009;113:451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 14.Lewis D, Bergstedt S, Edwards M, Burlison S, Gallaspy J, Brooks G, Adair CD. Successful magnesium sulfate tocolysis: Is "weaning" the drug necessary? Am J Obstet Gynecol. 1997;177:742–745. doi: 10.1016/s0002-9378(97)70261-8. [DOI] [PubMed] [Google Scholar]
- 15.Newton E. Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol. 2005;32:571–600. doi: 10.1016/j.clp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RK, Clark P, Locksmith GJ, Duff P. Performance characteristics of putative tests for subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 2001;9:209–214. doi: 10.1155/S1064744901000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–1341. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 18.Gray P, Michelsen KS, Sirois CM, Lowe E, Shimada K, Crother TR, Chen S, Brikos C, Bulut Y, Latz E, Underhill D, Arditi M. Identification of a novel human MD-2 splice variant that negatively regulates lipopolysaccharide-induced TLR4 signaling. J Immunol. 2010;184:6359–6366. doi: 10.4049/jimmunol.0903543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Ponchel FC, Toomes C, Bransfield K, Leong FT, Douglas SH, Field SL, Bell SM, Combaret V, Puisieux A, Mighell AJ, Robinson PA, Inglehearn CF, Isaacs JD, Markham AF. Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy KS, Miller LS, Cox EB, Konrath J, McCarthy KSSr. Estrogen receptor analysis: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 22.Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, Buhimschi IA. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol. 2009;182:7244–7253. doi: 10.4049/jimmunol.0803517. [DOI] [PubMed] [Google Scholar]
- 23.da Silva Correia J, Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem. 277:1845–1854. doi: 10.1074/jbc.M109910200. 202. [DOI] [PubMed] [Google Scholar]
- 24.Feng C, Stamatos NM, Dragan AI, Medvedev A, Whitford M, Zhang L, Song C, Rallabhandi P, Cole L, Nhu QM, Vogel SN, Geddes CD, Cross AS. Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS One. 2012;7:e32359. doi: 10.1371/journal.pone.0032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Re F, Strominger JL. Monomeric recombinant MD-2 binds toll-like receptor 4 and confers lipopolysaccharide responsiveness. J Biol Chem. 2002;277:23427–23432. doi: 10.1074/jbc.M202554200. [DOI] [PubMed] [Google Scholar]
- 26.LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, Labéta MO. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol. 2003;171:6680–6689. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Yu Y, Wang Y, Liu L, Zhang M, Sugano S, Wang Z, Chang Z. Both ERK and JNK are required for enhancement of MD-2 gene expression during differentiation of HL-60 cells. Biol Cell. 2008;100:365–375. doi: 10.1042/BC20070140. [DOI] [PubMed] [Google Scholar]
- 28.Jung HS, Yoon BH, Jun JK, Kim M, Kim YA, Kim CJ. Differential activation of mitogen activated protein kinases and nuclear factor-kappaB in lipopolysaccharide-treated term and preterm amnion cells. Virchows Arch. 2005;447:45–52. doi: 10.1007/s00428-005-1248-9. [DOI] [PubMed] [Google Scholar]
- 29.Roos T, Martin TR, Ruzinski JT, Leturcq DJ, Hillier SL, Patton DL, Eschenbach DA. Lipopolysaccharide binding protein and soluble CD14 receptor protein in amniotic fluid and cord blood in patients at term. Am J Obstet Gynecol. 1997;177:1230–1237. doi: 10.1016/s0002-9378(97)70044-9. [DOI] [PubMed] [Google Scholar]
- 30.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 31.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 32.Hyakushima N, Mitsuzawa H, Nishitani C, Sano H, Kuronuma K, Konishi M, Himi T, Miyake K, Kuroki Y. Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signalling. J Immunol. 2004;173:6949–6954. doi: 10.4049/jimmunol.173.11.6949. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuzawa H, Nishitani C, Hyakushima N, Shimizu T, Sano H, Matsushima N, Fukase K, Kuroki Y. Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J Immunol. 2006;177:8133–8139. doi: 10.4049/jimmunol.177.11.8133. [DOI] [PubMed] [Google Scholar]
- 34.Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109:739–749. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- 35.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Nishitani C, Mitsuzawa H, Ariki S, Takahashi M, Ohtani K, Wakamiya N, Kuroki Y. Mannose binding lectin and lung collectins interact with Toll-like receptor 4 and MD-2 by different mechanisms. Biochim. Biophys. Acta. 2009;1790:1705–1710. doi: 10.1016/j.bbagen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Deguchi A, Tomita T, Omori T, Komatsu A, Ohto U, Takahashi S, Tanimura N, Akashi-Takamura S, Miyake K, Maru Y. Serum amyloid A3 binds MD-2 to activate p38 and NF-κB pathways in a MyD88-dependent manner. J Immunol. 2013;191:1856–1864. doi: 10.4049/jimmunol.1201996. [DOI] [PubMed] [Google Scholar]
- 38.Duan G, Zhu J, Xu J, Liu Y. Landmark Targeting myeloid differentiation 2 for treatment of sepsis. Front Biosci. 2014;19:904–915. doi: 10.2741/4256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.