Abstract
Although antibiotics are a common treatment for acne, the difficulties inherent to effective antimicrobial penetration in sebum and selective antimicrobial action in skin are compounded by increasing resistance of Propionibacterium acnes clinical isolates. To address these problems, we engineered Pentobra, a peptide-aminoglycoside molecule which has multiple mechanisms of antibacterial action, and investigated whether it can be a potential candidate for the treatment of acne. Pentobra combines the potent ribosomal activity of aminoglycosides with the bacteria-selective membrane-permeabilizing abilities of antimicrobial peptides (AMPs). Pentobra demonstrated potent and selective killing of P. acnes, but not against human skin cells in vitro. In direct comparison, Pentobra demonstrated bactericidal activity and drastically outperformed free tobramycin (by 5–7 logs) against multiple P. acnes clinical strains. Moreover, EM studies showed that Pentobra had robust membrane activity, as treatment with Pentobra killed P. acnes cells and caused leakage of intracellular contents. Pentobra may also have potential anti-inflammatory effects as demonstrated by suppression of some P. acnes-induced chemokines. Importantly, the killing activity was maintained in sebaceous environments as Pentobra was bactericidal against clinical isolates in comedones extracts isolated from human donors. Our work demonstrates that equipping aminoglycosides with selective membrane activity is a viable approach for developing antibiotics against P. acnes that are effective in cutaneous environments.
Introduction
The commensal bacterium Propionbacterium acnes is a major etiological factor in acne vulgaris (McInturff et al., 2005; Ross et al., 2003; Williams et al., 2012). Although topical antibiotic therapies are used (Eady et al., 2003), increasing resistance have made acne treatment challenging. The incidence of P. acnes antibiotic resistance increased from 20% in 1978 to 72.5% in 1995 (Humphrey, 2012); widespread resistance has become a major dermatological issue (Cooper, 1998; Eady et al., 2003; Humphrey, 2012; Williams et al., 2012). Antibiotic resistance is compounded by the difficulties inherent to effective antimicrobial penetration in skin. Many antibiotics are charged and do not penetrate into the largely hydrophobic sebaceous environmental niche of P. acnes. Clearly, antibiotic therapies that are more tailored to these problems are needed.
It is difficult for an organism to simultaneously evolve resistance against multiple antimicrobial mechanisms. This observation has motivated the development of multi-drug strategies (antibiotic cocktails). While this approach has been effective for some diseases (i.e. leprosy), it has led to multi-drug resistant strains in others (i.e. tuberculosis), in part due to inherent problems from the use of multiple drugs. Rather than using a complex cocktail, we aim to engineer a P. acnes antibiotic by combining the potent ribosomal activity of aminoglycosides with the bacteria-selective membrane-permeabilizing abilities of antimicrobial peptides (AMPs), which can perforate prokaryotic membranes but not eukaryotic membranes. Aminoglycoside antibiotics target the 16S rRNA component of the bacterial ribosome leading to mistranslation, inhibition, and cell death (Fourmy et al., 1996; Vicens and Westhof, 2002). In vitro studies have shown that although aminoglycosides are usually potent antimicrobials, P. acnes is not strongly susceptible to them (Wang et al., 1977). As P. acnes is an anaerobic bacterium, it is hypothesized that its intrinsic resistance is a result of poor aminoglycoside uptake, not a lack of ribosomal activity (Davis, 1987; Taber et al., 1987). AMPs can selectively permeabilize bacterial membranes (Brogden, 2005; Hancock and Sahl, 2006; Schmidt and Wong, 2013; Zasloff, 2002). While many AMPs kill via lysis, many others combine membrane activity with additional mechanisms such as inhibition of metabolic functions by binding intracellular targets (Brogden, 2005). Aminoglycosides equipped with cell-penetrating abilities can have activity against slow-growing bacteria like P. acnes, which have little uptake. Moreover, the addition of AMP-like membrane activity will add an extra dimension of selectivity to the specific mechanisms inherent to aminoglycosides.
Here we report an aminoglycoside-based compound with bactericidal activity against P. acnes. Our design is informed by recent work which elucidate the roles of cationic and hydrophobic residues in AMP sequences (Schmidt et al., 2011; Schmidt et al., 2012b), Cell-penetrating peptide (CPP) sequences (Mishra et al., 2011), and non-peptidic AMP mimetic compositions (Hu et al., 2013; Schmidt et al., 2012a), and relate them to the geometric requirements of membrane permeabilization. Tobramycin, a potent aminoglycoside, is conjugated to a short 12AA peptide to equip the composite molecule, Pentobra (Figure 1A), with preferential activity against bacterial membranes like an AMP, so that the composite molecule has multiple levels of selectivity and multiple mechanisms of killing (Schmidt et al., 2014). The work presented herein, demonstrates that equipping aminoglycosides with selective membrane activity is a viable approach for developing antibiotics against P. acnes that are effective in cutaneous environments.
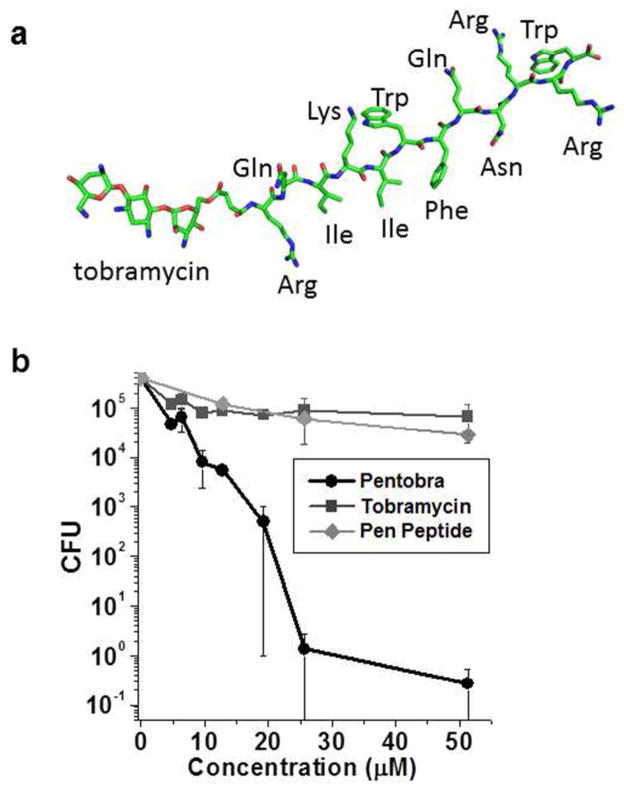
Figure 1. Pentobra is bactericidal against Propionibacterium acnes.
(A) Structure of Pentobra. (B) P. acnes ATCC 6919 was incubated with different concentrations of Pentobra, tobramycin, or pen peptide (0–52 μM) for 3 hours and tested for bactericidal activity using the CFU assay. Data show average CFU from three independent experiments (n = 3), error bars are ± SEM.
Results
Pentobra has potent and selective antimicrobial activity against P. acnes, but not against human skin cells
Since Pentobra is designed to permeabilize membranes, we hypothesized that Pentobra should be bactericidal against P. acnes. We tested the killing potency of Pentobra against P. acnes using CFU assays (Figure 1B). Pentobra displayed dose-dependent killing activity against P. acnes laboratory strain ATCC 6919. Concentrations as low as 8 μM Pentobra produced a ten-fold reduction in viable colonies, and 26 μM Pentobra led to a 5-log reduction in CFU. In contrast, tobramycin was not strongly bactericidal, as concentrations as high as 52 μM led to less than ten-fold reduction in CFU. These data show that membrane-active aminoglycosides can kill the P. acnes lab strain, whereas neither tobramycin nor the free pen peptides were effective. Importantly, Pentobra is not toxic to human skin cells as treatment did not affect the viability of human peripheral blood mononuclear cells (PBMCs), keratinoctyes, or sebocytes over 72 hrs (Supplementary Figure S1 A&B).
Pentobra is active against a wide variety of P. acnes clinical strains
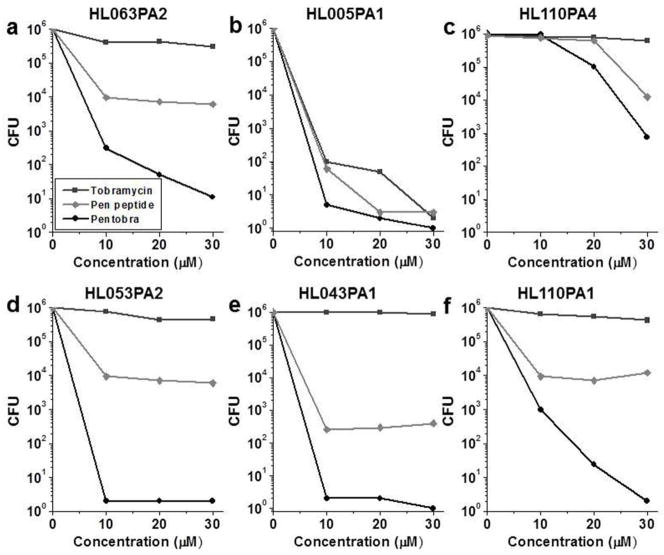
The predominant microbe found in the microcomedone content is P. acnes, which accounts for ~90% of the microbiota (Fitz-Gibbon et al., 2013). Moreover, microcomedones from healthy vs. diseased skin harbor P. acnes strains from distinct lineages and possess distinct nucleopeptide signatures of 16S rDNA sequences. While some P. acnes strains are found on healthy skin (phylotype III and ribotype 6), others are associated with acne disease (ribotypes 4, 5, 8, and phylotype IC) and with diseases such as medical device infections (phylotype II) (McDowell et al., 2013). To examine the antimicrobial activity of Pentobra against different P. acnes strains (Table 1), we conducted CFU assays on clinical isolates. In general, Pentobra exhibited robust bactericidal activity against all tested P. acnes strains (Figure 2). Against P. acnes clinical isolates HL063PA2 (healthy) and HL005PA1 (healthy) (Figure 2A&B), greater than 5-log reductions in CFU were observed at 26 μM Pentobra. While strain HL110PA4 (healthy) was less susceptible (Figure 2C), a 2-log reduction occurred at the highest concentration tested. Interestingly, this differential activity may allow Pentobra to shift slightly the ecology of P. acnes toward strains associated with healthy skin. Pentobra also killed P. acnes strains HL053PA2, HL043PA1, and HL110PA1 that are associated with acne skin (Figure 2D–F), as 13 μM Pentobra was sufficient to reduce CFU by greater than 5-log units for the first two strains and 3-log units for the third one. Similar to ATCC 6919, tobramycin did not exhibit significant antimicrobial activity against most of these clinical isolates, whereas the free pen peptide typically demonstrated moderate 2–3-log reductions in CFU. However, tobramycin was strongly bactericidal against strain HL005PA1, suggesting that aminoglycosides may be effective against certain strains of P. acnes. Our results demonstrate that Pentobra has potent activity against clinically relevant strains.
Table 1.
P. acnes clinical isolates used in the study
| Clinical isolate | Phylotype | Ribotype (RT) | Disease association |
|---|---|---|---|
| ATCC 6919 | IA-1 | RT1 | Neutral/commensala |
| HL005PA1 | IA-1 | RT1 | Healthyb |
| HL043PA1 | IA-2 | RT5 | Acnec |
| HL053PA2 | IB-1 | RT8 | Acne |
| HL063PA1 | IA-1 | RT1 | healthy |
| HL110PA1 | IB-1 | RT8 | Acne |
| HL110PA4 | II | RT6 | healthy |
P. acnes laboratory strain
Clinical isolates associated with healthy skin
Clinical isolates associated with acne skin
Figure 2. Pentobra is bactericidal against a broad range of P. acnes strains.
CFU assay results for Pentobra, pen peptide and tobramycin at varying concentrations (0–26 μM) incubated with P. acnes clinical isolates (A) HL063PA2 (health-associated), (B) HL005PA1 (health-associated) (C) HL110PA4 (health-associated), (D) HL053PA2 (acne-associated), (E) HL043PA1 (acne-associated) and (F) HL110PA1 (acne-associated) for 3 h. Data from one experiment is shown and the trends in antimicrobial activity of the compounds and activity differences between compounds are representative of three independent experiments.
Pentobra alters cell surface morphology, permeates and lyses P. acnes
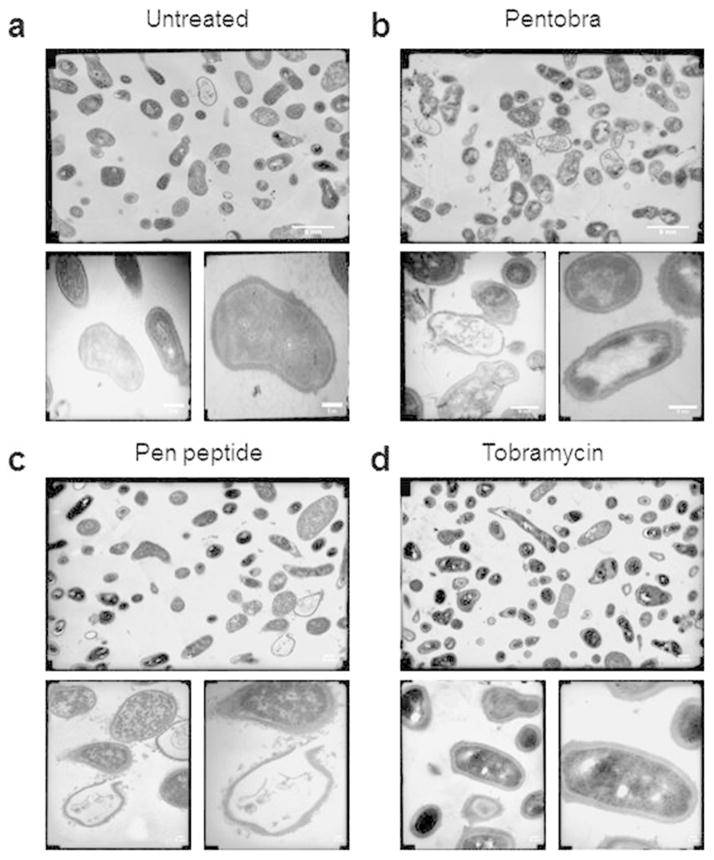
Pentobra is designed to have selective membrane permeation activity similar to AMPs. To determine its effects on the cell envelope, we examined P. acnes treated with Pentobra using electron microscopy (Figure 3). Transmission EM micrographs of untreated P. acnes illustrate their normal pleomorphic structure (Figure 3A). After a 3 hour treatment with Pentobra, different types of cell envelope disruptions are observed (Figure 3B). Cell surfaces appear ruffled from blebbing, and complete breaches in the envelope are observed, with externalized cytoplasmic contents characteristic of lysis. These morphological changes are consistent with those from membrane active molecules. The observed robust permeabilization of P. acnes membranes corresponds well with previous x-ray studies, which showed that Pentobra generates disruptive membrane curvatures in model bacterial cell membranes and permeabilizes E. coli inner membranes (Schmidt et al., 2014). To see how the different components of Pentobra impact P. acnes, EM studies were performed with free pen peptide and tobramycin. The pen peptide is lytic (Figure 3C), which is consistent with its strong membrane permeabilization profiles on E. coli cells (Schmidt, 2014). P. acnes treated with tobramycin are indistinguishable from control (Figure 3D), in line with our antibacterial assays and with the reduced uptake of aminoglycosides into anaerobic bacteria cells.
Figure 3. EM studies show Pentobra acts on P. acnes cell membranes.
Representative TEM micrographs of (A) untreated P. acnes control, (B) P. acnes after 3 h incubation with 25.7μM Pentobra, (C) P. acnes after 3 h incubation with 25.7μM pen peptide, and, (D) P. acnes after 3 h incubation with 25.7μM tobramycin. TEM were imaged at 10K magnification (top), 36K (bottom left), and 72K (bottom right). Compared with untreated control, the bacteria exposed to Pentobra and pen peptide exhibit cellular differences indicative of stresses on the membrane. Complete lysis of the cell membrane occurs, and the envelope boundary is now decorated with numerous blebbing events (bottom images in B and C). Scale bar = 5nm.
Pentobra maintains antimicrobial activity in human comedone extracts
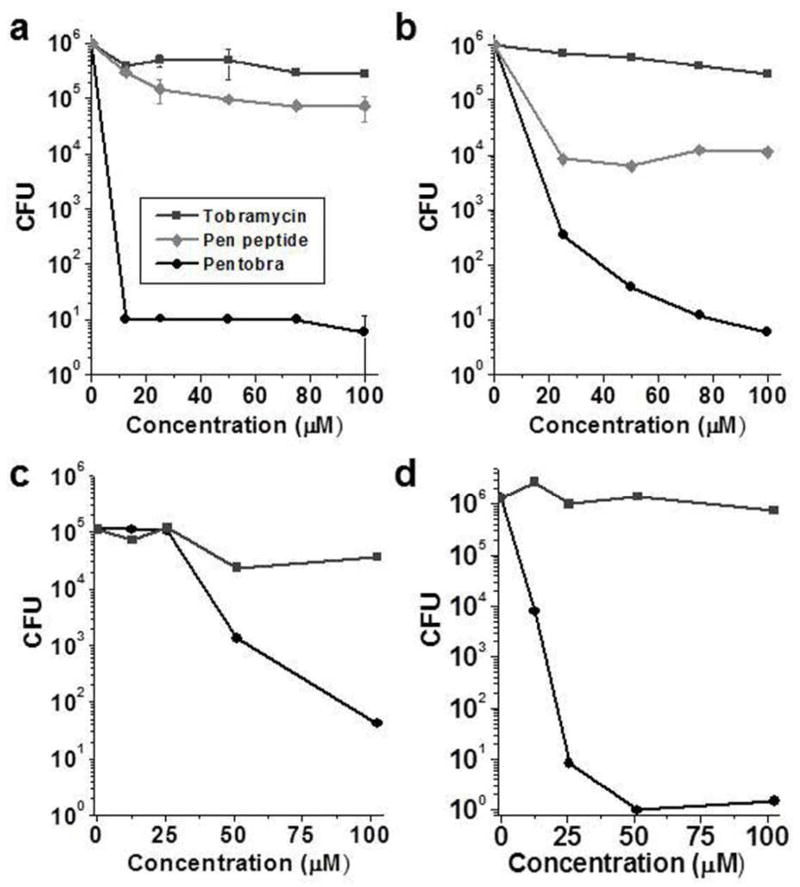
Acne therapeutics must maintain activity in lipid-rich environments. To assay the effectiveness of Pentobra against clinical isolates in sebaceous environments, we used comedone extracts isolated from human donors as an in vitro model (Figure 4). Pentobra showed dose-dependent bactericidal activity against P. acnes in comedone extracts from four donors, producing 5-log (Figure 4A&B), 3-log (Figure 4C), and 6-log (Figure 4D) reductions in CFUs. In contrast, tobramycin and the pen peptide alone were significantly less effective. That Pentobra is amphiphilic may contribute significantly to the enhanced activity in strongly hydrophobic environments. The amphiphilic peptide moiety of Pentobra has at least two beneficial effects: it drastically increases the activity of aminoglycoside antibiotics against P. acnes, and the lipophilicity aspect of Pentobra allows it to maintain activity in sebaceous environments.
Figure 4. Pentobra is bactericidal in the sebaceous microcomedone environment.
Antimicrobial activity against P. acnes in the microcomedones was determined using a CFU assay. Pentobra, pen peptide or tobramycin (0 – 102 μM) were incubated for 3 hours in collected lipid-rich microcomedones isolated from four donors’ faces (A – D) using deep cleaning pore strips. Pentobra exhibits strong bactericidal activity while tobramycin and pen peptide did not. Data in A represent mean ±SD of three experiments using microcomedones extracted on different days from the same donor. Data in B, C, and D are each from a different donor.
Immunomodulatory effect of Pentobra and tobramycin in human monocytes
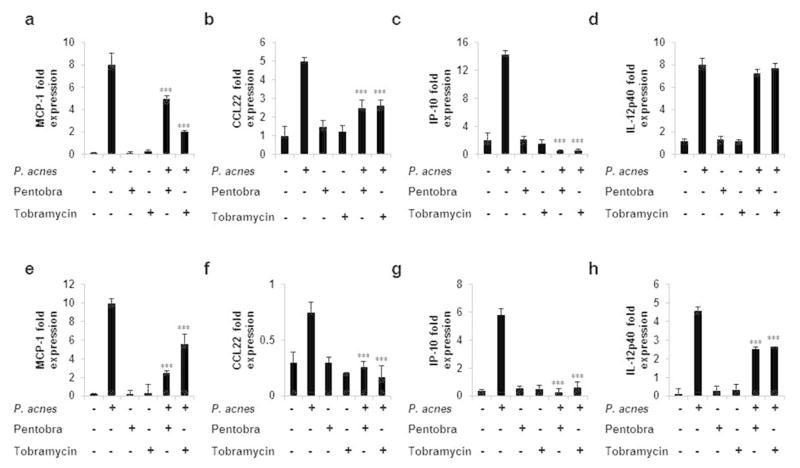
To determine if Pentobra has an immunomodulatory effect on host innate immune response, we isolated and co-treated human monocytes and keratinocytes with P. acnes in the presence of either Pentobra or tobramycin, and measured cytokine and chemokine expression levels by ELISA and real time PCR. P. acnes induced the expression of MCP-1, CCL22, IP-10 and IL-12p40 in both cell types (Figure 5) which in monocytes could be inhibited (40–100%) by both Pentobra and tobramycin except for IL-12p40 gene expression, where both antibiotics demonstrated little to no inhibition of IL-12p40 by P. acnes-stimulated monocytes. Tobramycin was more effective in inhibiting MCP-1 expression in monocytes compared to Pentobra. Both antibiotics completely abrogated P. acnes–induced IP-10 expression (p<0.001) in monocytes and keratinocytes. In contrast, no significant change in IL-6, IL-8, TNF-α and IL-12p40 cytokine production was found in P. acnes–activated monocytes in the presence of both antibiotics (Supplementary Figure S2 and data not shown). Therefore, both Pentobra and tobramycin appear to modulate specific cytokine and chemokine production induced by P. acnes but not all cytokines and chemokines that have been reported to play a role in the formation of inflammatory acne lesions. (McInturff et al., 2005; Vowels et al., 1995a; Vowels et al., 1995b)
Figure 5. Immunomodulatory effects of Pentobra on human monocytes and keratinocyte cell line stimulated with P. acnes.
Adherent monocytes (A–D)and HaCaT (E–H) cells were cultured (2–3 x 106/ml) with P. acnes in the presence of Pentobra (26 μM), and tobramycin (26 μM)). MCP-1 (A and E), CCL22 (B and F), IP-10 (C and G), and IL-12p40 (D and H) mRNA expression was analyzed 24 hours following P. acnes stimulation. Gene expression was normalized to the housekeeping genes GAPDH and quantified by the comparative method 2−ΔΔCT. Each panel is representative of three independent donors and experiments. Data represent mean ±SD (***p ≤ 0.001). Primers used in the study are listed in supplementary Table S1.
Discussion
The increasing antibiotic resistance in clinical isolates of P. acnes highlights the need for new therapeutic strategies. The performance of Pentobra against P. acnes suggests that equipping aminoglycosides with the ability to permeate membranes in the manner of AMPs can be broadly enabling. Aminoglycosides are known to generally target bacterial ribosomes by binding the decoding aminoacyl site on the 16S rRNA component of the 30S ribosomal subunit, leading to faulty protein translation and inhibition (Fourmy et al., 1996; Vicens and Westhof, 2002). This mechanism of action is potent and highly bactericidal against many types of clinically relevant bacteria. Empirically, aminoglycosides are known to have little effectiveness on P. acnes (Dréno et al., 2004; Wang et al., 1977; Williams et al., 2012) and other anaerobic bacteria. Aminoglycosides bind the ribosomes of anaerobic bacteria and bacteria that use oxygen with similar affinities (Bryan et al., 1979). It is therefore hypothesized that anaerobic bacteria are intrinsically resistant to aminoglycosides because the molecules cannot cross the cytoplasmic membranes of anaerobic cells to reach the bacterial ribosome (Allison et al., 2011; Taber et al., 1987). The lower proton-motive force (PMF) (Davis et al., 1986; Magnet and Blanchard, 2004) across the membranes of anaerobic bacteria in comparison with oxygen-using bacteria impairs internalization, since aminoglycoside uptake is proposed to be energy-dependent from reliance on a threshold PMF (Allison et al., 2011; Davis, 1987; Magnet and Blanchard, 2004; Taber et al., 1987). Although the general AMP membrane permeabilization mechanism is broad spectrum (Schmidt and Wong, 2013), and their activities are less sensitive to the metabolic status of the cell (Hurdle et al., 2011), AMPs often only display moderate potency. When juxtaposed, the distinct mechanisms of action of aminoglycosides and AMPs become complementary. Hybrid membrane-active aminoglycosides can kill bacteria by membrane disruption, and can also enter cells and interfere with ribosome translation. The direct killing of all tested P. acnes clinical isolates by Pentobra shows that our approach has promising general therapeutic value.
Bacteria must simultaneously evade both of pentobra’s killing mechanisms to develop resistance, similar to drug combination therapies used to minimize chances of bacterial resistance (Fischbach, 2011; Walsh, 2000; Worthington and Melander, 2013). While bacteria have well-developed pathways to decrease susceptibility to AMPs through phenotypic changes (Guo et al., 1997; Koprivnjak and Peschel, 2011; Li et al., 2007), these mechanisms do not usually confer complete resistance (Hancock and Sahl, 2006). This is believed to be a result of the ability of AMPs to target generic properties in the lipid composition of bacterial membranes (Hancock and Sahl, 2006; Schmidt and Wong, 2013; Zasloff, 2002). In fact, to evolve AMP resistance by eliminating these generic lipid properties of bacterial membranes amounts to a lethal mutation (Yang et al., 2008). By multiplexing the specificity of ribosomal activity from tobramycin with the general susceptibility of bacterial membranes to AMPs, Pentobra provides potent antimicrobial activity with reduced likelihood of P. acnes resistance.
A topical antimicrobial therapy must penetrate lipid-rich media and maintain activity in cutaneous environments. The initial stage of acne infection is a microcomedone, and previous work has used extracted microcomedone contents to determine the effectiveness of antimicrobial therapies for acne (Piérard-Franchimont et al., 2002). We performed similar microcomedone experiments to determine if Pentobra, which is amphipathic, can penetrate hydrophobic environments and remain active when presented with lipidic material from skin. Hydrophilic drugs like aminoglycosides may not be able to penetrate through the layers of fatty acids in human skin, while overly hydrophobic drugs may become irreversibly sequestered with fatty acids and will be unavailable to act on P. acnes. Our assays on P. acnes isolated from microcomedones show that Pentobra can kill P. acnes in a sebum-rich environment. Human skin widely expresses cathelicidin LL37 (Dürr et al., 2006), as well as defensins HBD-2 (Harder et al., 1997) and HBD-3 (Harder et al., 2001), which suggests that AMPs can offer protection in skin against microbial colonizers, and AMP gene knock out studies have shown these animals are more vulnerable to infection (Bowdish et al., 2006; Dürr et al., 2006). Many membrane-permeabilizing AMPs including HBD2 (Bals et al., 1998) and LL37 (Dürr et al., 2006) show loss of antimicrobial activity in elevated salt concentrations in vitro. Like these AMPs the bactericidal activity of Pentobra against P. acnes is attenuated with increasing sodium chloride concentrations (Panel C of supplementary Figure S1). This affords future engineering possibilities: While plate killing assays provide important measures of drug potency, it does not always mirror the performance of a drug in vivo. In principle, it is possible to engineer membrane-active aminoglycosides that are not as salt sensitive because some AMPs have in vitro salt tolerant antibacterial profiles (Harder et al., 2001). Furthermore, drug activities can be modulated by formulation and method of delivery. Overall, the microcomedone experiments highlight the efficacy of Pentobra in lipid-rich cutaneous environments, which is suggestive of its utility as a topical antimicrobial agent.
For a clear understanding of the immunomodulation by Pentobra, we explored its function at the transcriptional and protein levels. Many mammalian AMPs have immunomodulatory functions (Bowdish et al., 2006). For example, human cathelicidin LL37 can decrease cytokine production in response to LPS (Scott et al., 2002), which is a ligand for TLR4. Previous studies have proposed that the anti-endotoxin abilities of implicated cathelicidins are partly due to direct binding between the cationic peptides and anionic LPS (Rosenfeld and Shai, 2006; Scott et al., 2000), since the neutralized LPS is unavailable to associate with pattern recognition receptors. The 12AA pen peptide is derived from the protein transduction domain of the Drosophila antennapedia homeodomain (Derossi et al., 1994; Joliot et al., 1991). The polycationic nature of both Pentobra and tobramycin can lead to strong electrostatic interactions with anionic components of P. acnes. It is possible that the partial anti-inflammatory effects of Pentobra are derived from interference with aspects of the toll like receptor-2 (TLR2) pathway, such as NFkB activation, since P. acnes stimulates cells through TLR2 (Kim et al., 2002). While the ligands from P. acnes that activate TLR2 remain under investigation, the interaction of Pentobra with these molecules may reduce inflammation. Furthermore, the polyanionic charge of cell envelope components like peptidoglycan can promote complexation with Pentobra, which may prevent detection of peptidoglycan by intracellular sensors Nod1 and Nod2. Since inflammation is a key clinical feature of acne, the partial anti-inflammatory nature of Pentobra combined with its antibacterial activity increases its therapeutic potential.
The immunomodulatory capabilities of membrane-active aminoglycosides suggest potential clinical applications. Constraints on the amino acid content of AMPs under-determine the full peptide sequence (Schmidt et al., 2011; Schmidt and Wong, 2013), so other functions may be written into these peptide-aminoglycoside composites. LL37 is a notable natural example as it permeabilizes bacterial membranes and also has multiple immunomodulatory functions. In the same spirit, it should be possible to construct future generations of Pentobra which are not only potent bactericides against P. acnes, but also complex strongly with P. acnes ligands and thereby modulate immune responses.
In conclusion, we have demonstrated the efficacy of a hybrid antibiotic for P. acnes, Pentobra, by synergistically combining the antimicrobial functions and specificity mechanisms of two different classes of antimicrobials, aminoglycosides and AMPs. Pentobra exhibited bactericidal activity against a wide range of P. acnes clinical isolates, and effectively killed P. acnes from human donors in microcomedone sebum-rich environment in an in vitro assay. Furthermore, various skin cells treated with Pentobra showed no adverse effects, suggesting that Pentobra is not only an effective antimicrobial, but may also be a safe and less irritating topical agent. Pentobra displayed anti-inflammatory activity by suppressing the expression of inflammatory chemokines from monocytes and keratinocytes stimulated with P. acnes. The broad bactericidal activity against clinical strains of P. acnes in the lipid-rich cutaneous environment, in concert with their potential as anti-inflammmatory agents, shows that peptide-aminoglycoside antibiotics designed using rules for AMPs are promising therapeutics for infections caused by drug-resistant P. acnes.
Materials and methods
Pentobra
Pentobra is a composite peptide+aminoglycoside compound consisting of a 12AA peptide named the pen peptide, which is conjugated to tobramycin. (Schmidt et al., 2014). The five amine groups of tobramycin were Boc (tert-butyloxycarbonyl) protected, then the C6″ primary hydroxyl of tobramycin-Boc5 was selectively reacted with succinic anhydride to produce a terminal carboxyl function. Pen peptide was synthesized manually by solid phase synthesis. The carboxyl function extending from tobramycin was coupled with the N-terminal group of the fully protected resin-anchored pen peptide. The resulting Pentobra was cleaved from resin and fully deprotected by cleaving the side-chain protecting groups as well as Boc groups on tobramycin with a trifluoroacetic acid mixture containing scavengers. Pentobra was finally purified by preparative high-performance liquid chromatography (HPLC) and characterized by MALDI-TOF mass spectrometry.
The pen peptide, sequence RQIKIWFQNRRW, was designed with the cationic and hydrophobic motif shared by AMPs. It is cationic (+3 from 2 Arg and 1 Lys) and hydrophobic (2 Ile, 2 Trp, and 1 Phe, it is >30% hydrophobic). We designed Pentobra with the ability to disrupt bacterial membranes and cross them so that it can bind ribosomes through its tobramycin part. To ensure Pentobra permeates cell membranes the pen peptide sequence was derived from the 17AA sequence of antennapedia (ANTP) penetratin CPP (Derossi et al., 1994), such that the amounts of cationicity and hydrophobicity of the composite molecule (pen peptide+tobramycin) are consistent with design principles of CPP and AMP sequences (Mishra et al., 2011; Schmidt et al., 2011). Therefore, the sequence of the pen peptide was chosen such that the composite pen peptide plus tobramycin would be able to permeabilize and penetrate bacterial membranes thereby allowing it to enter bacteria cells and bind ribosomes using the mechanism of action from tobramycin.
P. acnes and clinical isolates
P. acnes strain ATCC 6919 was obtained from American Type Culture Collections (Manassas, VA). Clinical isolates were obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources) and from nasal skin microcomedones from patients attending the Division of Dermatology outpatient clinic at UCLA, after signed written informed consent as approved by the Institutional Review Board at UCLA in accordance with the Declaration of Helsinki Principles. The level of endotoxin contaminating the P. acnes was quantified with a Limulus Amoebocyte Lysate assay (BioWhittaker, Radnor, PA) and found to be <0.1 ng ml−1. P. acnes cultures were grown as previously described (Agak et al., 2014). P. acnes strains and clinical isolates used in the study are summarized in Table 1.
CFU assay
The CFU assay was performed as described previously (McInturff et al., 2005). P. acnes strains (Table 1) were grown under anaerobic conditions in Reinforced Clostridial Medium (Oxoid, Basingstroke, England) and collected in mid-log phase. The bacteria were washed with the assay buffer (10mM Tris pH 7.4, supplemented with 1% volume Trypticase soy broth, Tris-TSB), and enumerated by applying a conversion factor of 7.5 x 107 bacteria per mL=1 OD unit at 600 nm. Various concentrations of Pentobra, pen peptide or tobramycin were incubated with 3.75 x105 bacteria in a final volume of 100 μL at 37°C for 3 h. After incubation, 103–104-fold dilutions were prepared and plated on solid media comprised of Brucella broth (BD Biosciences, San Diego, California) with 5% sheep red blood cells (Remel, Lenexa, Kansas). Plates were incubated for 4 days at 37°C under anaerobic conditions, then individual colonies were counted and the number of CFU per tube was calculated.
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay
Three cell types, PBMCs, Keratinocytes (HaCaT) and Sebocytes cell lines were tested. Cell proliferation experiments were performed in 96-well plates (5 replicates). 26 μM of Pentobra, pen peptide and Tobramycin treatments were initiated at 24 hours post- seeding for 3 days, and assay developed as previously described (Agak et al 2014).
Electron Microscopy
P. acnes ATCC 6919 at 3x108 CFU/mL were incubated untreated or with 25.7μM of Pentobra, pen peptide or tobramycin for 3 hours, washed twice with PBS, and resuspended in PBS with 2% glutaraldehyde. The bacteria were fixed for 5 minutes with 0.05% OsO4, dehydrated in graded ethanol, and then embedded in Eponate 12 (Ted Pella). 60–70nm slices were cut with a Reichert-Jung Ultracut E ultramicrotome, which were picked up on formvar coated copper grids. Samples were stained with uranyl acetate and Reynolds lead citrate and visualized at 80 kV on a JEOL 100CX electron microscope.
Microcomedone assay
After informed consent was obtained, comedones were collected from human volunteers as previously described (McInturff et al., 2005). Briefly, individual plugs were removed from the strips with a fine point tweezer and pooled in a microfuge tube. These were then resuspended in 200 μL of assay medium (Tris-TSB) until the particulate material was broken up into a colloidal suspension. Then, 30 μL of this suspension was combined with either 30 μL of drug (Pentobra, pen peptide, tobramycin,) or 30 μL of clostridium medium (positive control). These were then incubated for 3 h at 37°C in an ambient air incubator. A serial dilution of each sample was prepared and 30 μL of each dilution was spotted on Brucella blood agar plates and incubated as previously described. The CFU per mL was determined by counting.
Monocyte isolation, stimulation and cytokine ELISAs
Monocytes were isolated as previously described (Agak et al., 2014; Qin et al., 2014). Adherent monocytes were stimulated (co-treated) with media or P. acnes in the presence of tobramycin or Pentobra and cytokine levels measured by ELISA as previously described (Agak et al., 2014; Qin et al., 2014) Samples were assayed in triplicates. Results are expressed as mean±SD of at least three independent experiments, with monocytes obtained from three independent donors.
RNA isolation, cDNA synthesis, and real-time PCR
Monocytes and keratinocytes (HaCaT) were stimulated with media or P. acnes in the presence of tobramycin or Pentobra. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and cDNA synthesis and real-time PCR reactions were done as previously described (Agak et al., 2014; Qin et al., 2014). The list of primers used in the study are summarized in Supplementary Table S1.
Statistical analysis
Results are expressed as the means±SD for the number of separate experiments indicated in each case (n≥3). One-way analysis of variance was used to compare variances within groups and among them. Post hoc two-tailed Student’s t-test was used for comparison between two groups. Significant differences were considered for those probabilities ≤ 5% (P≤ 0.05).
Supplementary Material
Acknowledgments
We would like to extend thanks to Marianne Cillufo for assistance with electron microscopy. This work was supported by NIH grants 1UO1 AI082192-01 (G.C.L.W.), R01-AR-053542 (J.K.) and T32 Training grant AR58921 (G.A.), NSF grants DMR1106106 (G.C.L.W.) and 1-DP2-OD008533 (A.M.K). UCLA has filed a patent application on membrane-active aminoglycoside peptide conjugates.
Abbreviations
- CPP
Cell-penetrating peptide
- AMPs
Antimicrobial peptides
- PBMCs
Peripheral blood mononuclear cells
- TNF-α
Tumor necrosis factor alpha
- MCP-1
Monocyte chemoattractant protein1
- IP-10
Interferon gamma-induced protein 10
- CCL22
C-C motif chemokine 22
- CFU
Colony forming unit
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Agak GW, Qin M, Nobe J, et al. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol. 2014;134:366–73. doi: 10.1038/jid.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473:216–20. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Wang X, Wu Z, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. Journal of Clinical Investigation. 1998;102:874. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish D, Davidson D, Hancock R. Antimicrobial Peptides and Human Disease. Springer; 2006. Immunomodulatory properties of defensins and cathelicidins; pp. 27–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Bryan L, Kowand S, Van Den Elzen H. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrobial agents and chemotherapy. 1979;15:7–13. doi: 10.1128/aac.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ. Systematic review of Propionibacterium acnes resistance to systemic antibiotics. The Medical Journal of Australia. 1998;169:259–61. doi: 10.5694/j.1326-5377.1998.tb140250.x. [DOI] [PubMed] [Google Scholar]
- Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiological reviews. 1987;51:341. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BD, Chen LL, Tai PC. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proceedings of the National Academy of Sciences. 1986;83:6164–8. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, et al. The third helix of the Antennapedia homeodomain translocates through biological membranes. Journal of Biological Chemistry. 1994;269:10444–50. [PubMed] [Google Scholar]
- Dréno B, Bettoli V, Ochsendorf F, et al. European recommendations on the use of oral antibiotics for acne. European Journal of Dermatology. 2004;14:391–9. [PubMed] [Google Scholar]
- Dürr UH, Sudheendra U, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Eady E, Gloor M, Leyden J. Propionibacterium acnes resistance: a worldwide problem. Dermatology. 2003;206:54–6. doi: 10.1159/000067822. [DOI] [PubMed] [Google Scholar]
- Fischbach MA. Combination therapies for combating antimicrobial resistance. Current opinion in microbiology. 2011;14:519–23. doi: 10.1016/j.mib.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu B-H, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. Journal of investigative dermatology. 2013 doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourmy D, Recht MI, Blanchard SC, et al. Structure of the Ä Site of Escherichia coli 16S Ribosomal RNA Complexée! with an Aminoglycoside Antibiotic. Science. 1996;274:1367. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- Guo L, Lim KB, Gunn JS, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–3. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature biotechnology. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. Journal of Biological Chemistry. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Hu K, Schmidt NW, Zhu R, et al. A critical evaluation of random copolymer mimesis of homogeneous antimicrobial peptides. Macromolecules. 2013;46:1908–15. doi: 10.1021/ma302577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S. Antibiotic resistance in acne treatment. Skin Therapy Lett. 2012;17:1–3. [PubMed] [Google Scholar]
- Hurdle JG, O’Neill AJ, Chopra I, et al. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nature Reviews Microbiology. 2011;9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, et al. Antennapedia homeobox peptide regulates neural morphogenesis. Proceedings of the National Academy of Sciences. 1991;88:1864–8. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ochoa M-T, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. The Journal of Immunology. 2002;169:1535–41. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnjak T, Peschel A. Bacterial resistance mechanisms against host defense peptides. Cellular and Molecular Life Sciences. 2011;68:2243–54. doi: 10.1007/s00018-011-0716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lai Y, Villaruz AE, et al. Gram-positive three-component antimicrobial peptide-sensing system. Proceedings of the National Academy of Sciences. 2007;104:9469–74. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S, Blanchard JS. Molecular Insights into Aminoglycoside Action and Resistance. Chemical reviews. 2004;105:477–98. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- McDowell A, Nagy I, Magyari M, et al. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One. 2013;8:e70897. doi: 10.1371/journal.pone.0070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInturff JE, Wang S-J, Machleidt T, et al. Granulysin-derived peptides demonstrate antimicrobial and anti-inflammatory effects against Propionibacterium acnes. Journal of investigative dermatology. 2005;125:256–63. doi: 10.1111/j.0022-202X.2005.23805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Lai GH, Schmidt NW, et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proceedings of the National Academy of Sciences. 2011;108:16883–8. doi: 10.1073/pnas.1108795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard-Franchimont C, Goffin V, Arrese JE, et al. Lymecycline and Minocycline in Inflammatory Acne. Skin Pharmacology and Physiology. 2002;15:112–9. doi: 10.1159/000049398. [DOI] [PubMed] [Google Scholar]
- Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes Induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381–8. doi: 10.1038/jid.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld Y, Shai Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2006;1758:1513–22. doi: 10.1016/j.bbamem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Ross J, Snelling A, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. British journal of Dermatology. 2003;148:467–78. doi: 10.1046/j.1365-2133.2003.05067.x. [DOI] [PubMed] [Google Scholar]
- Schmidt NW, Deshayes S, Hawker S, et al. Engineering Persister-Specific Antibiotics with Synergistic Antimicrobial Functions. ACS nano. 2014;8:8786–93. doi: 10.1021/nn502201a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NW, Lis M, Zhao K, et al. Molecular basis for nanoscopic membrane curvature generation from quantum mechanical models and synthetic transporter sequences. J Am Chem Soc. 2012a;134:19207–16. doi: 10.1021/ja308459j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NW, Mishra A, Lai GH, et al. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J Am Chem Soc. 2011;133:6720–7. doi: 10.1021/ja200079a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NW, Tai KP, Kamdar K, et al. Arginine in α-Defensins: Differental Effects on Bactericidal Activity Correspond to Geometry of Membrane Curvature and Peptide-Lipid Phase Behavior. Journal of Biological Chemistry. 2012b;287:21866–72. doi: 10.1074/jbc.M112.358721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NW, Wong GC. Antimicrobial peptides and induced membrane curvature: Geometry, coordination chemistry, and molecular engineering. Current Opinion in Solid State and Materials Science. 2013;17:151–63. doi: 10.1016/j.cossms.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, Gold MR, et al. The Human Antimicrobial Peptide LL-37 Is a Multifunctional Modulator of Innate Immune Responses. The Journal of Immunology. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Scott MG, Vreugdenhil AC, Buurman WA, et al. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. The Journal of Immunology. 2000;164:549–53. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- Taber HW, Mueller J, Miller P, et al. Bacterial uptake of aminoglycoside antibiotics. Microbiological reviews. 1987;51:439. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens Q, Westhof E. Crystal structure of a complex between the aminoglycoside tobramycin and an oligonucleotide containing the ribosomal decoding a site. Chemistry & biology. 2002;9:747–55. doi: 10.1016/s1074-5521(02)00153-9. [DOI] [PubMed] [Google Scholar]
- Vowels B, Yang S, Leyden J. Pro-inflammatory cytokines are inducible by a soluble factor of propionibacterium acnes: evidence of peptidoglycan involvement. Journal of investigative dermatology. 1995a:104. [Google Scholar]
- Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infection and immunity. 1995b;63:3158–65. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–81. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- Wang WLL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to Seventeen Antibiotics. Antimicrobial agents and chemotherapy. 1977;11:171–3. doi: 10.1128/aac.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. The Lancet. 2012;379:361–72. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends in biotechnology. 2013;31:177–84. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Gordon VD, Trinkle DR, et al. Mechanism of a prototypical synthetic membrane-active antimicrobial: Efficient hole-punching via interaction with negative intrinsic curvature lipids. Proceedings of the National Academy of Sciences. 2008;105:20595–600. doi: 10.1073/pnas.0806456105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.