Abstract
Traumatic injury to the knee leads to the development of posttraumatic osteoarthritis. The objective of this study was to characterize the effects of a single intra-articular injection of a non-ionic surfactant, Poloxamer 188 (P188), in preservation of meniscal tissue following trauma through maintenance of meniscal glycosaminoglycan (GAG) content and mechanical properties. Flemish Giant rabbits were subjected to a closed knee joint, traumatic compressive impact with the joint constrained to prevent anterior tibial translation. The contralateral limb served as an un-impacted control. Six animals (treated) received an injection of P188 in phosphate buffered saline (PBS) post trauma, and another six animals (sham) received a single injection of PBS to the impacted limb. Histological analyses for GAG was determined 6 weeks post trauma, and functional outcomes were assessed using stress relaxation micro-indentation. The impacted limbs of the sham group demonstrated a significant decrease in meniscal GAG coverage compared to non-impacted limbs (p < 0.05). GAG coverage of the impacted P188 treated limbs was not significantly different than contralateral non-impacted limbs in all regions except the medial anterior (p < 0.05). No significant changes were documented in mechanics for either the sham or treated groups compared to their respective control limbs. This suggests that a single intra-articular injection of P188 shows promise in prevention of trauma induced GAG loss.
Keywords: Knee, Glycosaminoglycan
Introduction
Menisci are critical fibrocartilaginous structures that dissipate forces between the femoral condyles and the tibial plateau in the tibiofemoral joint43. Meniscal structure is composed of fibrochondrocytes, extracellular matrix (ECM), and fluid components. The ECM structure is mainly composed of collagen and proteoglycans, which dictate the biomechanical properties through matrix-fluid interactions2. Meniscal integrity is largely dependent on the proper maintenance and remodeling of this tissue matrix which is primarily regulated by fibrochondrocytes1.
Acute injury to a joint can induce post-traumatic osteoarthritis (PTOA), which has been attributed to 12% of the 21 million cases of OA each year in the U.S.5 and is often caused by meniscal damage followed by a subsequent degradation of the structure 12,14,35,36. Importantly, 15% of knee injuries in sport involve the menisci, indicating the importance of exploring new treatment modalities in an attempt to prevent or delay the development of PTOA 26. Current clinical treatment for meniscal injury focuses on repairing macroscopic acute tissue damage, but does not treat for aberrant cellular activity that may be crucial to development of PTOA. Interventions that mitigate this chronic disease in the joint may best be administered early before significant changes occur in the functional properties of the menisci.
Previous studies have shown that severe traumatic impact to the knee may lead to meniscal damage including gross tears, the loss of fibrochondrocytes and a decrease in glycosaminoglycan (GAG) content 14,19,20. In vitro studies of impact loading to meniscal explants have even shown cell death and the release of GAGs from the tissue following impact without the observation of gross damage to the tissue, such as fissuring, cracking, or other sudden failure of the ECM 17,22. Damage to the menisci alters the homeostasis of the entire knee joint by allowing more force to be transmitted to the underlying articular cartilage, which may lead to cartilage degradation and development of osteophytes 8,18,29,41. The effects of trauma are compounded by the mainly avascular nature of menisci which limits the ability of the tissue to regenerate. Therefore, preventing cell death via necrosis or apoptosis has a twofold effect on the tissue: it preserves the limited supply of fibrochondrocytes in the meniscus and it may prevent the release of matrix degenerating compounds upon cell death. Therapeutic strategies that preserve both fibrochondrocytes and the tissue matrix may hold promise in the prevention or limitation of PTOA.
A non-ionic surfactant, P188, has shown promise in preventing cell death in articular cartilage and neurons of the brain by inserting into cell membranes of damaged cells and restoring their integrity19,23,31,34,38. P188 is an 8400 Da tri-block polymer with a poly(oxyethylene) – poly(oxypropylene) – poly(oxyethylene) structure. This structure imparts hydrophilic and hydrophobic qualities which allow P188 to interact with and seal plasma membranes. Normal cell membranes have a surface pressure of 30 mN/m, while damaged cells have surface pressures of 20–26 mN/m27. P188 self-inserts at a surface pressure of 22 mN/m, and thus will not insert into normal cell membranes. Previous studies have investigated the effects of P188 on impacted articular cartilage explants 34 and on articular cartilage following constrained blunt tibiofemoral impact 19. Both investigations report increased cell viability at time points immediately following trauma, and Isaac et al. report increased viability of P188 treated cells after 6 weeks 19,34.
The objective of the current study was to characterize the effects of a single intra-articular injection of P188 on the preservation of meniscal tissue following blunt trauma. The study tests the hypothesis that a single intra-articular, post traumatic injection of P188 will help maintain the GAG content and mechanics of meniscal tissue following a constrained, blunt tibiofemoral impact.
Materials and Methods
Twelve skeletally mature Flemish Giant rabbits (5.6 ± 0.2 kg) were used for this study. The experiment was approved by an All University Committee on Animal Use and Care. The tibiofemoral joint of left limbs was subjected to a constrained impact, as detailed by Isaac et al., so as to prevent ACL and meniscal damages by limiting tibial translation 19. In brief, animals were anesthetized using 2% isoflurane and oxygen. The closed-joint tibiofemoral impact, with the limb positioned at 90° flexion, was performed with a gravity accelerated mass of 1.75 kg attached to a pre-crushed, deformable impact interface (Hexcel, 3.76 MPa crush strength). A load transducer (Model AL311CV, 1000lb capacity, Sensotec, Columbus, Ohio USA,) fixed to the impact interface, was used to measure peak load, time to peak, and duration of contact. The impact sled was arrested electronically after impact to ensure a single insult to the joint. The right limb served as a paired, non-impacted control.
The animals were divided into two groups; treated (n=6) and sham (n=6). Immediately after impact, treated animals received a single 1.5mL injection of 8 mg/mL P188 in sterile phosphate buffered saline (PBS) into the left tibiofemoral (TF) joint (impacted treated) 3,34,38. Right limbs of the treated animals, “non-impacted treated”, received a single 1.5 mL injection of sterile PBS. Sham animals received a single 1.5 mL injection of sterile PBS into TF joints in both impacted and non-impacted limbs. The impacted limb of sham animals were referred to as “impacted sham” and the non-impacted contralateral limb of these animals were referred to as “non-impacted sham”. Limbs were flexed several times following injection to distribute the solution. Rabbits were allowed free range in cages for duration of the study, and were euthanized 6 weeks post impact. Immediately following euthanasia, left and right limb menisci were removed from the joints. Previous studies indicate mechanical and histologic regional variations in the meniscus 6,21,42. Therefore, menisci were accordingly sectioned into anterior and posterior regions for analysis.
Gross Morphological Assessment
Morphological damage scoring was performed by three blinded graders. A grading system was developed based on previous methodologies 28,33. Anterior and posterior regions of all specimens were graded as follows; 0 = normal, 1= surface damage, 2 = un-displaced tears, 3 = displaced tears, and 4 = tissue maceration. The average of the three individual scores (rounded to nearest whole number) for each category and each specimen were used for all data presentation.
Micro-Indentation
Relaxation indentation testing (Mini Bionix 858 MTS Corp, Eden Praire, MN) was performed on anterior and posterior regions in a limited number of specimens, n = 4 each group, with exception of lateral posterior and medial hemijoint of the impacted treated group (n =3) (due to uncontrollable circumstances i.e. material test system malfunctioning). Specimens were mounted using cyanoacrylate in a PBS bath attached to an x-y translation base and two degree of freedom camera mount to allow orientation of the specimen such that the surface of indentation was normal to the indenter, similar to previous studies24,42. An indentation depth of 0.25 mm was chosen, well below the radius of the 1.59 mm diameter spherical indenter but sufficient to penetrate the superficial layer of the meniscus, which has been reported to be 100–200 µm thick 30. Load data was acquired using an 8.9 N load cell (Futek LSB200, Irvine, California). Displacement control was used to preload each specimen to 20mN, indent 0.25 mm, and maintain the displacement for 900 seconds which allowed the tissue to reach equilibrium. Hertzian contact was assumed. A MATLAB (Mathworks, Natick, MA) routine, based on a Hertzian contact equation, was used to extrapolate instantaneous and equilibrium moduli. The Hertzian contact formula is based on the theory of linear elasticity. It has been shown under small deformations that the theory can be applied to biological soft tissues25. The authors assessed the linear fit of the model during meniscal loading. The indenter moduli and Poisson’s ratio were assumed to be 210 GPa and 0.3 respectively, and Poisson’s ratio of the meniscus was taken to be 0.01 based on a regional average from a previous study42.
Histology
Meniscal sections were fixed in 10% formalin. Menisci were then immersed in 30% sucrose solution for up to 72 hours for cryoprotection. Sections were then embedded in Optimal Cutting Temperature Compound (OCT) (Pelco, Redding, CA) and flash frozen using liquid nitrogen. The meniscal cross-sections were then cryosectioned (6µm thick) and stained for GAG coverage using Hematoxlyin, Safranin-O (Saf-O), and Fast Green (FG) staining. Slides were imaged using an Olympus BH2 Microscope (Olympus, Center Valley, PA) and MicroPublisher 5.0 RTV camera (Qimaging, Surrey,BC, Canada). Meniscal GAG coverage was analyzed semi-quantitatively using Image J (NIH, Bethesda, MD). Briefly, images were trimmed and converted to 8 bit images, allowing a threshold to be applied. Total area of the meniscal section was calculated using the ‘Analyze Particles’ tool and summing particles. To separate out the red associated with GAG coverage, the original color images were color deconvoluted using the ‘ColourDeconvolution’ tool37. Area of GAG coverage was calculated by thresholding the meniscal section of the deconvoluted image corresponding to the color channel selected for GAG and analyzing and summing particles. A percent area metric was calculated and used in all statistical analyses11.
Statistics
Paired Student t-tests were performed on the impact data (peak load, time to peak load and contact duration) to investigate any potential differences between the treated and sham groups of data. Two-factor (hemijoint and region) repeated measures analysis of variance (ANOVA) with post hoc Tukey tests using Minitab software (Minitab15, State College, PA) was used to compare GAG coverage, instantaneous and equilibrium moduli between the impacted and contralateral unimpacted limbs for each treatment group. A one factor nested ANOVA was used to compare the impacted limbs of the treated and sham groups, and the control limbs of the treated and sham groups for GAG coverage, instantaneous and equilibrium moduli. Significance was taken to be p < 0.05 for all metrics.
Results
Impaction
Impact parameters, peak impact force, time to peak force and contact duration, for the impacted, treated group were 1046 ± 197 N, 6.6 ± 1.7 ms and 19.1 ± 5.3 ms, respectively, which were not significantly different from the impacted sham group values of 1026 ± 92 N, 6.5 ± 1.1ms and 19.7 ± 6.0 ms.
Gross Morphological Assessment
Morphological assessment outcomes are shown in Table 1. Gross morphological assessments showed no high grade (≥ grade 2) damage to non-impacted treated or non-impacted sham menisci. Grade 3 damage was observed in two medial impacted sham menisci in both anterior and posterior regions and in one lateral impacted treated meniscus in both anterior and posterior regions. High grade damage (≥ grade 3) was present in three lateral impacted sham menisci and occurred in both anterior and posterior regions of the same meniscus.
Table 1.
Morphological meniscus damage grading
| Meniscal Grade Score |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| P188 | Medial | A | 6/6 | - | - | - | - | |
| Control | P | 6/6 | - | - | - | - | ||
| A | 6/6 | - | - | - | - | |||
| Impacted | P | 6/6 | - | - | - | - | ||
| Lateral | A | 6/6 | - | - | - | - | ||
| Control | P | 5/6 | 1/6 | - | - | - | ||
| A | 5/6 | - | - | 1/6 | - | |||
| Impacted | P | 4/6 | 1/6 | - | 1/6 | - | ||
| Sham | Medial | A | 6/6 | - | - | - | - | |
| Control | P | 6/6 | - | - | - | - | ||
| A | 4/6 | - | - | 2/6 | - | |||
| Impacted | P | 4/6 | - | - | 2/6 | - | ||
| Lateral | A | 6/6 | - | - | - | - | ||
| Control | P | 6/6 | - | - | - | - | ||
| A | 3/6 | 2/6 | - | - | 1/6 | |||
| Impacted | P | 4/6 | - | - | 1/6 | 1/6 | ||
Micro-Indentation
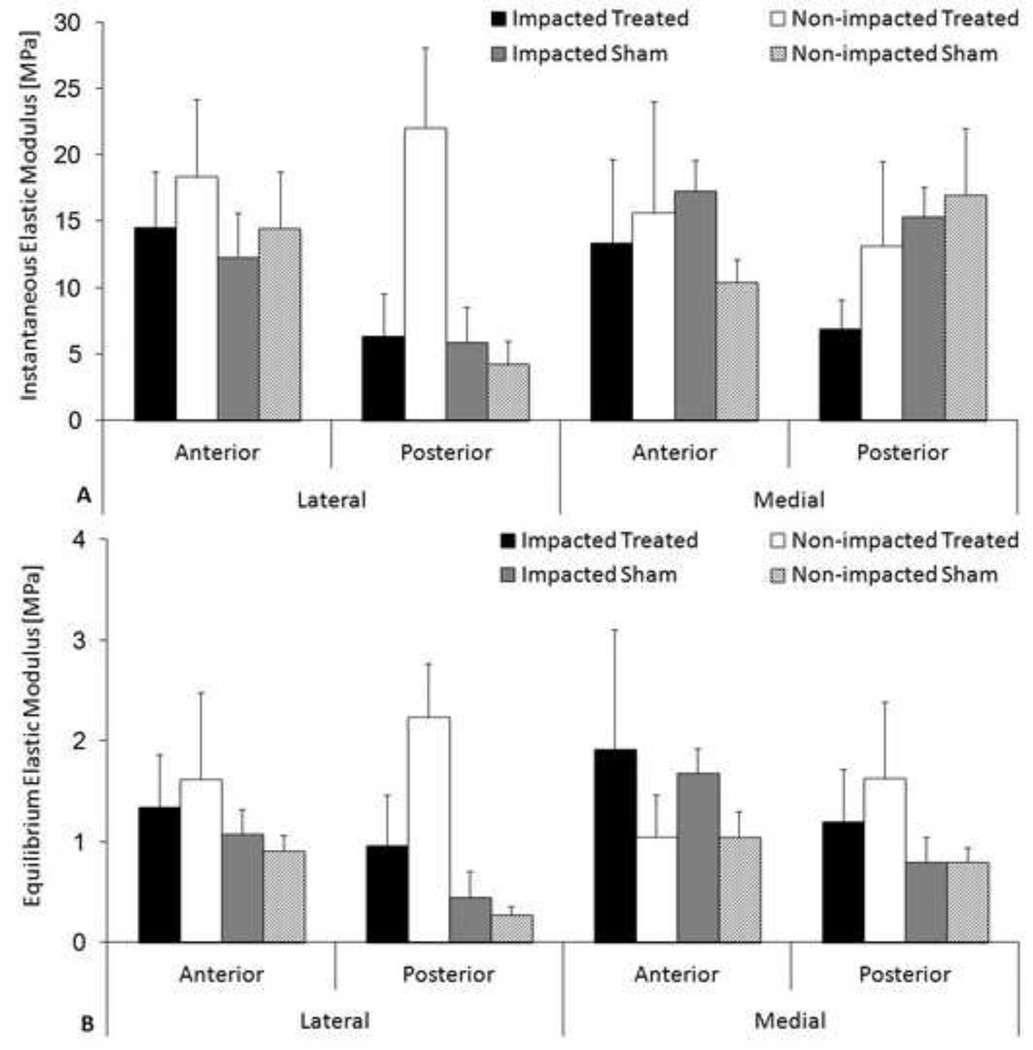
The Hertzian contact equation fit the data well resulting in an average R2 value of 0.97 ± 0.02. No significant differences were documented in instantaneous or equilibrium moduli for either impacted treated or impacted sham groups compared to their respective non-impacted controls (Figure 1). The highest equilibrium and instantaneous moduli were found in the lateral posterior horn for the non-impacted treated group.
Figure 1.
Micro-indentation results. A) Instantaneous elastic modulus B) Equilibrium elastic modulus. Mean + standard error of the mean. ANOVA revealed no statistically significant differences between contralateral controls limbs and either the impacted sham or impacted treated limbs.
GAG coverage
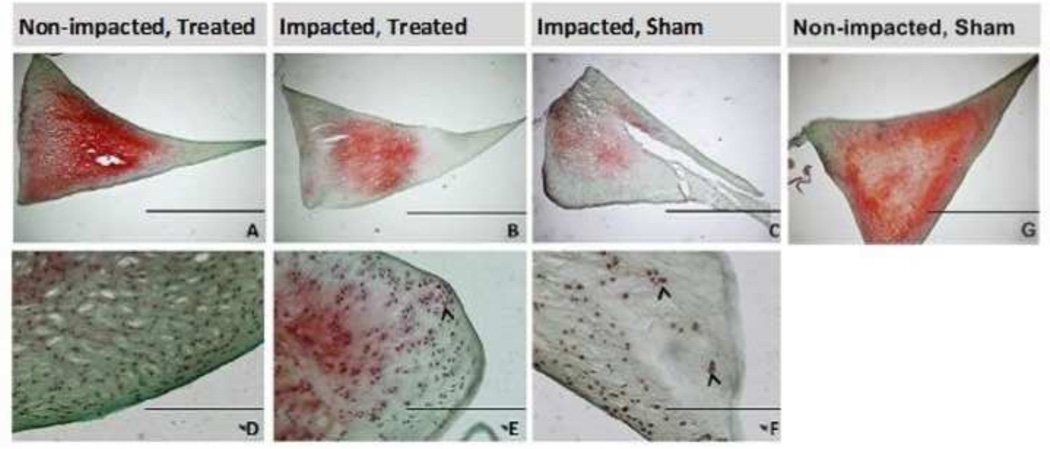
Histologically, the impacted sham group (Figure 2C, 2F) demonstrated a marked decrease in GAG coverage compared to the paired non-impacted shams. In contrast, the impacted treated (Figure 2B, 2E) and non-impacted treated (Figure 2A, 2D) menisci demonstrated less difference in GAG coverage. Localized cell clustering was also observed in the impacted groups for both sham and treated animals (Figure 2E, 2F), often along the surface and at sites of gross damage.
Figure 2.
Morphological comparisons of sections from control (A,D), impacted treated (B,E), impacted sham (C,F), and Non-impacted, Sham (G) lateral posterior menisci. Scale bar = 2mm (A,B,C) and 200µm (D,E,F). Arrowheads highlight cell clustering. Radial tear in (C) is damage from impact.
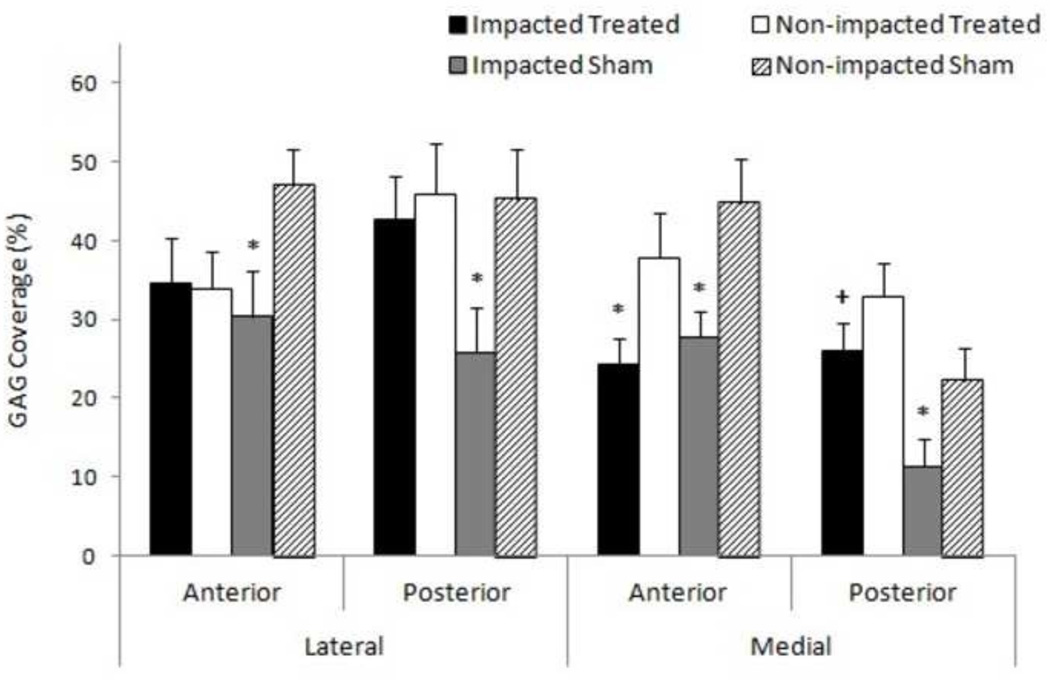
All impacted sham regions (anterior and posterior) in both lateral and medial hemijoints showed significantly lower (p < 0.05) percent GAG coverage than their respective paired controls (Figure 3). A decrease in percent GAG coverage was observed between the impacted treated group compared to non-impacted treated only in the medial anterior region (p < 0.05). There were no significant differences between non-impacted treated and non-impacted sham groups. However, percent GAG coverage was significantly higher in the medial posterior region of the impacted treated group compared to the same region of the impacted sham group (p < 0.05). Overall, GAG coverage decreased less in the impacted treated group than the impacted sham group compared to respective non-impacted controls.
Figure 3.
Comparison of meniscal GAG coverage 6 weeks post impact. * denotes significant difference between respective impact group and paired control (p<0.05). + represents statistical difference between impacted treated and impacted sham groups of same region on respective hemijoint, (p<0.05). Mean + standard error of the mean.
Discussion
To the best of our knowledge, this is the first report investigating P188 efficacy in the meniscus using a traumatic impaction model. GAG was targeted as an indicator of matrix integrity in this study as it is largely responsible for the uptake, retention, and dissipation of the fluid component in menisci and therefore essential to proper biomechanical function. In this study a blunt, constrained tibiofemoral impact caused significant loss of GAG six weeks post trauma in all impacted sham regions compared to non-impacted sham paired controls. A single intra-articular injection of P188 prevented significant GAG loss post trauma in all regions, except the medial anterior.
It is proposed that P188 prevents GAG loss via preservation of injured fibrochondrocytes by sealing damaged cell membranes and preventing necrosis and apoptosis, based on previous studies using P188 in articular cartilage 19,34,38,39. Fibrochondrocytes are crucial to proper meniscal function as they produce matrix constituents which are responsible for maintaining mechanical integrity of menisci. Mechanical insult has been shown to cause the release of catabolic mediators that contribute to tissue matrix breakdown and the induction of cellular apoptosis13,17,32. By sealing the membranes of fibrochondrocytes which have entered the aforementioned pathway, P188 may inhibit the continued release of these mediators and curb additional GAG and matrix degeneration. It is unclear though whether these ‘saved’ cells continue normal GAG production, halt production of matrix degenerating enzymes, or both. A previous study on acutely injured cartilage has indicated that P188 acts not only on the cell membrane, but also through inhibition of p38 and Interleukin-6 signaling4.
This study showed a significant decrease in overall GAG content (anterior and posterior regions of both medial and lateral menisci) in the impacted sham group, suggesting that the impact in this model was able to induce molecular degradation in the menisci in addition to the observed macroscopic damage. The lateral posterior region of the impacted sham group was also one of the most frequent sites of meniscal tearing in this constrained animal model, and was the location of the highest grade damage. Clinical studies often indicate significant damage to the lateral meniscus following impact landings7,9,40.
The observed cell clustering phenomena has been related to the disruption of the tissue matrix15,16. Le Graverand, et al. proposed that post traumatic matrix degeneration disrupts the network of cellular interactions, causing an isolation of fibrochondrocytes, which when separated from their normal signaling environments undergo a change in phenotype, resulting in proliferation of these aberrant cells to a localized area15. These clusters are also an origin of calcification in damaged menisci 15 which is detrimental to meniscal function and observed in many OA patients 41. The observation of these clusters in both the impacted sham and impacted treated groups suggests that while P188 maintains the GAG content through fibrochondrocyte interaction, some matrix breakdown likely occurred in the treated group. Direct mechanical damage to the matrix may have caused a reduction of the GAG that would not be mitigated by acute pharmacological intervention. Taken together, these findings suggest that additional intervention may be necessary to fully counteract any potential enzymatic degradation of the matrix, such as administration of IL-1ra (Interleukin 1 receptor antagonist) or an aggrecanase inhibitor.
A limitation of the current study was sample size available for determination of the mechanical properties. Despite the decrease in GAG in the impacted sham group, no significant changes were noted in material properties. It was likely that the large statistical variations observed in the lateral posterior and both regions of the medial hemijoint of the treated group are attributable to the low sample number (n=3) for those groups. While there were definitive decreases in GAG in the impacted sham group, the decreases observed may not have been sufficient enough to cause mechanical changes. Tissue swelling, indicative of matrix disruption, allowing greater fluid uptake by the tissue and less resistance to fluid dispersion under applied load, was also not observed. This study utilized the Hertzian contact solution to determine both the instantaneous and equilibrium modulus, when there is likely little to no fluid flow and hence for small deformation the linear elastic theory is applicable. The strong relation between load and deformation validates the use of Hertzian contact to model this data.
Intervention with P188 has shown promise in being able to prevent a loss of meniscal GAG, presumably by the repair of damaged fibrochondrocytes from blunt impact, but the viability of these cells is currently uncharacterized, and is a limitation of the current study. Future investigations should be conducted to understand the implications of these ‘saved’ cells on the surrounding matrix and adjacent cells. Additionally, future investigations are needed to characterize the mode, rate of absorption, and depth of P188 penetration into menisci and surrounding tissues. Another limitation of this study was administration of a single dose of P188 immediately post impact. While the single dosage volume was previously optimized10,38, optimizing dosage frequency may improve results and should be considered in future studies.
Overall, this study has shown that P188 can prevent a significant loss of GAG in menisci following traumatic impaction. Clinical treatment of meniscal damage following injury is limited to macroscopic methodologies often including debridement, suturing, and partial meniscectomy. Current treatment does not address the biomolecular responses and events following injury which help drive the progression of PTOA. Pharmaceutical intervention to address the molecular pathways of meniscal tissue degradation following trauma may provide a new avenue for curbing or slowing the progression of PTOA both within the meniscus and surrounding joint tissues.
Acknowledgments
Acknowledgement of funding:
This work was supported by grants from the National Institutes of Health (R21 AR060464, F31AG039975).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams ME, Hukins DW. The extracellular matrix of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee Meniscus;Basic and Clinical Foundations. New York: Raven Press; 1992. [Google Scholar]
- 2.Arnoczky SP, Adams ME, Mow VC, DeHaven KE, Eyre D. The meniscus. In: Buckwalter JA, Woo SL, editors. The injury and repair of musculoskeletal soft tissue. Park Ridge, IL: Am. Acad. Orthop. Surg.; 1988. pp. 487–537. [Google Scholar]
- 3.Baars DC, Rundell SA, Haut RC. Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech Model Mechanobiol. 2006;5:133–139. doi: 10.1007/s10237-006-0024-3. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj S, Shoemaker T, Hakimiyan AA, Rappoport L, Pascual-Garrido C, Oegema TR, Wimmer MA, Chubinskaya S. Protective effect of P188 in the model of acute trauma to human ankle cartilage: the mechanism of action. J Orthop Trauma. 2010;24:571–576. doi: 10.1097/BOT.0b013e3181ec4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma. 20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 6.Chia HN, Hull ML. Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J Orthop Res. 2008;26:951–956. doi: 10.1002/jor.20573. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla M, Scala A, Gianni E, Puddu G. Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee Surg Sport. Traumatol Arthrosc. 1995;3:130–134. doi: 10.1007/BF01565470. [DOI] [PubMed] [Google Scholar]
- 8.Crema MD, Guermazi A, Li L, Nogueira-Barbosa MH, Marra MD, Roemer FW, Eckstein F, Le Graverand MPH, Wyman BT, Hunter DJ. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–43. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Duncan JB, Hunter R, Purnell M, Freeman J. Meniscal injuries associated with acute anterior cruciate ligament tears in alpine skiers. Am J Sport. Med. 1995;23:170–172. doi: 10.1177/036354659502300208. [DOI] [PubMed] [Google Scholar]
- 10.Ewers BJ, Haut RC. Polysulphated glycosaminoglycan treatments can mitigate decreases in stiffness of articular cartilage in a traumatized animal joint. J. Orthop. Res. 2000;18:756–761. doi: 10.1002/jor.1100180512. [DOI] [PubMed] [Google Scholar]
- 11.Fischenich KM, Coatney GA, Haverkamp JH, Button KD, DeCamp C, Haut RC, Haut Donahue TL. Evaluation of meniscal mechanics and proteoglycan content in a modified anterior cruciate ligament transection model. J. Biomech. Eng. 2014;136 doi: 10.1115/1.4027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda Y, Takai S, Yoshino N, Murase K, Tsutsumi S, Ikeuchi K, Hirasawa Y. Impact load transmission of the knee joint-influence of leg alignment and the role of meniscus and articular cartilage. Clin Biomech (Bristol, Avon) 2000;15:516–521. doi: 10.1016/s0268-0033(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 13.Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-lbeta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J. Biol. Chem. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis Rheum. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Hellio Le Graverand MP, Sciore P, Eggerer J, Rattner JP, Vignon E, Barclay L, Hart DA, Rattner JB. Formation and phenotype of cell clusters in osteoarthritic meniscus. Arthritis Rheum. 2001;44:1808–1818. doi: 10.1002/1529-0131(200108)44:8<1808::AID-ART318>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Hellio Le Graverand MP, Vignon E, Otterness IG, Hart DA. Early changes in lapine menisci during osteoarthritis development: Part I: cellular and matrix alterations. Osteoarthr. Cartil. 2001;9:56–64. doi: 10.1053/joca.2000.0350. [DOI] [PubMed] [Google Scholar]
- 17.Hufeland M, Schiinke M, Grodzinsky AJ, Imgenberg J, Kurz B. Response of mature meniscal tissue to a single injurious compression and interleukin-1 in vitro. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, Guermazi A, Grigorian M, Gale D, Felson DT. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 19.Isaac DI, Golenberg N, Haut RC. Acute repair of chondrocytes in the rabbit tibiofemoral joint following blunt impact using P188 surfactant and a preliminary investigation of its long-term efficacy. J Orthop Res. 2010;28:553–558. doi: 10.1002/jor.21022. [DOI] [PubMed] [Google Scholar]
- 20.Killian ML, Isaac DI, Haut RC, Dejardin LM, Leetun D, Haut Donahue TL. Traumatic Anterior Cruciate Ligament Tear and its Implications on Meniscal Degradation: A Preliminary Novel Lapine Osteoarthritis Model. J. Surg. Res. 2010;164:234–241. doi: 10.1016/j.jss.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Killian ML, Lepinski NM, Haut RC, Haut Donahue TL. Regional and zonal histo-morphological characteristics of the lapine menisci. Anat. Rec. (Hoboken) 2010;293:1991–2000. doi: 10.1002/ar.21296. [DOI] [PubMed] [Google Scholar]
- 22.Kisiday JD, Vanderploeg EJ, Mcllwraith CW, Grodzinsky AJ, Frisbie DD. Mechanical injury of explants from the articulating surface of the inner meniscus. Arch Biochem Biophys. 2010;494:138–144. doi: 10.1016/j.abb.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Lee RC, Hannig J, Matthews KL, Myerov A, Chen CT. Pharmaceutical therapies for sealing of permeabilized cell membranes in electrical injuries. Ann N YAcad Sci. 1999;888:266–273. doi: 10.1111/j.1749-6632.1999.tb07961.x. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J. Bone Joint Surg. Am. 2006;88:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 25.Lin DC, Shreiber DI, Dimitriadis EK, Horkay F. Spherical indentation of soft matter beyond the Hertzian regime: numerical and experimental validation of hyperelastic models. Biomech. Model. Mechanobiol. 2009;8:345–358. doi: 10.1007/s10237-008-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Maskarinec SA, Hannig J, Lee RC, Lee KYC. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys. J. 2002;82:1453–1459. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matyas JR, Atley L, lonescu M, Eyre DR, Poole AR. Analysis of cartilage biomarkers in the early phases of canine experimental osteoarthritis. Arthritis Rheum. 2004;50:543–552. doi: 10.1002/art.20027. [DOI] [PubMed] [Google Scholar]
- 29.McCann L, Ingham E, Jin Z, Fisher J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthr. Cartil. 2009;17:995–1000. doi: 10.1016/j.joca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Moyer JT, Abraham AC, Haut Donahue TL. Nanoindentation of Human Meniscal Surfaces. J. Biomech. 2012;45:2230–2235. doi: 10.1016/j.jbiomech.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natoli RM, Athanasiou KA. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J Biomech Eng. 2008;130:41012. doi: 10.1115/1.2939368. [DOI] [PubMed] [Google Scholar]
- 32.Ollivierre F, Gubler U, Towle CA, Laurencin C, Treadwell BV. Expression of IL-1 genes in human and bovine chondrocytes: a mechanism for autocrine control of cartilage matrix degradation. Biochem Biophys Res Commun. 1986;141:904–911. doi: 10.1016/s0006-291x(86)80128-0. [DOI] [PubMed] [Google Scholar]
- 33.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, Lotz MK, D'Lima DD. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011;19:1132–1141. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips DM, Haut RC. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J Orthop Res. 2004;22:1135–1142. doi: 10.1016/j.orthres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Robertson CM, Pennock AT, Harwood FL, Pomerleau AC, Allen RT, Amiel D. Characterization of pro-apoptotic and matrix-degradative gene expression following induction of osteoarthritis in mature and aged rabbits. Osteoarthr. Cartil. 2006;14:471–476. doi: 10.1016/j.joca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 38.Rundell SA, Baars DC, Phillips DM, Haut RC. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop. 2005;Res 23:1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 39.Serbest G, Horwitz J, Jost M, Barbee K. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. Faseb J. 2006;20:308–310. doi: 10.1096/fj.05-4024fje. [DOI] [PubMed] [Google Scholar]
- 40.Spindler KP, Schils JP, Bergfeld JA, Andrish JT, Weiker GG, Anderson TE, Piraino DW, Richmond BJ, Medendorp SV. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sport. Med. 1993;21:551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, Jr, Gruber HE. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord. 2010;11:19. doi: 10.1186/1471-2474-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweigart MA, Zhu CF, Burt DM, DeHoll PD, Agrawal CM, Clanton TO, Athanasiou KA. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng. 2004;32:1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- 43.Welsh RP. Knee joint structure and function. Clin Orthop Relat Res. 1980:7–14. [PubMed] [Google Scholar]