Abstract
The storage of sperm in mated females is important for efficient reproduction. After sperm are transferred to females during mating, they need to reach and enter into the site(s) of storage, be maintained viably within storage, and ultimately be released from storage to fertilize eggs. Perturbation of these events can have drastic consequences on fertility. In Drosophila melanogaster, females store sperm for up to two weeks after a single mating. For sperm to be released normally from storage, Drosophila females need to receive the seminal fluid protein (SFP) sex peptide (SP) during mating. SP, which binds to sperm in storage, signals through the sex peptide receptor (SPR) to elicit two other effects on mated females: the persistence of egg laying and a reduction in sexual receptivity. However, it is not known whether SPR is also needed to mediate SP’s effect on sperm release. By phenotypic analysis of flies deleted for SPR, and of flies knocked down for SPR, ubiquitously or in specific tissues, we show that SPR is required to mediate SP’s effects on sperm release from storage. We show that SPR expression in ppk+ neurons is needed for proper sperm release; these neurons include those that mediate SP’s effect on receptivity and egg laying. However, we find that SPR is also needed in the spermathecal secretory cells of the female reproductive tract for efficient sperm release. Thus, SPR expression is necessary in both the nervous system and in female reproductive tract cells to mediate the release of stored sperm.
Keywords: sex peptide, sex peptide receptor, sperm storage, spermathecal secretory cells
1. Introduction
A common misconception is that reproduction is a process involving only sperm and eggs. In reality, numerous molecules—both male and female-derived—are involved before and after fertilization to ensure successful reproduction. A major reproductive process requiring both male- and female-derived components is the storage of sperm by mated females. Female sperm storage is an integral step in the fertility of a number of animals, from insects to mammals (Orr and Zuk, 2012). Male-derived seminal fluid proteins (SFPs) are transferred to females with sperm during mating and are important for sperm storage to occur normally (Avila and Wolfner, 2009; Dean, 2013; Gwathmey et al., 2006; Rogers et al., 2009). In addition to their roles in sperm storage, SFPs also contribute to a variety of reproductive processes in mated females. For example, SFPs induce a series of behavioral and physiological changes in mated female insects that include increasing ovulation and egg laying rates and reducing sexual receptivity (reviewed in Avila et al., 2011; Gillott, 2003; Poiani, 2006). In Drosophila, these postmating changes are collectively referred to as the female post-mating response.
Drosophila melanogaster females store sperm in two types of specialized organs—the seminal receptacle and the spermathecae. SFPs have been identified that regulate several aspects of sperm storage in this species. For example, SFPs are required for sperm to enter into storage (Avila and Wolfner, 2009; Bloch Qazi and Wolfner, 2003; Neubaum and Wolfner, 1999), for sperm maintenance within storage (Wong et al., 2008), and for sperm to be properly released from storage (Avila et al., 2010; Ravi Ram and Wolfner, 2007, 2009). Storage of sperm by mated females also has another significant effect in this species: sperm storage is required for the persistence of female post-mating responses long-term (e.g. reducing sexual receptivity and prolonged egg laying; Liu and Kubli, 2003; Peng et al., 2005). The requirement of sperm storage to prolong the female post-mating response is due to the association of sperm with the SFP sex peptide (SP; Peng et al., 2005). SP is transferred to females, and is detectable in the female’s hemolymph (short term) (Pilpel et al., 2008) and in her reproductive tract (Peng et al., 2005). Within the female’s reproductive tract SP is bound to the tails of sperm, providing females with a long-term source of this SFP (Peng et al., 2005). In storage, the active region of sperm-bound SP is gradually cleaved from sperm, presumably freeing it to induce its long-term effects of the post-mating response (Peng et al., 2005).
SP is required for the release of sperm from storage; females that do not receive SP during mating fail to release sperm efficiently (Avila et al., 2010). SP also triggers a number of additional post-mating responses in D. melanogaster females (Gioti et al., 2012). Receiving SP reduces female receptivity and sustains long-term egg laying (Chapman et al., 2003; Liu and Kubli, 2003), increases female feeding (Carvalho et al., 2006), decreases the rate of intestinal transit (Apger-McGlaughon and Wolfner, 2013; Cognigni et al., 2011) and decreases ‘siesta’ sleep (Isaac et al., 2010).
How SP effects sperm release from storage is not known. In contrast, SP’s effects on increased egg laying and reduced receptivity have been shown to require the sex peptide receptor (SPR), a G-protein coupled receptor (Yapici et al., 2008) that is highly expressed in the nervous system and in tissues of the female reproductive tract (Prokupek et al., 2010; Prokupek et al., 2009; Yapici et al., 2008). Knockdown of SPR solely in the nervous system results in a failure to mediate SP’s effects on egg laying and receptivity (Yapici et al., 2008). Within the nervous system, SPR is only required in a few female reproductive tract neurons that co-express the markers fruitless, pickpocket, and doublesex in order to induce these post-mating responses (Hasemeyer et al., 2009; Rezaval et al., 2012; Yang et al., 2009). However, the involvement of SPR in mediating SP’s effects on the release of sperm from storage, and where it does so, is unknown.
Here, we report that SPR mediates SP’s effect on sperm release from storage. Using a deletion of the SPR locus, as well as ubiquitous and localized RNAi to knock down SPR expression, we found that SPR is required for efficient release of sperm from storage. SPR null females and global knockdown of SPR resulted in the retention of sperm in storage at 4d post-mating, similar to what is seen in females that do not receive SP during mating (Avila et al., 2010). Additionally, removal of SPR from the nervous system or from the spermathecal secretory cells each affected sperm release independently. Our results suggest that SP signals though SPR to prompt sperm release from storage and that SPR expression in ppk+ neurons and the spermathecal secretory cells is important to mediate this effect.
2. Materials and Methods
2.1. Flies
The Df(1)Exel6234/FM7 line (Parks et al., 2004) was used to collect homozygous Df(1)Exel6234 (“SPR;”) females and their heterozygous control sibs (Bloomington Drosophila Stock Center (BDSC) #7708). The UAS/GAL4 system (Brand and Perrimon, 1993) was used to drive RNAi-mediated knockdown of SPR. UAS-SPR dsRNA/CyO (Vienna Drosophila RNAi Center (VDRC) #106804) and, where noted, UAS-antares dsRNA flies (VDRC #100513; (Findlay et al., 2014) were crossed to the specified driver strain to generate knockdown females. tubulin-GAL4/TM3 (BDSC #5138) drives ubiquitous expression; elav-GAL4 (BDSC #8760) drives expression in the nervous system (Soller and White, 2004); ppk-GAL4 (kind gift of Drs. Lily and Yuh Nung Yan, University of California San Francisco) drives expression in a subset of reproductive tract neurons that mediate SP’s effect on receptivity and egg laying (Hasemeyer et al., 2009; Yang et al., 2009); send1-GAL4 (kind gift of Dr. Mark Siegal, New York University) drives expression in the spermathecal secretory cells (Schnakenberg et al., 2011); OAMBGAL4 (kind gift of Dr. Kyung-An Han, University of Texas El Paso) drives expression in the common oviduct (Lee et al., 2003). We recombined the send1-GAL4 and OAMBGAL4 drivers onto the same chromosome (“send1, OAMB-GAL4”). Control flies were generated by crossing each driver to the attP2 line (VDRC #60100). Knockdown and control females were mated to wild-type, Canton S males. Flies were raised at 23°C on standard yeast-glucose media and a 12:12 LD cycle and aged 3–5 days before use in each experiment.
2.2. Sperm Counts
Virgin experimental and control females were individually mated to males. Matings were observed, and males were removed after mating ended. Mated females were flash frozen in liquid nitrogen at 2hrs, 4d, and, where noted, 10d after mating. Sperm were stained as in Bloch Qazi and Hogdal (2010). In brief, reproductive tracts were dissected in 50% acetic acid and stained with 2% orcein in 50% acetic acid. Sperm in the seminal receptacle were counted under 1000x magnification using a Zeiss 47 30 11-9901 stereo microscope. To avoid bias, we blind-coded samples before counting sperm. We had a repeatability of >93% for each experiment, based on duplicate counts of a subset of samples. Counts were analyzed using Wilcoxon tests in JMP software (JMP 9.02).
2.3. RT-PCR
Where possible, we verified knockdown of SPR. We extracted RNA from ~20 whole flies (to test tub-GAL4) or dissected ~25 lower reproductive tracts (send1, OAMBGAL4), lower reproductive tracts minus the spermathecae (OAMB-GAL4) or lower reproductive tracts minus the common oviduct (send1-GAL4) from experimental and the control groups using TRIzol® reagent (Invitrogen). Isolated RNA was treated with RQ1 DNase (Promega) to degrade any possible contaminating DNA in the samples. cDNA from 1ug of RNA was synthesized using the SMARTScribe Reverse Transcriptase (Clontech). PCR was run for 35 cycles using SPR primers (SPR-5’: TGA TTG TGC TCG TTT TGA GC; SPR-3’: AAT GTA ATC GTG CAC CCA CA) to amplify cDNA derived from SPR transcripts. Actin5C primers (Actin 5': AGC GCG GTT ACT CTT TCA CCA C; Actin 3': GTG GCC ATC TCC TGC TCA AAG T) were used in parallel to serve as the loading control. PCR products were run on a 1% agarose gel and visualized using ethidium bromide; that SPR was amplified in the RT-PCRs as expected was confirmed by direct sequencing of the RT-PCR product, by the Cornell Bioresource Center. The level of knockdown achieved for the genotypes tested are shown in Figure S1.
3. Results and Discussion
3.1. The role of SPR in mediating SP’s regulation of sperm release from storage
3.1.1. SPR regulates sperm release from storage
SP is required for the efficient release of sperm from the female storage organs; excess retention of sperm is apparent by 4d after the start of mating (ASM) in females that do not receive SP during mating (Avila et al., 2010). While both types of sperm storage organ showed excess sperm retention at 4d after mating to an SP-less male, the magnitude of the effect is much larger in the seminal receptacle, and by 10 days after such a mating, significant over-retention of sperm is only seen in the seminal receptacle (Avila et al., 2010). Preventing release of SP from sperm also impaired sperm release from the seminal receptacle, suggesting that liberation of SP’s C-terminus from sperm signals the female to release stored sperm (Avila et al., 2010). Because the G protein-coupled receptor, SPR, is required for SP to mediate its effects on receptivity and egg laying (Yapici et al., 2008), we asked if SPR mediates SP’s activity in regulating the long-term release and utilization of stored sperm.
To determine whether SPR plays a role in the release of sperm from storage, we first tested the sperm storage/release phenotypes of females homozygous for a deletion of the SPR locus (SPR; Parks et al., 2004). Because SP’s effect on sperm release was most evident in the seminal receptacle (Avila et al., 2010), we focused our experiments on this storage organ. We compared the total number of sperm stored in the seminal receptacles of SPR and control females at two time points post-mating: 2hrs and 4d. These time points were chosen as they accurately assess both sperm entry into (2hr) and release from (4d) the seminal receptacle: mutants that perturb sperm entry into or release from storage show phenotypes at these time points, respectively (entry: Bloch Qazi and Wolfner, 2003; release: Avila et al., 2012; Avila et al., 2010).
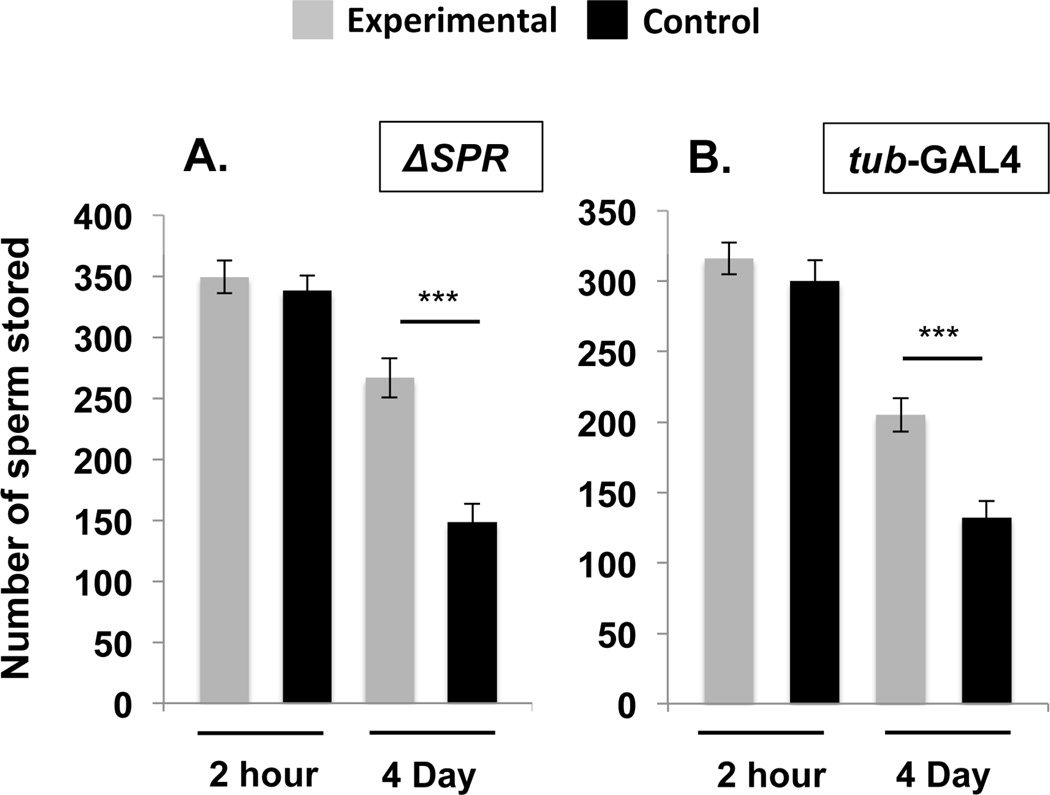
Absence of SPR did not affect the entry of sperm into storage: SPR and control females stored similar numbers of sperm at 2hrs ASM (Fig. 1A; Ncont = 13, NSPR = 12, Z = 0.571, p = 0.55). However, SPR females retained significantly more sperm in storage than controls at 4d ASM (Fig. 1A; Ncont = 14, NSPR = 13, Z = −3.664, p = 0.0002). These results, which parallel those reported for mates of SP-null males (Avila et al., 2010), show that SPR is required for the release of stored sperm from the seminal receptacle.
Figure 1. SPR is required to mediate SP’s effect on sperm release from storage.
Total sperm stored (mean ± standard error) in the seminal receptacles of control females or females homozygous for an SPR deletion (SPR) (A), or females in whom SPR was knocked down using the ubiquitous driver tubulin-GAL4 (B) at 2hrs and 4d ASM. Statistical significance denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005). N = 12–22 for each time point and genotype, see Results.
SPR is expressed in multiple female tissues, and SPR females lack the protein in all tissues. To determine where SPR expression is required for sperm release from storage, we needed to selectively remove SPR from particular tissues. This could be accomplished by tissue-specific RNAi-mediated knockdown of SPR. Prior to beginning these experiments, we demonstrated that ubiquitous knockdown of SPR (using the tub-GAL4 driver) recapitulated the results using the SPR deletion: tub>SPRRNAi and control females stored similar numbers of sperm at 2hrs ASM (Fig. 1B; Ncont = 16, NTub = 15, Z = 0.751, p = 0.4525) but retained significantly more sperm at 4d ASM compared to control females (Fig. 1B; Ncont = 21, NTub = 22, Z = −3.827, p = 0.0001). Sperm retention in storage was not due simply to induction of a dsRNA or usage of the RNAi machinery: females in which we used tub-GAL4 to drive dsRNA for a control gene (the male-specific SFP antares; Findlay et al., 2014) showed normal sperm entry and release (Fig. S2). Taken together our results indicate that in addition to its roles in other SP-induced post-mating behaviors, SPR is required to mediate SP’s effect on sperm release.
3.1.2. SPR action in the nervous system regulates sperm release from storage
Our results with a deletion of the SPR locus and with ubiquitous SPR knockdown showed that SPR is required in females for proper sperm release from storage, but did not reveal the site where SPR is necessary for this effect. SPR is expressed in the nervous system and tissues of the female reproductive tract (Prokupek et al., 2009; Yapici et al., 2008). To determine whether SPR is required in the nervous system, the female reproductive tract, or both to regulate sperm release, we assessed the effect of removing SPR from nervous system tissues or from two tissues of the female reproductive tract (the common oviduct and spermathecal secretory cells) that have high levels of SPR expression (Yapici et al., 2008). SPR expression is also reported in the seminal receptacle (Prokupek et al., 2009) but as no GAL4 driver is currently available to target this tissue, we were unable to address its expression there.
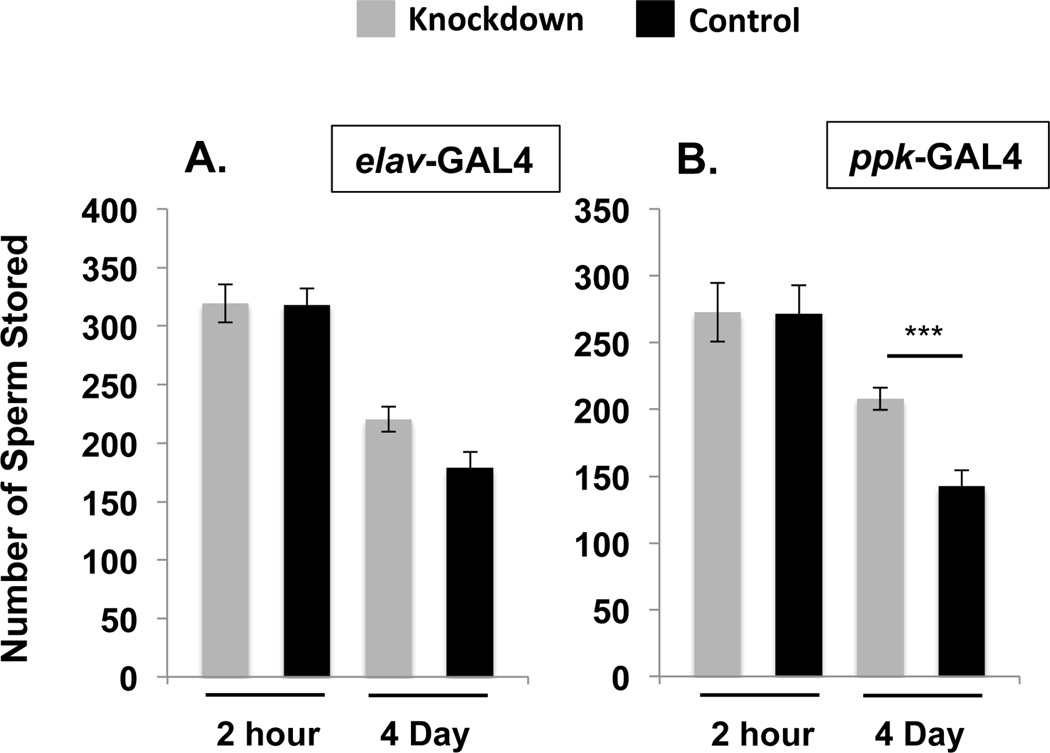
To examine whether neural expression of SPR regulates sperm release from storage we used the elav-GAL4 driver (Soller and White, 2004) to induce RNAi knockdown of SPR throughout the nervous system. elav> SPRRNAi females contained equivalent amounts of stored sperm compared with control females at 2 hrs ASM (Fig. 2A; Ncont = 23, Nelav = 16, Z = −0.086, p = 0.93). At 4d ASM a slight, but non-significant increase in the retention of sperm was observed (Fig. 2A; Ncont = 22, Nelav = 18, Z = 1.930, p = 0.053). To test whether the trend seen at 4d reflected the beginning of a detectable effect on sperm release, we examined sperm in storage at a much later time point: 10d ASM. At this time, few sperm typically remain in the seminal receptacle unless females do not receive SP during mating (Avila et al., 2010). We found that at this late time point, elav>SPRRNAi females retained significantly more sperm than did control females (Fig. S3; Ncont = 13, Nelav = 14, Z = −1.991, p = 0.0465). These results indicate that removal of SPR from the nervous system affects sperm release from the seminal receptacle.
Figure 2. SPR is required in the nervous system to mediate SP’s effect on sperm release from storage.
Total sperm stored (mean ± standard error) in the seminal receptacles of control females or females where SPR was knocked down in the nervous system using the neuronal driver elav-GAL4 (A), or the ppk+ fru+ neuronal driver ppk-GAL4 (B) at 2hrs or 4d ASM. Statistical significance denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005). N = 15–24 for each time point and genotype, see Results.
SP’s effect on post-mating levels of receptivity and egg laying requires SPR in a subset of female reproductive tract sensory neurons that co-express the sodium channel pickpocket (ppk) and the sex-specific transcript fruitless (fru) (Hasemeyer et al., 2009; Yang et al., 2009; Yapici et al., 2008). We used the ppk-GAL4 driver to target SPR knockdown to ppk-neurons neurons; the neurons in which this driver is expressed includes the overlapping ppk+ fru+ neurons of the female reproductive tract. Knocking down SPR with the ppk-GAL4 driver recapitulates the receptivity and egg laying defects observed when SPR is knocked down ubiquitously (Hasemeyer et al., 2009; Yang et al., 2009). At 2 hrs ASM, ppk> SPRRNAi and control females stored equal amounts of sperm (Fig. 2B; Ncont = 17, Nppk= 16, Z = 0.00, p = 1.0) indicating no need for SPR in these neurons for sperm entry into storage. However, knocking down SPR in ppk+ neurons led to a sperm retention defect evident at 4d ASM; ppk> SPRRNAi females retained significantly more sperm in storage than control females at this time (Fig. 2B; Ncont = 24, Nppk = 15, Z = −3.551, p = 0.0004). Because the sperm retention defect was more severe than retention in elav>SPRRNAi females, we examined sperm in storage at 10d ASM in ppk> SPRRNAi as well as tub>SPRRNAi females (as a control) to determine the severity of sperm retention in these backgrounds. At 10d ASM, ppk> SPRRNAi females had retained significantly more sperm than control females (Fig. S3; Ncont = 21, Nppk = 18, Z = 3.367, p = 0.0007) although this effect was not as severe as that seen in tub>SPRRNAi females (Fig. S3; Ncont = 21, NTub = 22, Z = −4.143, p < 0.001). It is possible that the ppk-GAL4 driver is more efficient in inducing RNAi knockdown than the elav-GAL4 driver. Alternatively, the differing genetic backgrounds might contribute to the observed differences in sperm retention. However, these results show that SPR expression in the nervous system, and specifically in ppk+ neurons, is required to effect sperm release from storage.
3.1.3. SPR action in spermathecae secretory cells also regulates sperm release from storage
While knockdown of SPR in ppk+ neurons led to significant retention of sperm in storage (Fig. 2B), the magnitude of this phenotype was less severe than that seen when SPR was knocked down ubiquitously (Fig. 1B). In addition to its nervous system expression, SPR expression has been reported in the secretory cells of the spermathecae and in the common oviduct of the female reproductive tract (Yapici et al., 2008). Because of these existing sources of SPR in close proximity to stored sperm, we asked whether SPR expression in these tissues (individually or combined) contributes to the regulation of sperm release. To test this possibility, we used GAL4 drivers specific to the common oviduct (OAMB-GAL4; Lee et al., 2003) and the spermathecal secretory cells (send1-GAL4; Schnakenberg et al., 2011) to knock down SPR in these tissues. We also combined these drivers into a single fly line to induce RNAi knockdown in both tissues simultaneously (“send1, OAMB-GAL4”).
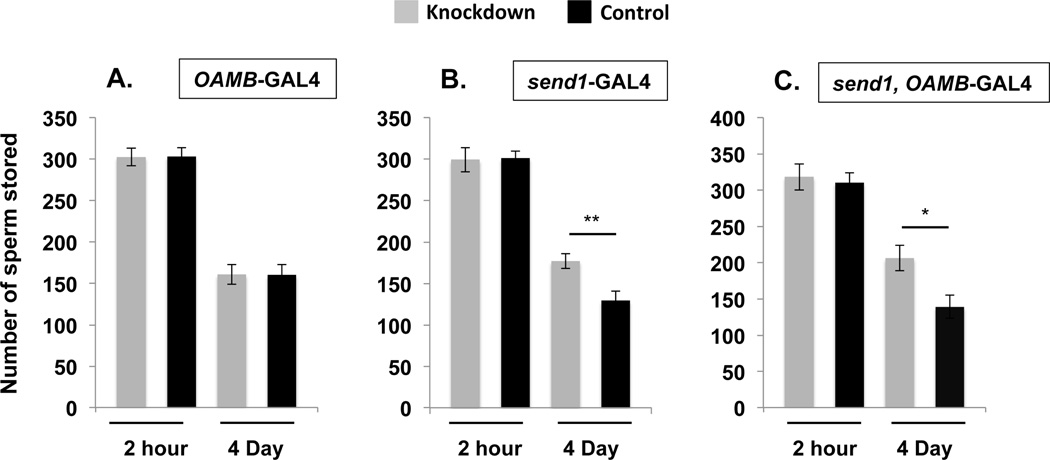
Knockdown of SPR in the common oviduct alone did not affect sperm entry into or release from storage as both OAMB>SPRRNAi and control females had similar levels of sperm in the seminal receptacle at 2hrs (Fig. 3A; Ncont = 14, NOAMB= 15, Z = 0.196, p = 0.84) and 4d ASM (Fig. 3A; Ncont = 17, NOAMB = 18, Z = −0.149, p = 0.88). However, knockdown of SPR in the spermathecal secretory cells affected sperm release from (but not entry into) storage. While both send1> SPRRNAi and control females stored similar levels of sperm at 2hrs ASM (Fig. 3B; Ncont = 15, Nsend1 = 14, Z = −0.327, p = 0.74), send1> SPRRNAi females retained significantly more sperm in their seminal receptacles by 4d ASM (Fig. 3B; Ncont = 16, Nsend1 = 17, Z = −2.739, p = 0.0062). Consistent with our finding that knockdown of SPR in the oviduct did not affect sperm release from storage, no differences in sperm levels were observed between send1, OAMB> SPRRNAi and control females at 2hrs ASM (Fig. 3C; Ncont = 13, NOAMB,send1 = 14, Z = −0.243, p = 0.81). By 4d ASM however, send1, OAMB> SPRRNAi females had retained significantly more sperm when compared to control females (Fig. 3C; Ncont = 17, Nsend1, OAMB = 15, Z = 2.511, p = 0.012). Taken together, our data show that SPR plays a role in the spermathecal secretory cells, but not in the common oviduct, in regulating sperm release from storage.
Figure 3. SPR is required in the spermathecal secretory cells to mediate SP’s effect on sperm release from storage.
Total sperm stored (mean ± standard error) in the seminal receptacles of control females or females where SPR was knocked down using the common oviduct driver OAMB-GAL4 (A), the spermathecal secretory cell driver send1-GAL4 (B), and both the send1 and OAMB-GAL4 drivers (C) at 2hrs or 4d ASM. Statistical significance denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005). N = 13–18 for each time point and genotype, see Results.
3.1.4. Conclusions
How sperm are stored and released from storage is not well understood in any insect. Here, we identify a critical female molecule for this process: the G-protein coupled receptor SPR. We found that SPR action in ppk+ neurons and in the spermathecal secretory cells is required to regulate sperm release from storage. SPR has several ligands (Kim et al., 2010; Poels et al., 2010), not all of which have apparent reproductive roles (Oh et al., 2014). Previously we showed that a particular SPR ligand, the male-derived seminal peptide SP, is essential for release of sperm from storage (Avila et al., 2010). The qualitative and quantitative parallels between the results of the present study of SPR and those of Avila et al. (2010) on SP strongly support that SPR is triggered by SP for this reproductive process. SP’s effect on sperm release requires that the active region of sperm-bound SP is cleaved from sperm (Avila et al., 2010). The liberated active region of SP could interact with SPR directly within the sperm storage organs or perhaps could leave the reproductive tract and bind nervous system SPR to moderate sperm release.
SPR is expressed in many adult tissues (Prokupek et al., 2009; Yapici et al., 2008). We showed here that SPR is needed in at least two of these tissues to mediate SP’s effect on sperm release. The nervous system role that we discovered for SPR in sperm storage is in keeping with previous studies that pointed to a role of the female nervous system in sperm-related processes such as sperm competition (Chow et al., 2013) and sperm storage events in Drosophila (Arthur et al., 1998; Avila et al., 2012) and sperm storage in Tribolium (Qazi et al., 1998). A role for SPR expression in the spermathecal secretory cells could not be predicted from prior studies and is the first demonstration of a reproductive function for SPR outside the nervous system. Spermathecal secretory cells synthesize potentially secreted proteins (Allen and Spradling, 2008; Prokupek et al., 2009) that are important for sperm storage (Schnakenberg et al., 2011) as well as for egg laying and ovulation (Schnakenberg et al., 2011; Sun and Spradling, 2013). Our finding that SPR is needed in these cells for proper rates of sperm release raised the intriguing possibility that SP/SPR signaling in these cells might participate in coordinating sperm release rates with other reproductive phenomena, such as the SP/SPR-mediated increase in egg production and egg release.
Supplementary Material
Figure S1. Knockdown of SPR using the ubiquitous tub-GAL4 driver (A) and the female reproductive tract drivers OAMB-GAL4 (B), send1-GAL4 (C) and send1, OAMB-GAL4 (D). For each genotype, a dilution series of SPR PCR products from the cDNA of control females (cont) is compared to the SPR PCR product from knockdown (KD) females. This gives an estimate of the level of knockdown achieved in our experimental females. All PCR products were obtained at 35 cycles except where noted. Actin5C (Act) PCR products from both knockdown and control female cDNA was used as a loading control. For the drivers tested, we estimate the following levels of expression relative to controls: tub-GAL4 <10%; send1-GAL4 ~50%; OAMB-GAL4 <25%; send1, OAMB-GAL4 >25%.
Figure S2. Induction of the RNAi machinery does not affect the entry or release of sperm from storage. The expression of dsRNA of a male-specific gene (the SFP antares) does not affect sperm storage in the seminal receptacle in females relative to their non-dsRNA expressing control sibs at 2hrs or 4d ASM (2hr: Ncont = 12, Nantr = 12, Z = 0.693, p = 0.49; 4d: Ncont = 14, Nantr = 15, Z = 0.894, p = 0.37).
Figure S3. Sperm retention at 10d ASM in tub>SPRRNAi, elav> SPRRNAi and ppk> SPRRNAi females. Total sperm stored (mean ± standard error) in the seminal receptacles of control females or females where SPR was knocked down using the ubiquitous driver tubulin-GAL4, the nervous system driver elav-GAL4 and the ppk+ fru+ neuronal driver ppk-GAL4. Statistical significance denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005). N = 13–22 for each time point and genotype, see Results.
Drosophila SP is a seminal peptide that regulates release of sperm stored by females.
SPR is a receptor known to mediate other effects of SP (egg-laying and receptivity).
We show that females’ SPR has another function: it regulates release of stored sperm.
SPR in neurons and spermathecal secretory cells is needed to regulate sperm release.
Acknowledgements
We thank K. Han for the OAMB-GAL4 driver, M. Siegal for the send1-GAL4 driver, Lily and Yuh Nung Jan for the ppk-GAL4 driver, the Bloomington Drosophila Stock Center for the tubulin-GAL4 driver and SPR, and the Vienna Drosophila Stock Center for UAS-SPR-RNAi line. We thank J. Morin, N. Buchon, J. Apger-McGlaughon, and anonymous reviewers for comments on the manuscript, M Shulman for encouragement, and S. Guerrier for assistance with the initial experiments for this study. We are grateful to NIH/NICHD grant R01-HD038921 for support of this work. A.L.M. was a Biology Research Scholar supported by NSF grant 0933921 to M. Shulman and R. Harris-Warrick.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- Apger-McGlaughon J, Wolfner MF. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J Insect Physiol. 2013;59:1024–1030. doi: 10.1016/j.jinsphys.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur BI, Hauschteck-Jungen E, Nothiger R, Ward PI. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. Proceedings of the Royal Society B-Biological Sciences. 1998;265:1749–1753. [Google Scholar]
- Avila FW, Bloch Qazi MC, Rubinstein CD, Wolfner MF. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc Natl Acad Sci U S A. 2012;109:4562–4567. doi: 10.1073/pnas.1117689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Ram KR, Qazi MCB, Wolfner MF. Sex Peptide Is Required for the Efficient Release of Stored Sperm in Mated Drosophila Females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA. 2009;106:15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi MC, Hogdal L. Hold on: females modulate sperm depletion from storage sites in the fly Drosophila melanogaster. J Insect Physiol. 2010;56:1332–1340. doi: 10.1016/j.jinsphys.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206:3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted Gene-Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female postmating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Wolfner MF, Clark AG. Large Neurological Component to Genetic Differences Underlying Biased Sperm Use in Drosophila. Genetics. 2013;193:177–185. doi: 10.1534/genetics.112.146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric Neurons and Systemic Signals Couple Nutritional and Reproductive Status with Intestinal Homeostasis. Cell Metabolism. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MD. Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility. Plos Genet. 2013;9 doi: 10.1371/journal.pgen.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Sitnik JL, Wang WK, Aquadro CF, Clark NL, Wolfner MF. Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Drosophila melanogaster Female Post-Mating Responses. Plos Genet. 2014;10 doi: 10.1371/journal.pgen.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annual Review of Entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gioti A, Wigby S, Wertheim B, Schuster E, Martinez P, Pennington CJ, Partridge L, Chapman T. Sex peptide of Drosophila melanogaster males is a global regulator of reproductive processes in females. Proc Biol Sci. 2012;279:4423–4432. doi: 10.1098/rspb.2012.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod. 2006;75:501–507. doi: 10.1095/biolreprod.106.053306. [DOI] [PubMed] [Google Scholar]
- Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Isaac RE, Li CX, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proceedings of the Royal Society B-Biological Sciences. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Bartalska K, Audsley N, Yamanaka N, Yapici N, Lee JY, Kim YC, Markovic M, Isaac E, Tanaka Y, Dickson BJ. MIPs are ancestral ligands for the sex peptide receptor. Proc Natl Acad Sci U S A. 2010;107:6520–6525. doi: 10.1073/pnas.0914764107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octoparnine receptor OAMB is required for ovulation in Drosophila melanogaster. Developmental Biology. 2003;264:179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Yoon SE, Zhang Q, Chae HS, Daubnerova I, Shafer OT, Choe J, Kim YJ. A homeostatic sleep-stabilizing pathway in Drosophila composed of the sex Peptide receptor and its ligand, the myoinhibitory Peptide. PLoS Biol. 2014;12:e1001974. doi: 10.1371/journal.pbio.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr TJ, Zuk M. Sperm storage. Curr Biol. 2012;22:R8–R10. doi: 10.1016/j.cub.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Pilpel N, Nezer I, Applebaum SW, Helfetz Y. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem Molec. 2008;38:320–330. doi: 10.1016/j.ibmb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Poels J, Van Loy T, Vandersmissen HP, Van Hiel B, Van Soest S, Nachman RJ, Vanden Broeck J. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cellular and Molecular Life Sciences. 2010;67:3511–3522. doi: 10.1007/s00018-010-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behavioral Ecology and Sociobiology. 2006;60:289–310. [Google Scholar]
- Prokupek AM, Eyun SI, Ko L, Moriyama EN, Harshman LG. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. Journal of Evolutionary Biology. 2010;23:1386–1398. doi: 10.1111/j.1420-9101.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 2009;18:465–475. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- Qazi MCB, Aprille JR, Lewis SM. Female role in sperm storage in the red flour beetle, Tribolium castaneum. Comparative Biochemistry and Physiology a-Molecular and Integrative Physiology. 1998;120:641–647. [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. Plos Genet. 2007;3:e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaval C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 2012;22:1155–1165. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-Mediated Semen Coagulation Controls Sperm Storage in the Malaria Mosquito. PLoS biology. 2009;7 doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9:e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, White K. Elav. Curr Biol. 2004;14:R53–R53. doi: 10.1016/j.cub.2003.12.041. [DOI] [PubMed] [Google Scholar]
- Sun JJ, Spradling AC. Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. Elife. 2013;2 doi: 10.7554/eLife.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Albright SN, Giebel JD, Ram KR, Ji S, Fiumera AC, Wolfner MF. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008;180:921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the postmating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Knockdown of SPR using the ubiquitous tub-GAL4 driver (A) and the female reproductive tract drivers OAMB-GAL4 (B), send1-GAL4 (C) and send1, OAMB-GAL4 (D). For each genotype, a dilution series of SPR PCR products from the cDNA of control females (cont) is compared to the SPR PCR product from knockdown (KD) females. This gives an estimate of the level of knockdown achieved in our experimental females. All PCR products were obtained at 35 cycles except where noted. Actin5C (Act) PCR products from both knockdown and control female cDNA was used as a loading control. For the drivers tested, we estimate the following levels of expression relative to controls: tub-GAL4 <10%; send1-GAL4 ~50%; OAMB-GAL4 <25%; send1, OAMB-GAL4 >25%.
Figure S2. Induction of the RNAi machinery does not affect the entry or release of sperm from storage. The expression of dsRNA of a male-specific gene (the SFP antares) does not affect sperm storage in the seminal receptacle in females relative to their non-dsRNA expressing control sibs at 2hrs or 4d ASM (2hr: Ncont = 12, Nantr = 12, Z = 0.693, p = 0.49; 4d: Ncont = 14, Nantr = 15, Z = 0.894, p = 0.37).
Figure S3. Sperm retention at 10d ASM in tub>SPRRNAi, elav> SPRRNAi and ppk> SPRRNAi females. Total sperm stored (mean ± standard error) in the seminal receptacles of control females or females where SPR was knocked down using the ubiquitous driver tubulin-GAL4, the nervous system driver elav-GAL4 and the ppk+ fru+ neuronal driver ppk-GAL4. Statistical significance denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005). N = 13–22 for each time point and genotype, see Results.