Abstract
Background
Recent literature suggests that some patients may develop addictive disorders after bariatric surgery, in particular following Roux-en-Y gastric bypass (RYGB). These may include traditional addictions and so called “behavioral addictions”, although prevalence data on the latter have not been published.
Objectives
To establish prevalence of addictive behaviors in adults following RYGB.
Setting
2 university hospitals and 1 not-for-profit research institute in the U.S.
Methods
Participants from a large observational study of bariatric surgery who had undergone RYGB were recruited to complete additional measures. Of 241 consented participants, 201 provided data (i.e., Structured Clinical Interview for DSM-IV Axis I [SCID], additional Impulsive Control Disorder Modules, and various self-report measures, including the Alcohol Use Disorder Identification Test [AUDIT]) to assess status prior to surgery and in the first three post-operative years.).
Results
Based on the SCID, 16 (8.0%) developed alcohol use disorder [AUD] within three years post-RYGB, 7 (43.8%) of whom had no history of AUD. When both the SCID and AUDIT were used to identify AUD, the corresponding numbers/percentages were 32 (18.4%) and 13 (40.6%). Data on other behavioral addictive disorders indicated 19 (9.5%) had a post-surgery disorder, 6 (31.6%) of whom had no history.
Conclusions
These data add to a growing literature suggesting there is a substantial risk for the development of AUD after bariatric surgery. Understanding the risk for non-drug related addictive disorders requires more data from larger studies before clear conclusions can be drawn.
Introduction
In recent decades severe obesity has markedly increased in prevalence in the United States and has reached epidemic proportions (1). Unfortunately most treatments for severe obesity are only minimally effective, and the resultant weight loss is often followed by weight regain (2). Bariatric surgery is often the exception, and is now considered the most effective treatment for severely obese patients, with surgery resulting in substantial weight reductions, improvements in comorbidities and reductions in long-term mortality (2–4). The morbidity and mortality risks associated with these procedures are low. In the Longitudinal Assessment of Bariatric Surgery-1 (LABS-1) Study, the 30-day mortality rate was 0.3% and 4.3% of patients had at least one major adverse event (5).
Despite the great benefits of bariatric surgery, recent literature suggests that some patients may develop addictive or impulse control disorders following bariatric surgery (6–12). Much of this literature has focused on the development of problems with alcohol use. Until recently, published empirical literature in this area has been quite limited, and generally has involved cross-sectional or retrospective studies with relatively small sample sizes (13–16), with several notable exceptions. Three recent prospective reports are of particular interest. King and colleagues (17) published data from the multicenter Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) prospective cohort study, which included 1,945 patients who completed pre-operative and annual post-operative assessments using the Alcohol Use Disorders Identification Test (AUDIT). In this cohort the prevalence of alcohol use disorder (AUD) symptoms increased in the second post-operative year, as alcohol intake per typical drinking episode resumed to pre-operative levels (after an initial decrease in the first postoperative year), and frequency of alcohol intake increased compared to pre-operative and first year levels. A number of pre-operative factors were found to independently increase risk of post-operative AUD symptoms, including male sex, younger age, positive smoking status, regular alcohol consumption (defined as ≥2 drinks/week), recreational drug use, and lower perceived interpersonal support. It was also found that those who underwent Roux-en-Y gastric bypass (RYGB), as opposed to laparoscopic adjustable banding (LAGB), had an increased risk of AUD symptoms, a finding that may be explained at least partially by other research suggesting more rapid absorption, and/or higher peak alcohol concentrations and/or a longer half-life for alcohol after RYGB (18–22). Further evidence of a surgical effect comes from the Swedish Obese Subjects (SOS) Study. Svensson and colleagues (23) found that compared to controls, alcohol consumption, the prevalence of alcohol problems, and alcohol abuse diagnoses were higher in the 8–22 years following RYGB, or vertical banded gastroplasty, a procedure no longer widely utilized, whereas the findings regarding LAGB were not different from controls. The third report concerned the risk of inpatient treatment for alcohol abuse in Sweden from 1980 through 2006 (24). The sample included 11,115 patients who had undergone bariatric surgery. After a mean follow-up of 8.6 years, those who had undergone RYGB had an increased risk of inpatient treatment for alcohol abuse versus those having a LAGB (hazard rating = 2.3; 95% CI 1.7–3.2).
Other impulse control or “addictive” disorders that have been mentioned in the literature as occurring post-operatively in bariatric surgery recipients include skin picking, compulsive buying behavior, and intermittent explosive disorder, as well as sexual promiscuity, compulsive gambling and compulsive exercise (6,9,10,25). It has been hypothesized that when such “addictive” phenomena occur after surgery, they represent an “addiction transfer”, the presumed pre-operative addiction to food transforming into an addiction to other substances or behaviors after surgery (7). However, empirical support for this hypothesis is lacking, and other than the data concerning alcohol problems, there have been very little or no substantial data published on any of these other behaviors of interest apart from alcohol misuse.
The current study brings together researchers in the areas of obesity, bariatric surgery, psychopathology, eating behavior, impulse control disorders and substance use disorders to examine the emergence of such behaviors in individuals who underwent RYGB for severe obesity. This study used a subset of the LABS-2 cohort previously mentioned, who were intensively interviewed.
The study capitalized on the ongoing LABS-2 data collection system (26,27) to conduct an in-depth interview assessment for a history of or development of such behaviors among a subgroup of men and women who were studied at a minimum of 2 years and at most 4 years post-RYGB, on at least two occasions separated by 1 year. This time frame was chosen since it was hypothesized that many of the problems of interest would occur after subjects achieved their weight loss nadir, and that some would begin to experience weight regain. This choice was also based on the results contained in our prior report using the complete LABS-2 cohort, in which the prevalence of AUD increased in the second year after surgery among those undergoing a RYGB (17). We maximized sample size in this report by focusing on the year 3 assessment (N = 201). Also, while we acknowledge that labeling a wide range of behaviors as “addictive” can be regarded as controversial, our purpose is not to address the validity of that concept here, but instead to group these behaviors together using the term addictive for the sake of convenience.
Methods
Participants
This observational study included a subgroup of participants from the LABS-2 cohort. LABS-2 is designed to assess the risks and benefits, including long-term outcomes, of patients undergoing bariatric surgery (27). LABS-2 includes patients at least 18 years of age, who underwent a first bariatric surgical procedure between March 2006–April 2009 performed by one of the participating LABS surgeons at one of the ten LABS hospitals in the United States. All of the subjects were originally recruited into the LABS-2 sample between February 2006 and February 2009. All participating centers had Institutional Review Board approval for the LABS-2 protocol, and for this additional protocol.
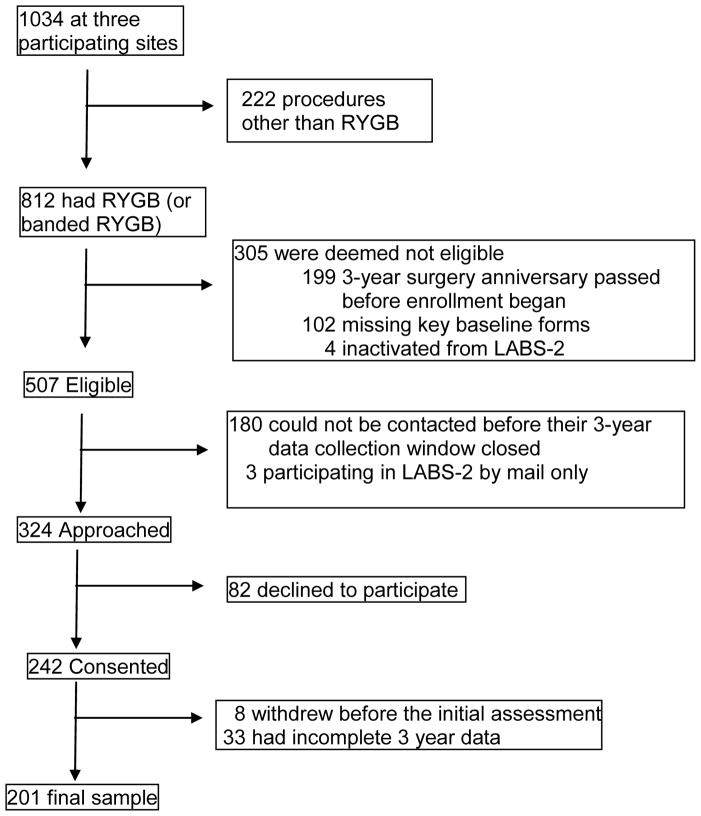
Given the complexity of the assessments and financial limitations, the target recruitment for this subproject of LABS-2 was 300. Participants who had undergone a RYGB alone or a banded RYGB were recruited from three of the LABS centers, the Neuropsychiatric Research Institute in Fargo, the University of Pittsburgh, and Oregon Health and Science University, in 2010–2011. The numbers eligible, approached, consented and excluded are summarized in Figure 1. The sample size for this analysis was 201.
Figure 1.
Participant flow
Measures
The following interviews were utilized, with data obtained either in person or by phone:
The alcohol and substance abuse/dependence eating disorders, affective disorders and anxiety disorders sections from the Structured Clinical Interview for DSM-IV Axis I (SCID-I) (28).
The kleptomania, pathological gambling, impulse-compulsive buying, trichotillomania, impulsive-compulsive non-paraphilic sexual behavior, and impulsive-compulsive internet use sections from the Impulse Control Modules developed by First for the DSM-IV-TR (personal communication). A section modeled on these modules was added for exercise dependence using questions adopted from the Exercise Dependence Assessment Scale (EDAS) (29).
All interviewers were experienced masters-level mental health clinicians who had been carefully trained in the use of the instruments, including the auditing training interviews and completing tape-monitored practice interviews, which were assessed and scored. All interviewers had completed the structured interviews in prior trials. To ensure consistency throughout the assessment period, interviewers participated in monthly conference calls in which a supervisor led a discussion regarding ambiguous responses.
As part of the LABS-2 assessment anthropometric data were measured, and participants self-reported socio-demographics, smoking status, and treatment for psychiatric and emotional issues (27). They also completed the Alcohol Use Disorders Identification Test (AUDIT) (30), the Beck Depression Inventory V.1 (BDI-1) (31), the Short Form-36 Health Inventory (SF-36) (32), the Impact of weight on Quality of Life-Lite (IWQOL-lite) (33), and the Interpersonal Support Evaluation List (ISEL) (34,35).
The Structured Clinical Interview for DSM-IV was used as the primary instrument for assessing alcohol abuse and dependence. However, SCID criteria require a positive response to having ingested five or more drinks on one occasion as a screening question. Prior research described above, much of which has come to light since the LABS protocol was designed, indicated that pharmacokinetic changes result in more rapid and/or higher levels of alcohol absorption following RYGB. At the time of data analysis this criteria was judged to be excessively high in post-RYGB surgery patients. Therefore, both DSM-IV SCID criteria and the criteria which had been utilized in the LABS-2 report for AUD, based on the AUDIT, were used to identify AUDs. Criteria utilized in the LABS-2 report included an AUDIT score of ≥8, endorsement of any alcohol dependence symptoms and/or endorsement of any alcohol-related harm symptom (17). Of note, assessment tools other than the AUDIT assessed preoperative lifetime history, whereas the AUDIT assessed only the past year both at the pre-operative and annual post-operative assessments.
Statistical Analysis
Analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, N.C.). Statistical significance was set at p<0.05. Frequencies and percentages were reported for categorical data. Medians, 25th and 75th percentiles were reported for continuous data which were not normally distributed. Pearson’s chi-square test of association for categorical variables and Wilcoxon rank-sum test for continuous variables were used to assess statistical significance of differences among those included vs. excluded from the analysis.
Based on their responses to the interviews and self-report measures subjects were classified into one of four groups for each addictive disorder/behavior: no pre-operative/no post-operative occurrence; pre-operative but no post-operative occurrence; no pre-operative but post-operative occurrence; and both pre-operative and post-operative occurrence. The pre-operative period included the entire “lifetime,” except when the AUDIT, which assessed the past-year only, was used. The post-operative period was the first three post-operative years. Because the AUDIT assessed past-year only, responses from the 1, 2 and 3 year assessments were combined to determine post-operative status. If any one of those three assessments was missing, post-operative status could not be determined for the purpose of reporting prevalence. Due to the way the interviews were structured and/or the way the data were recorded, it was not possible to ascertain exactly when the disorder or symptoms began or ended, during either the pre- or post-operative periods examined.
Descriptive statistics were used to report prevalence of common (i.e., at least 5% sample prevalence) psychiatric conditions (affective and anxiety disorders, eating disorders and impulse control/”addictive” disorders) at various time points (i.e., pre-operative lifetime, post-operative first three years, post-operative past 30 days) cross-tabulated with presence or absence of post-operative (first three years) AUD as determined by the combination of the SCID and AUDIT. Pearson’s chi-square test was used to test for differences in prevalence in conditions. Due to the rarity of some conditions statistical power to detect clinically meaningful differences was low. Wilcoxon rank-sum test for continuous variables was used to assess statistical significance of weight change differences by post-operative status of addictive disorders and/or behaviors, specifically: 1) any addictive disorder as determined by the SCID; 2) AUD as determined by the SCID; 3) AUD as determined by the SCID and AUDIT; 4) any addictive disorder not including substance use disorders; and 5) impulsive/compulsive buying. Given the sample size and prevalence of these addictive disorders and behaviors, the analysis had 80% power to detect a difference in weight loss of at least 7–8%. Other addictive behaviors were too uncommon to investigate.
Results
Patient recruitment and participant flow are shown in Figure 1. Baseline characteristics of the analysis sample (n=201) are shown in Table 1. We compared characteristics of the analysis sample to LABS-2 participants who underwent RYGB or banded RYGB at one of the three participating sites, but who were not included in the analysis sample for various reasons (Figure 1; n = 611). There was not a significant difference (ps>.05) between samples with respect to sex, race, ethnicity, marital status, employment, income, education, alcohol consumption, symptoms of AUD, illegal drug use, treatment for alcohol or drugs in the past year, BMI, BDI score, SF-36 PCS score, all IWQOL-lite scores except self-esteem, and ISEL appraisal and tangible scores at baseline. However, those included in the study sample were somewhat older (median = 48 versus 43 years, p < .0001), and prior to surgery, were less likely to be current or recent smokers (8.0% versus 19.1%, p <.001). Although these variables are not shown in Table 1, those not included in the analysis were less likely to have been treated (medication, counseling, hospitalization) in the prior 12 months for psychiatric or emotional problems (41.8% versus 51.1%, p = .02), and endorsed less impairment and better support on some subscales evaluating quality of life and interpersonal support. These included the SF-36 mental component subscale (median 53.4 versus 50.6, p < .01); the Self-Esteem subscale of the Impact of Weight on Quality of Life-Lite (median = 39.3 versus 35.7, p <.01), and the “Belonging” subscale of the Interpersonal Support Evaluation List (both medians = 14, but the distributions differed, p = .02). At three years post-surgery, those included in the analysis sample were less likely to report consumption at a hazardous level on the AUDIT (18.1% versus 22.2%, p = .04) and fewer had been treated for psychiatric or emotional problems in the prior 12 months (39.3% vs. 62.7%; p<.0001), but there was not a significant difference in other measures of post-operative alcohol intake/effect (ps >.05) or % weight loss (p = .64).
Table 1.
Baseline Characteristics of Adults Undergoing RYGB or Banded RYGB (n=201)a.
| n(%)/median (25th, 75th percentile)
|
|
|---|---|
| Male | 38(18.9) |
| Age, years | |
| median(25th, 75th percentile) | 48(39,56) |
| range | 22–75 |
| Race | |
| White | 191(95.5) |
| Black | 3(1.5) |
| Other | 6(3.0) |
| Hispanic ethnicity | 5(2.5) |
| Married or living as married | 144(71.6) |
| Employed for pay | 145(72.9) |
| Household income | |
| Less than $25,000 | 27(13.8) |
| $25,000–$49,000 | 68(34.7) |
| $50,000–$74,999 | 50(25.5) |
| $75,000–$99,999 | 29(14.8) |
| $100,000 or more | 22(11.2) |
| Education | |
| High school or less | 45(22.4) |
| Some college/post high school education | 84(41.8) |
| College degree or higher | 72(35.8) |
| Current or recent smoker | 16(8.0) |
| BMI, kg/m2 | |
| median(25th, 75th percentile) | 45.4(41.3,50.4) |
| range | 33.8–76.8 |
RYGB= Roux-en-Y Gastric Bypass.
Missing: race (n=1), employed for pay (n=2), household income (n=5).
Distribution of participants by patterns of substance use disorder prior to and in the first three years following RYGB, is shown in Table 2. Based on the SCID, 16 (8.0%) participants developed AUD within the first three years of RYGB; just over half of those participants (n=9; 56.3%) had a history of AUD prior to surgery, whereas the others (n=7; 43.8%) were classified as “new AUD.” Another way to consider these data is that 13.4% (9/67) with a pre-operative history of AUD developed postoperative AUD, compared to 5.2% (7/134) without a pre-operative history of AUD. When AUDIT responses were also taken into account, 32 (18.4%) participants were identified as having post-operative AUD. Thirteen of these participants (40.6%) were “new AUD,” while 7 (12.5%) experienced “continued AUD” (i.e., met AUD criteria on the AUDIT for the year prior to surgery and continued to meet SCID or AUDIT criteria after surgery), and 12 (37.5%) experienced “recurrent AUD” (i.e., negative for AUD on the AUDIT for the year prior to surgery but positive for lifetime AUD before surgery (SCID) and after surgery (SCID and/or AUDIT). Another way to consider these data is that 29.2% (19/65) with a pre-operative history of AUD developed postoperative AUD, compared to 11.9% (13/109) without a pre-operative history of AUD.
Table 2.
Status of alcohol and substance use disorders prior to (lifetime history) and in the first three years following RYGB (n=201).
| Alcohol and Substance Use Disorders | no pre-op/no post-op | pre-op/no post-op | no pre-op/post-op | pre-op/post-op |
|---|---|---|---|---|
| Alcohol Use Disorder-SCID criteria | 127 (63.2%) | 58(28.9%) a | 7(3.5%) b | 9(4.5%) c |
| Alcohol Use Disorder-SCID + AUDIT criteria (missing 27)d | 96 (55.2%) | 46(26.4%) | 13(7.5%) | 19(10.9%) e |
| Any Other Substance Use Disorder (1 missing) | 178 (89.0%) | 20(10.0%) | 1(0.5%) | 1(0.5%) |
| Sedatives/Hypnotics/Anxiolytics | 200 (99.5%) | 0(0.0%) | 0(0.0%) | 1(0.5%) |
| Cannabis (1 missing) | 185 (92.5%) | 15(7.5%) | 0(0.0%) | 0(0.0%) |
| Stimulants | 194 (96.5%) | 7(3.5%) | 0(0.0%) | 0(0.0%) |
| Opioids | 197 (98.0%) | 3(1.5%) | 1(0.5%) | 0(0.0%) |
| Cocaine | 197 (98.0%) | 4(2.0%) | 0(0.0%) | 0(0.0%) |
| Hallucinogen/Phencyclidine | 200 (99.5%) | 1(0.5%) | 0(0.0%) | 0(0.0%) |
| Poly Drug | 199 (99.0%) | 2(1.0%) | 0(0.0%) | 0(0.0%) |
SCID= Structured Clinical Interview for DSM-IV; AUDIT=Alcohol Use Disorder Identification Test; PCP= Phencyclidine.
42 had pre-op alcohol abuse; 16 had pre-op alcohol dependence
7 had post-op alcohol dependence
3 had pre-op and post-op alcohol abuse; 6 had pre-op and post-op alcohol dependence
Alcohol Use Disorder (AUD) status for the SCID was recoded from “no” to “yes” if the AUDIT data indicated AUD. Because the AUDIT only assesses the past 12 months, data was set to missing if AUDIT data from pre-op, 1, 2, or 3 years was incomplete, and pre-op status was only updated based on the 12 months prior to surgery.
7 had indication of AUD in the 12 months pre-op (AUDIT), whereas 12 did not, but had indication of AUD pre-op (SCID).
Other substance use disorders, as determined by the SCID, were less common, with 11.0% of participants experiencing a non-AUD substance use disorder prior to or following surgery. Prior to surgery, cannabis (7.5%), stimulant (3.5%), and cocaine (2.0%) disorders were most common, but no participants experienced these disorders following surgery. Of the two participants with post-surgery non-AUD substance use disorders, one individual (0.5%) reported abuse or dependence of sedatives/hypnotics/anxiolytics both prior to and after surgery, and one (0.5%) reported opioid dependence after surgery only. The former, but not the later, also had postoperative AUD.
Data on other behavioral addictive disorders and behaviors obtained using the SCID impulse control disorder module (Table 3) indicated that 23 (11.4%) had at least one such disorder in their lifetime prior to surgery and 19 (9.5%) in the first three years following surgery, 6 (31.6%) of whom had no history of that problem. The only disorders that occurred in at least 2.0% (n=4) of participants were impulsive-compulsive buying, pathological gambling and impulsive-compulsive internet use.
Table 3.
Status of Non-drug Related Addictive Disorders/Behaviors Prior to (Lifetime History) and in the First Three Years Following RYGB (n=201).
| Non-drug Related Addictive Disorders/Behaviors | no pre-op/no post-op | pre-op/no post- op | no pre-op/post-op | pre-op/post-op |
|---|---|---|---|---|
| Any non-drug related addictive behavior | 172 (85.6%) | 10(5.0%) | 6(3.0%) | 13(6.5%) |
| Kleptomania | 198 (98.5%) | 1(0.5%) | 1(0.5%) | 1(0.5%) |
| Pathological gambling | 197 (98.0%) | 0(0.0%) | 2(1.0%) | 2(1.0%) |
| Trichotillomania | 200 (99.5%) | 1(0.5%) | 0(0.0%) | 0(0.0%) |
| Impulsive-compulsive buying | 181 (90.1%) | 6(3.0%) | 3(1.5%) | 11(5.5%) |
| Impulsive-compulsive non-paraphilic sexual behavior | 198 (98.5%) | 2(1.0%) | 1(0.5%) | 0(0.0%) |
| Impulsive-compulsive internet use | 196 (97.5%) | 1(0.5%) | 4(2.0%) | 0(0.0%) |
| Exercise dependence | 199 (99.0%) | 2(1.0%) | 0(0.0%) | 0(0.0%) |
Table 4 depicts the prevalence of the most common psychiatric conditions (affective and anxiety disorders, eating disorders and impulse control disorders) at various time points (pre-operative lifetime, post-operative 3 years, and post-operative past 30 days) cross tabulated by the presence or absence of post-operative (first three years) AUD as determined by the SCID and AUDIT. Those with post-operative AUD had a higher prevalence of mood disorder, and specifically major depressive disorder when pre-operative lifetime, post-operative 3 years, or post-operative past 30 days, time periods were considered (ps < .05). Similarly, they had a higher prevalence of generalized anxiety disorder for all three time periods (ps < .05). They also had a higher prevalence of any anxiety disorder post-operative past 30 days (7.1% vs. 20.6%; p=.02), and panic disorder post-operative 3 years (4.9% vs. 14.7%; p=.04). There was not a statistically significant difference in post-traumatic stress disorder prevalence or specific phobia prevalence by post-operative AUD status at any time point.
Table 4.
Prevalence of the most common psychiatric conditions during various time periods by whether participants had postoperative (first three years) alcohol use disorder (SCID/AUDIT criteria) (n=176)
| No Post-op Alcohol Use Disorder (n=142) | Post-op Alcohol Use Disorder (n=34) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-op | Post-op | Pre-op | Post-op | |||
| Lifetime | 3 years | Past 30 days | Lifetime | 3 years | Past 30 days | |
| MOOD DISORDERS | ||||||
| Any Mood Disorder | 61(43.0)a | 25(17.6)c | 9(6.3)c | 21(61.8)a | 16(47.1)c | 9(26.5)c |
| Major Depressive Disorder | 56(39.4)a | 22(15.5)c | 9(6.3)b | 20(58.8)a | 14(41.2)c | 8(23.5)b |
| ANXIETY DISORDERS | ||||||
| Any Anxiety Disorder (1 missing pre-op status) | 41(29.1) | 25(17.7) | 10(7.1)a | 11(32.4) | 10(29.4) | 7(20.6)a |
| Panic Disorder | 22(16.1) | 7(4.9)a | 3(2.1) | 5(14.7) | 5(14.7)a | 3(8.8) |
| Specific Phobia | 12(8.5) | 12(8.5) | 7(4.9) | 4(11.8) | 4(11.8) | 2(5.9) |
| Posttraumatic Stress (1 missing pre-op status) | 21(14.9) | 6(4.3) | 2(1.4) | 6(17.6) | 3(8.8) | 2(5.9) |
| Generalized Anxiety | 5(3.5)a | 5(3.5)a | 2(1.4)b | 5(14.7)a | 5(14.7)a | 4(11.8)b |
| EATING DISORDERS | ||||||
| Binge Eating Disorder | 30(21.1)a | 2(1.4)b | 0(0.0)b | 12(35.3)a | 4(11.8)b | 2(5.9)b |
| IMPULSIVE DISORDERS | ||||||
| Impulsive-compulsive Buying | 11(7.7)b | 8(5.6) | 4(2.8)a | 8(23.5)b | 5(14.7) | 4(11.8)a |
SCID= Structured Clinical Interview for DSM-IV; AUDIT=Alcohol Use Disorder Identification Test.
Pearson’s chi-square test used to test whether prevalence of psychiatric conditions during specified time periods differed by postop alcohol use disorder status.
Statistical power was limited to detect clinically significant differences of less common conditions. P-value:
<.05,
<.01,
<.001.
We also examined whether 3 year weight loss, measured as percentage of initial weight lost, differed between those with and without addictive disorders (Table 5). No statistically significant differences were detected (ps >.05).
Table 5.
Percentage weight change by whether participants had postoperative (first three years) addictive behaviors
| Percentage weight change by additive behavior | p-(quartiles) | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| n | Median (quartiles) | n | Median(quartiles) | ||
| Any Addictive Behavior Disorder-SCID criteria | 167 | −30.7 (−38.0,−25.4) | 34 | −30.3 (−36.4,−26.7) | 0.94 |
| Alcohol Use Disorder-SCID criteria | 185 | −30.7 (−38.0,−25.4) | 16 | −29.6 (−36.6,−25.9) | 0.59 |
| Alcohol Use Disorder- SCID/AUDIT criteria | 142 | −31.7 (−38.8,−26.1) | 34 | −29.9 (−34.4,−26.7) | 0.44 |
| Non-Substance Use Addictive Disorder | 182 | −30.7 (−37.9,−25.4) | 19 | −30.0 (−36.4,−26.7) | 0.71 |
| Impulsive/Compulsive Buying | 187 | −30.7 (−37.7,−25.2) | 14 | −32.4 (−42.3,−27.6) | 0.30 |
RYGB=Roux-en-Y Gastric Bypass; SCID= Structured Clinical Interview for DSM-IV; AUDIT=Alcohol Use Disorder Identification Test.
These analyses had 80% power to detect a difference in % weight loss of 7–8%, i.e., the analysis was underpowered to detect a real difference smaller than this amount.
Discussion
These results suggest that overall 8% of patients develop AUD in the first three years following RYGB, as diagnosed by the SCID, or 18.4% as diagnosed by the combination of both the SCID and the AUDIT. While post-operative AUD prevalence appears higher among patients with a pre-operative history of AUD (i.e., 13% or 29%, depending on the criteria), a notable group of patients with no history of such a problem at baseline also experience post-operative AUD (i.e., 5% or 12%, depending on the criteria employed). Relative to comparison to large population based data sets, comparison data on severely obese groups are lacking. However, studies from the U.S. (36), Germany (37) and Italy report lifetime rates of alcohol abuse and dependence of 18.6%, 4.6% and 1.1% respectively. Also a study from Germany reported a lifetime prevalence rate of 4.7% in obese individuals not seeking treatment. We were unable to find data on the assessment of such problems over a 3-year period.
Assuming that this one area is significant, the reasons for this are unclear. This might be attributable to the kinetic change in alcohol, or other factors such as not meeting one’s target weight goal, experiencing weight regain or global dissatisfaction with the result. Further research in the area is needed.
Relative to the issue of non-drug addictive behavioral disorders, the protocol used diagnostic criteria using modules developed as an addition to the SCID-IV, and they only measured certain addictive behaviors. Several of these disorders are not included in DSM-5, and other addictive behaviors (e.g., skin picking) have been suggested in the literature, which are not addressed by these criteria. The data do suggest that other addictive disorders may develop, but without a control group it is difficult to interpret the data. Data from Koran et al., (36) found that compulsive buying occurred in 5–8% of individuals in consumer societies. However, comparison data on the prevalence of the non-drug addictive disorders in the general population, and in obese, age and gender-matched populations are lacking. Therefore, this should be seen as an early examination of such problems, which clearly requires further work with updated criteria and larger sample sizes. The issue of impulsive-compulsive internet use is interesting, and it is worth mentioning that over the course of this study internet usage accelerated dramatically in the general population, which may have contributed to the number of cases that were reported post-operatively. However, the sample size for this disorder was too modest to allow for meaningful conclusions.
We also examined whether there was an association between certain psychiatric conditions during both pre- and post-operative time periods, and post-operative AUD. The data indicate that those with post-operative AUD were more likely to have pre-operative lifetime and post-operative diagnoses of certain psychiatric conditions, including any mood disorder, major depressive disorder, generalized anxiety disorder, and binge eating disorder. The results also indicate increased rates of panic disorder post-operative, and impulsive/compulsive buying both pre-operative lifetime and post-operative in the past 30 days.
Finally, we investigated whether addictive disorders in the post-operative time period were related to post-operative weight loss during the first three years. Addictive disorders in general, and AUD in particular, did not appear to have a significant impact on the amount of post-operative weight loss. Neither did the non-substance use addictive disorders overall, including impulsive/compulsive buying. However, it must be remembered that the data only address the first few years after surgery.
The strengths of this study include a reasonable, though not large, sample size and the comprehensive assessment, using both interview and self-report measures. Limitations include that most data were collected at one and/or two time points (3 and possibly also 2 years post RYGB) to get retrospective data on pre- and post-RYGB time periods. All the results whether by interview or self-report measures were based on patient self-report. It is possible that patients under reported substance use disorders, including AUD, and addictive behaviors, when reporting status prior to surgery, and/or after surgery. However, because all assessments other than the AUDIT were done retrospectively following surgery, participants should not have been concerned regarding whether their responses would impact surgery eligibility. In interpreting these data it must be born in mind that the assessment presented includes data from the Structured Diagnostic Interview (the SCID) and from the self-report form, the AUDIT, for reasons that are explained in the methods section. While the SCID, given its five drinks on one occasion minimum criteria, is suspected to underestimate post-operative AUD, the AUDIT may overestimate AUD, given that it is meant as a screening, rather than a diagnostic tool. Additionally, pre-surgery assessments use lifetime data (SCID) or past year (AUDIT) while post-operative data were for the prior 3 years; thus the lengths of the pre- and post-operative periods differed. It should also be remembered that all these patients had undergone RYGB, and therefore no comparison is possible with the development of such problems in patients undergoing other bariatric surgery procedures such as LAGB, sleeve gastrectomy or biliary pancreatic diversion. Likewise, the rate in the general population of the development of AUDs in this range of BMIs, at this age, with this sex and minority/ethnic distribution, could not be ascertained from the literature, and therefore the results must be seen as interesting but speculative without a control population.
Conclusion
There is a substantial risk for the development of AUD following RYGB, especially among those with a history of AUD. The frequency of the development of such problems appears to be modest. However, whether or not behavioral addictive disorders increase following RYGB requires further study, with a larger sample size.
Acknowledgments
Funding/Support: LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants U01 DK066557 (data coordinating center); U01-DK66667 and UL1-RR024996 (Columbia-Presbyterian in collaboration with Cornell University Medical Center Clinical and Translational Research Center [CTRC]); U01-DK66568 and M01RR-00037 (University of Washington in collaboration with Cornell University Medical Center CTRC); U01-DK66471 (Neuropsychiatric Research Institute); U01-DK66526 (East Carolina University); U01-DK66585 and UL1-RR024153 (University of Pittsburgh Medical Center in collaboration with Cornell University Medical Center CTRC); and U01-DK66555 (Oregon Health & Science University). This particular part of the LABS-2 study was also funded by a Supplement from the National Institute of Drug Abuse and the National Institute For Alcoholism and Alcohol Abuse to U01-DK66471 (Neuropsychiatric Research Institute.
Footnotes
Role of the Sponsor: The NIDDK scientists contributed to the design and conduct of the study, which included collection, and management of data. The project scientist from the NIDDK served as a member of the steering committee, along with the principal investigator from each clinical site and the data coordinating center. The data coordinating center housed all data during the study and performed data analyses according to a pre-specified plan developed by the data coordinating center biostatistician and approved by the steering committee and independent data and safety monitoring board. The decision to publish was made by the Longitudinal Assessment of Bariatric Surgery-2 steering committee, with no restrictions imposed by the sponsor. As a coauthor, an NIDDK scientist contributed to the interpretation of the data and preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–1933. doi: 10.1007/s11695-013-1070-4. [DOI] [PubMed] [Google Scholar]
- 3.Courcoulas AP, Flum DR, Miles CW, et al. the Longitudinal Assessment of Bariatric Surgery Consortium Writing Group. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;36:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer J. After weight-loss surgery, some find new addictions. Wall Street Journal. 2006 [Google Scholar]
- 7.Sogg S. Alcohol misuse after bariatric surgery: Epiphenomenon or “Oprah” phenomenon? Surg Obes Relat Dis. 2007;3:366–368. doi: 10.1016/j.soard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Müller A, Mühlhans B, Silbermann A, et al. Compulsive buying and psychiatric comorbidity. Psychother Psychosom Med Psychol. 2009;59:291–299. doi: 10.1055/s-2008-1067438. [DOI] [PubMed] [Google Scholar]
- 9.Moorehead MK, Alexander CL. Transfer of addictive and preventive measures in bariatric surgery: Part II. Bariatric Times. 2011 [Google Scholar]
- 10.Moorehead M, Alexander C. Transfer of addiction and considerations for preventive measures in bariatric surgery. Bariatric Times. 2007;4:22–24. [Google Scholar]
- 11.Karim R, Chaudhri P. Behavioral addictions: An overview. J Psych Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- 12.Berczik K, Szabo A, Griffiths MD, et al. Exercise addiction: Symptoms, diagnosis, epidemiology, and etiology. Sub Use Misuse. 2012;47:403–417. doi: 10.3109/10826084.2011.639120. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell JE, Lancaster KL, Burgard MA, et al. Long-term follow-up of patients’ status after gastric bypass. Obes Surg. 2001;11:464–468. doi: 10.1381/096089201321209341. [DOI] [PubMed] [Google Scholar]
- 14.Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM. Alcohol abuse and dependence before and after bariatric surgery: A review of the literature and report of a new data set. Surg Obes Relat Dis. 2008;4:647–650. doi: 10.1016/j.soard.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg. 2012;22:201–207. doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- 16.Fogger SA, McGuinness TM. The relationship between addictions and bariatric surgery for nurses in recovery. Persp Psychi Care. 2012;48:10–15. doi: 10.1111/j.1744-6163.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 17.King W, Chen J, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagedorn JC, Encarnacion B, Brat GA, Morton JM. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 2007;3:543–548. doi: 10.1016/j.soard.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Klockhoff H, Näslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54:587–591. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: A case-crossover trial. J Am Coll Surg. 2011;21:209–214. doi: 10.1016/j.jamcollsurg.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Avena NA, Gold MS. Sensitivity to alcohol in obese patients: a possible role for food addiction. J Am Coll Surg. 2011;5:451. doi: 10.1016/j.jamcollsurg.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Steffen KJ, Engel SG, Pollert GA, Li C, Mitchell JE. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:470–473. doi: 10.1016/j.soard.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson P, Anveden A, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish Obese Subjects Study. Obesity. 2013;21:2444–2451. doi: 10.1002/oby.20397. [DOI] [PubMed] [Google Scholar]
- 24.Östlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148:374–377. doi: 10.1001/jamasurg.2013.700. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt F, Körber S, de Zwaan M, Müller A. Impulse control disorders in obese patients. Eur Eat Disorders Rev. 2012;20:144–147. doi: 10.1002/erv.2162. [DOI] [PubMed] [Google Scholar]
- 26.Belle S. The NIDDK Bariatric Surgery Clinical Research Consortium (LABS) Surg Obes Relat Dis. 2005;1:145–147. doi: 10.1016/j.soard.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Oges Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition (SCIP-I/P, Version 2.0) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 29.Klein DA, Bennett AS, Schebendach J, Foltin RW, Devlin MJ, Walsh BT. Exercise “addiction” in anorexia nervosa: Model development and pilot data. CNS Spectr. 2004;9:531–537. doi: 10.1017/s1092852900009627. [DOI] [PubMed] [Google Scholar]
- 30.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: the Alcohol Use Disorders Identification Test. Guidelines for use in primary care. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 31.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AL, Ware JE. Measuring functioning and Well-Being: the medical outcomes study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 33.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 34.Brummett BH, Babyak MA, Barefoot JC, et al. Social support and hostility as predictors of depressive symptoms in cardiac patients one month after hospitalization: a prospective study. Psychosom Med. 1998;60:703–713. doi: 10.1097/00006842-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Brummett BH, Babyak MA, Siegler IC, et al. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and non-caregivers. Health Psychol. 2006;25:220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 37.Herpertz S, Burgmer R, Stang A, de Zwaan M, Wolf AM, Chen-Stute A, et al. Prevalence of mental disorders in normal-weight and obese individuals with and without weight loss treatment in a German urban population. J Psychsom Res. 2006;61:95–103. doi: 10.1016/j.jpsychores.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.de Girolamo G, Polidori G, Morosini P, Scarpino V, Reda V, Serra G. Prevalence of common mental disorders in Italy: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) Soc Psychiatry Psychiatr Epidemiol. 2006;41:853–861. doi: 10.1007/s00127-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 39.Koran LM, Faber RJ, Aboujaoude E, Large MD, Serpe RT. Estimated prevalence of compulsive buying behavior in the United States. Am J Psych. 2006;163:1806–1812. doi: 10.1176/ajp.2006.163.10.1806. [DOI] [PubMed] [Google Scholar]