Abstract
Various aspects of immune response exhibit 24-hour variations suggesting that infection susceptibility and treatment efficacy may vary by time of day. Whether these 24-hour variations are endogenous or evoked by changes in environmental or behavioral conditions is not known. We assessed the endogenous circadian control and environmental and behavioral influences on ex-vivo lipopolysaccharide stimulation of whole blood in thirteen healthy participants under 48 hours of baseline conditions with standard sleep-wake schedules and 40–50 hours of constant environmental and behavioral (constant routine; CR) conditions. Significant 24-hour rhythms were observed under baseline conditions in Monocyte Chemotactic Protein, Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin 8 but not Tumor Necrosis Factor alpha whereas significant 24-hour rhythms were observed in all four immune factors under CR conditions. The rhythm amplitudes, expressed as a percentage of mean, were comparable between immune factors and across conditions. In contrast, the acrophase time (time of the fitted peak) was different between immune factors, and included daytime and nighttime peaks and changes across behavioral conditions. These results suggest that the endogenous circadian system underpins the temporal organization of immune responses in humans with additional effects of external environmental and behavioral cycles. These findings have implications for understanding the adverse effects of recurrent circadian disruption and sleep curtailment on immune function.
Keywords: Human, circadian, sleep, immune response, cytokine, chemokine, lipopolysaccharide, melatonin
1. INTRODUCTION
Various immune factors have been shown to exhibit daily rhythms in both humans and animal models including lymphocyte proliferation (Esquifino et al., 1996), number of leukocytes, hematocrit, and white blood cell subsets (Abo et al., 1981; Born et al., 1997; Kawate et al., 1981) and cytokine levels (Born et al., 1997; Young et al., 1995). In addition, a time-of-day dependent modulation has been reported for infection susceptibility (Shackelford and Feigin, 1973), survival rate after endotoxic shock in mice (Halberg et al., 1960), disease severity in rheumatoid arthritis (Cutolo, 2012) and adverse cardiovascular events and asthma (Litinski et al., 2009). The immune system in mice (Gibbs et al., 2012), Drosophila (Stone et al., 2012) and the flowering plant Arabidopsis (Wang et al., 2011a) is regulated by endogenous circadian clocks, suggesting that the endogenous clock-based regulation of immune response is conserved across phyla. In addition, recent studies in mice have demonstrated the circadian regulation of innate immune response in the presence of LPS, and that it is mediated by macrophage-specific molecular clocks (Gibbs et al., 2012; Keller et al., 2009).
An evolutionarily conserved molecular clock is also found in human peripheral blood mononuclear cells (PBMCs), which include most immune cell types. The phase of gene expression in these PBMC clocks is sensitive to light/dark schedules (Boivin et al., 2003; James et al., 2007). This suggests that these molecular clocks in the PBMCs may be controlled by the central circadian pacemaker, which drives endogenously generated rhythms that persist in the absence of external or behavioral time cues (Mills, 1966; Pittendrigh, 1960). Whether innate immunity in humans is also controlled by the circadian pacemaker remains to be elucidated, however. Nycthemeral (24-hour) rhythms that are observed under normal conditions may not be intrinsic, and therefore not circadian, if they are induced by external cycles such as the light/dark cycles, sleep, posture and activity. Prior studies in humans of immune response and/or basal immune function rhythmicity have not controlled for external factors such as sleep or meal times (e.g., (Born et al., 1997; Young et al., 1995)), and therefore have not determined whether immune function is intrinsically circadian. For example, circulating cytokine levels in the presence of LPS can be altered based on meal frequency (Dixit et al., 2011; Manning et al., 2008) and sleep (Born et al., 1997) that could influence their rhythmic expression patterns.
The aim of the study was to assess whether cytokine and chemokine levels in the presence of LPS exhibit endogenous circadian rhythmicity in humans. Our primary interest was in Monocyte Chemotactic Protein (MCP-1) as it has been shown to be regulated by the molecular clock (Gibbs et al., 2012; Hayashi et al., 2007; Sato et al., 2014) and has been implicated in diverse health disorders including diabetes (Kang et al., 2010; Seok et al., 2013) and cardiovascular disease (Niu and Kolattukudy, 2009; Seijkens et al., 2014), which are common in shift work that induces recurrent circadian disruption (Puttonen et al., 2011; Scheer et al., 2009; Wang et al., 2011b). Therefore we wanted to establish if MCP-1 is endogenously circadian, which could make MCP-1 a potential target of circadian disruption leading to the adverse health effects associated with MCP-1 function. We studied Interleukin 8 (IL-8) as a secondary marker of chemokine response and because previous studies have suggested that it is not under the control of the circadian molecular clock but does demonstrate rhythmicity in vivo (Gibbs et al., 2012). As a classical marker of LPS induced cytokine response, we studied Tumor Necrosis Factor alpha (TNF-α) and as a secondary cytokine marker we studied Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF). In addition, GM-CSF expression was shown to be disrupted by recurrent circadian misalignment (Castanon-Cervantes et al., 2010). We employed the gold-standard method for determining circadian rhythmicity, namely the constant routine (CR) protocol (Duffy and Dijk, 2002; Mills et al., 1978), and assessed two cytokines (TNF-α and GM-CSF) and two chemokines (IL-8 and MCP-1) every four hours over a 40 to 50-hour CR protocol. We also measured the same parameters under standard sleep-wake baseline conditions with standard sleep-wake schedules conditions to assess potential differences in circadian versus evoked characteristics of the rhythms.
2. METHODS
We studied 13 healthy participants (mean age ± SD = 24.5 ± 3.1 years; range 20–30 years; six females, three in follicular and three in luteal phase determined by self-report) in the Intensive Physiology Monitoring Unit in the Center for Clinical Investigation at Brigham and Women’s Hospital. The study was approved by the institutional Human Research Committees at Brigham and Women’s Hospital, and participants gave their written informed consent. All participants were healthy as determined by comprehensive physical, psychological and ophthalmologic exams. A modified version of the Horne and Ostberg’s Morningness-Eveningness Questionnaire (Horne and Östberg, 1976) was used to exclude subjects with extreme chronotypes (excluded: 70<score<30; mean ± SD chronotype score 53.1 ± 6.9). Potential participants were also excluded for night/shift work in the past 3 years or travel across more than two time zones in the previous 3 months. To stabilize circadian rhythmicity before starting the inpatient phase of the study, all participants maintained a self-selected, constant 8 h sleep/rest/dark schedule confirmed with calls to a time- and date-stamped voicemail at bedtime and wake time for 3 weeks and actigraphy (Actiwatch-L, Minimitter, Inc., Bend, OR) for at least 1 week prior to entering the unit. The average (± SD) for bedtime (2329 ± 0:58h) and waketime (7:26 ± 0:55 h) and the average duration of sleep (7:56 ± 0:15 h) during the last seven days prior to admission were used to calculate the scheduled sleep times maintained during the inpatient portion of the study and are referenced as scheduled sleep. Participants were instructed to refrain from use of prescription or nonprescription medications, supplements, recreational drugs, caffeine, alcohol, or nicotine. Compliance was verified by urine toxicology tests during screening and upon starting the inpatient phase of the study.
2.1 Study Protocol
Participants were studied for 7 or 9 days in an environment free of time cues (no access to windows, clocks, watches, live television, radio, internet, telephones, and newspapers and continually supervised by staff trained not to reveal information about the time of day). We analyzed the first 5 days of the protocol, that were identical for both the 7- and 9-day studies. Both protocols consisted of a 3-day baseline (8 h:16 h sleep:wake cycle based on average sleep times in the 7 days prior to study entry). The baseline days were followed by a 40-h CR in the 7-day study and a 50-h and 10 min CR in the 9-day study (Figure 1). During the CR episodes, participants were asked to remain awake while supervised in constant dim light in a semirecumbent posture, with daily nutritional intake divided into hourly portions (150 mEq Na+/100 mEq K+ (± 20%) controlled nutrient, isocaloric [basal energy expenditure x 1.3] diet, 2000–2500 mL fluids/24 h) (Lockley et al., 2006).
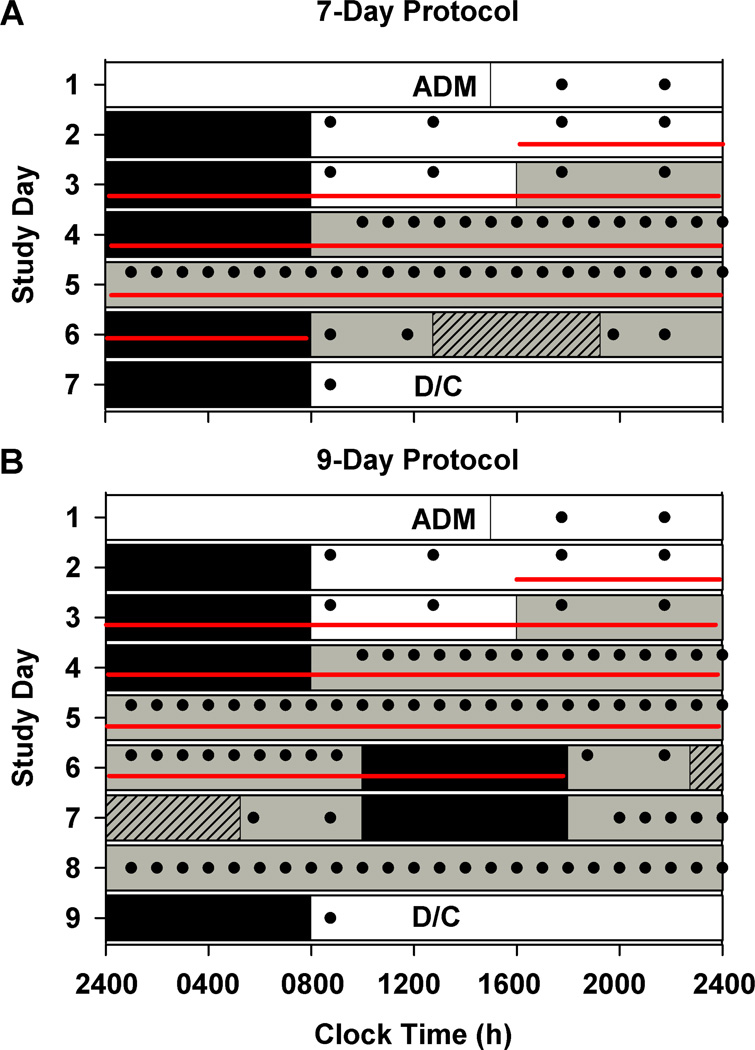
Figure 1. Study protocol to assess daily rhythms under baseline (standard sleep-wake schedules) conditions and endogenous circadian rhythmicity of innate immune response in humans.
This is an example protocol for a participant with a bedtime of midnight. Participants were enrolled in a 7-day (n = 3; A) or 9-day (n = 10; B) inpatient protocol in an environment free of time cues. White bars indicate exposure to ambient room light (~90 lux) and gray bars indicate exposure to dim ambient light (<3 lux). Black bars show scheduled sleep episodes in darkness. The schedule consisted of a 3-day baseline including admission (ADM), with 8:16 h sleep:wake cycle (based on average sleep times in the 7 days prior to study entry) and four meals per 24-hours (filled circles); an initial 40 h (A) or 50 h and 10 min (B) constant routine under dim light, semi-recumbent constant posture, forced wakefulness, and hourly space isocaloric meals (filled circles), a 16 h light exposure day (gray hatched bar); followed by 8 h sleep and then discharge (A) or a second 29 h 50 min constant routine followed by an 8 h sleep episode and then discharge (D/C) (B). In the present study data were analyzed from the baseline and initial CR components only using blood samples collected at 4 h intervals shown by the red lines.
During the first 2.5 baseline days, maximum ambient light during scheduled wake was 48 µW/cm2 (~190 lux) when measured in the horizontal plane at a height of 187 cm and 23 µW/cm2 or (~88 lux) when measured in the vertical plane (137 cm). Midway through day 3, maximum ambient light was decreased to 0.4 µW/cm2 (< 3 lux; ~1.5 lux) when measured in the horizontal plane and 0.1 µW/cm2 (~0.6 lux) when measured in the vertical plane and maintained at that level for the remainder of the study. Room light was switched off during scheduled bedrest episodes. Ambient room lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/HO/EW, 95W; F32T8/ADV841/A, 32W; F25T8/TL841, 25W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA). Routine illuminance and irradiance measures were conducted using an IL1400 radiometer/powermeter with an SEL-033/Y/W or SEL-033/F/W detector, respectively (International Light, Inc., Newburyport, MA).
2.2 Cytokine and chemokine measurement
Whole blood was drawn every 4 hours through an indwelling cannula in a forearm vein kept patent via a heparinized saline infusion (5 IU heparin/mL 0.45% NaCl, infused at 40 mL/h). Blood collection started 8 h after wake time on baseline Day 2 and continued throughout the CR and subsequent recovery sleep episode on Day 6 (Figure 1). We used a validated ex-vivo whole-blood LPS challenge assay to investigate the circadian regulation of LPS induced cytokine and chemokine production in humans (Born et al., 1997) although we used a shorter incubation time, which has been validated in murine models (Adams et al., 2013) and room temperature incubation as described below Samples of whole blood (500 µl) were collected in EDTA-coated tubes containing 31.25, 62.5 or 625 µg of LPS from Escherichia coli O111:B4 (SIGMA, Saint Louis, MI, USA) diluted in RPMI 1640 medium (ATCC, Manassas, VA, USA) supplemented with 2mM L-Glutamine and 10mM HEPES [final LPS concentration was 50 µg/ml (n = 3); 100 µg/ml (n = 8); and 1000 µg/ml (n = 2)] and incubated at room temperature (RT) for 4 h. Incubation was stopped by centrifuging the sample at 1500 g x 10 minutes at 4°C and then immediately freezing the samples at −20° C for storage prior to batch assays at a later time. The different doses of LPS were used to construct a dose response curve to determine the optimum dose for LPS challenge. Plasma samples were analyzed in duplicate for the expression of multiple cytokines using a Milliplex MAP kit (Millipore, Bedford, MA; USA) ran on a Luminex xMAP platform per manufacturer’s instructions. For each cytokine measured, the assay had a detection range from 0.64 to 10000 pg/mL. The intra-assay coefficient of variation (%CV) generated as the mean of the %CV’s from eight reportable results across two different concentration of cytokines in one experiment was 7.1% for IL-8, 10.5% for TNF-α, 6.1% for MCP-1 and 10.4% for GM-CSF. Inter-assay %CV generated as the mean of the %CV’s from two reportable results each for two different concentrations of cytokine across four different experiments was 11.6% for IL-8, 15.9% for TNF-α, 12.0% for MCP-1 and 12.6% for GM-CSF.
2.3 Circadian Phase Markers
Core body temperature (CBT) was measured throughout the protocol via a rectal thermistor (Yellow Springs Instruments Inc., Yellow Springs, OH) with temperature values recorded every minute. This variable was used for estimation of circadian phases associated with the blood samples. In addition, the dim light melatonin onset (DLMO) was used as an additional circadian phase marker based on plasma melatonin collected every 30–60 minutes from the second baseline wake episode until the end of the study. Melatonin concentration was determined by double-antibody radioimmunoassay with the Kennaway G280 antiserum (Vaughan, 1993) by a laboratory blind to experimental conditions (Specialty Assay Research Core Laboratory, Brigham and Women’s Hospital, Boston, MA). The plasma melatonin intra-assay coefficient of variation (%CV) was 10.0% at 1.9 pg/mL and 7.2% at 21.9 pg/mL, and the interassay %CV was 12.65% at 3.06 pg/mL and 12.12% at 22.36 pg/mL.
2.4 Data Analysis
Data are expressed as mean ± SEM unless otherwise specified. The first 5 h of data from the CR were excluded from analysis to eliminate transitory masking effects from the prior sleep episode and posture changes (Brown and Czeisler, 1992). Values greater than 3 standard deviations of the mean were removed for each immune factor (Scheer et al., 2010), leading to 2.1% loss in total data. The cytokine and chemokine levels were significantly different between individuals independent of differences in LPS concentration (p<0.01; one-way mixed model analysis; Supplemental Figure 1). Therefore, data were transformed to Z-scores and percentage-of-mean as appropriate for the type of analysis. Transformed values were not different between individuals (Supplemental Figure 1). While there was a significant effect of LPS concentration on the absolute levels of cytokine and chemokine expression (p<0.01; one-way mixed model analysis; Supplemental Figure 2), the transformed data did not show a significant dose response (Supplemental Figure 2) therefore subsequent analysis was performed on pooled data.
Two different types of analyses were performed to assess (i) the endogenous circadian regulation and (ii) the differences in immune response under standard sleep-wake/baseline and CR conditions. In both analyses, all individual values were assigned a time relative to scheduled wake time for each individual. Values were binned every 4-hours within each individual and values from matching bins were averaged across individuals for group analyses. For each individual, each cytokine and chemokine value was first transformed to a percentage of the mean calculated as the average concentration over the entire 96-h collection interval (Scheer et al., 2010). The transformed values were then analyzed separately for baseline and constant routine conditions, with data from the first 48 h of the 96-h profiles as baseline and data from the next 48 h as constant routine. CBT and melatonin data were Z-transformed (Chua et al., 2013) before fitting with a single harmonic cosinor regression model with one 24-h fundamental component and a linear term [Y= µ + A*cos*((2π(x− φ))/24) + ((m*x)+b)] or a dual harmonic cosinor regression model with one 24-h fundamental component and 12-h harmonic component [Y = µ + (A*cos*((2π(x−φ))/24)) + (A*cos*((2π(x- φ))/12)) + ((m*x)+b)], where µ = Mesor, A = amplitude, φ = acrophase, m= gradient of the linear term, b = vertical intercept of the linear term. The regression was considered significant if the amplitude was significantly different from 0. Whenever a significant nadir was detected by the regression model then the acrophase time was calculated as the peak 12-hours earlier for a single harmonic model or 6-hours earlier for a dual harmonic model. A linear component was included in the model to estimate the influence of secular changes associated with the CR protocol (e.g., effects of accumulating sleep loss, time in semi-recumbent posture, repetitive meals, etc.). Residual error was assumed to be independent and to have a normal distribution εi ~ N(0,σ2) (Fortier et al., 2011). If the linear or harmonic components were not significant, a simpler single harmonic cosinor regression model with one 24-h fundamental component was used. Therefore, four different regression models were used in total: (i) single harmonic (ii) single harmonic with linear component (iii) dual harmonic (iv) dual harmonic with linear component. If multiple models were significant or none of the models were significant then the final model was selected based on the Akaike Information Criterion results.
Differences in rhythm acrophases and amplitudes between different immune factors using values from each individual were assessed using one-way General Linear Model analysis followed by Student-Newman-Keuls post-hoc analysis under baseline and CR conditions separately. Differences in rhythm acrophases and amplitudes between different immune factors using values from group fitted results were assessed using one-way ANOVA followed by Student-Newman-Keuls post hoc analysis under baseline and CR conditions separately.
To assess the differences in immune response under standard sleep-wake/baseline and CR conditions Z-transformed data were subjected to repeated-measures, random intercept two-way mixed model analysis of variance with restricted maximum likelihood (REML) estimates of the variance components. Data were analyzed for the main and interaction effects of time awake and behavioral state (baseline and CR).
The estimated circadian phase of the core body temperature minimum (CBTmin) was evaluated from data collected during the CR using the previously validated method of fitting a dual-harmonic regression with correlated noise model (Brown and Czeisler, 1992). Melatonin phase was assessed as the dim light melatonin onset (DLMOn) defined as the clock time at which the melatonin rhythm crossed a threshold value of 25% of the three-harmonic peak-to-trough fitted amplitude (half the standard amplitude) (Gooley et al., 2010). The cosinor regression models used to fit the immune data were also applied to core body temperature and plasma melatonin. These values were then compared with CBTmin and DLMOn assessments derived using validated methods for assessing each of these parameters (Brown and Czeisler, 1992; Gooley et al., 2010). Melatonin rhythm acrophases derived using the current cosinor regression models and DLMOn values derived using the previously validated method were significantly correlated (p<0.01; Pearson r2=0.90) and CBT rhythm acrophases derived using the current cosinor regression models and CBTmin values derived using the previously validated method were also significantly correlated (p<0.01; Pearson r2=0.81). All linear statistical analyses were computed using SAS version 9.2 (Cary, NC, USA) and all circular statistical analysis used to compute group temporal means was computed using R 3.1.0 (2014) with the packages psych and circular. For all statistical analyses, significance was set to p<0.05.
3. RESULTS
3.1 Regression model selection
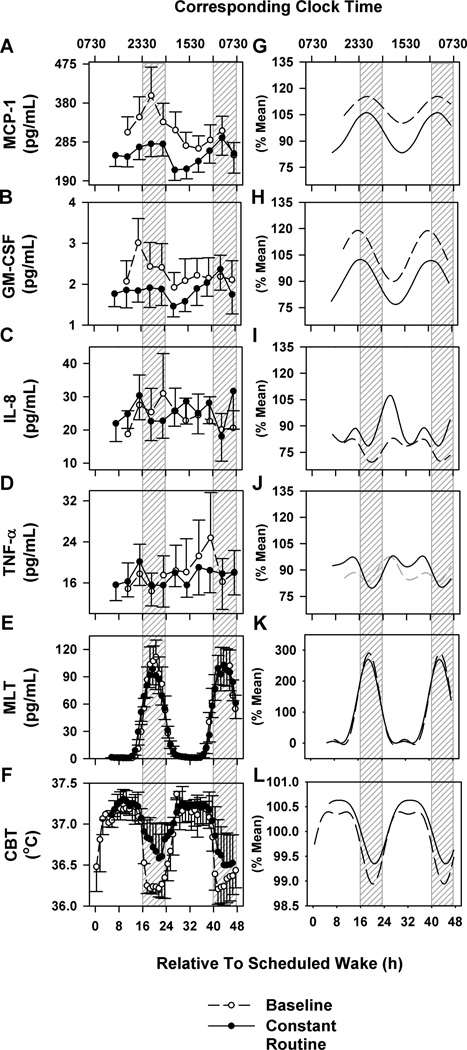
The immune factors IL-8, TNF-α, and MCP-1 were detected in all 13 participants, whereas only 8 participants had detectable GM-CSF. Under baseline conditions immune measures could not be assessed for two individuals due to missing blood samples. The absolute rhythm profiles under standard sleep-wake (48 h) and CR (48 h) conditions are shown in Figure 2A–F. The complementary profiles shown in Figure 2G–L were derived from transformed data and fitted with cosinor regression models.
Figure 2. Daily baseline and circadian rhythms in LPS induced cytokine and chemokine production in humans.
Group data mean ± SEM (left panel) and group-mean fitted cosinor functions under baseline conditions with standard sleep-wake schedules (dashed lines) and constant conditions (solid lines) of MCP-1 (A, G), GM-CSF (B, D), IL-8 (C, I), TNF-α (D, J), plasma melatonin (E, K) and core body temperature (CBT; F, L). Non-significant regression fits are marked in gray lines. Corresponding clock times are reported relative to scheduled wake. Time = 0 relative to scheduled wake was defined as 0730 h based on the group mean (mean ± SD: 0727 ± 0056 h) for illustrative purposes. Hashed gray bars represent group mean scheduled sleep times. Throughout the constant routine participants remained awake.
In fitting the four regression models described earlier, only a minority (≤ 50%) of individual rhythms had improved fits when including the linear component under CR (Percent subjects with a significant linear component in the cosinor regression model: MCP-1 30.8%; GM-CSF 12.5%; IL-8 23.1%; TNF-α 38.5%; Supplemental Table 1) and therefore the linear component was removed and only the single or dual harmonic cosinor regression models used. Under baseline conditions, the majority of individual rhythms were best modeled with a dual harmonic regression (Percent subjects: MCP-1 63.6%; GM-CSF 50.0%; IL-8 54.6%; TNF-α 63.6%; Supplemental Table 1). In contrast, under CR conditions, the majority of the individual rhythms for immune factors except TNF-α were best modeled with a single harmonic regression (MCP-1 61.5%; GM-CSF 62.5%; IL-8 69.2%; TNF-α 38.5%; Supplemental Table 1). Therefore, the dual harmonic regression model was used for all individuals for all measures under baseline conditions, and the single harmonic regression used under CR conditions, except for TNF-α which was modeled with dual harmonic regression under both baseline and CR conditions.
The immune rhythm acrophases and amplitudes from individual profiles, the group mean values (averaged across values derived from individual profiles) and group fitted values (regression models applied to the cytokine and chemokine group average profiles) are shown under baseline conditions in Table 1 and under CR conditions in Table 2.
Table 1.
Acrophase times and amplitudes of the individual and population fitted curves of immune parameters under standard sleep-wake conditions*.
| MCP-1 | GM-CSF | IL-8 | TNF-α | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | WAKE TIME (hh:mm) |
CBT PH (hh:mm) |
MEL PH (hh:mm) |
PH (hh: mm) |
AMP (%) |
P | PH (hh: mm) |
AMP (%) |
p | PH (hh: mm) |
AMP (%) |
p | PH (hh: mm) |
AMP (%) |
p |
| 3090V | 8:59 | 6:26 | 23:50 | 2:40 | 25.75 | <0.01 | 14:34 | 21.23 | 0.21 | 4:52 | 31.27 | 0.14 | |||
| 30H6V | 6:30 | 4:39 | 21:28 | 2:38 | 8.90 | 0.19 | 1:35 | 10.01 | 0.17 | 5:07 | 42.61 | <0.01 | 5:04 | 35.63 | <0.01 |
| 3174V | 8:00 | 4:40 | 23:57 | ||||||||||||
| 3175V | 8:09 | 4:50 | 23:29 | 9:56 | 12.28 | 0.13 | 23:33 | 32.24 | 0.03 | 23:19 | 42.35 | 0.11 | 10:46 | 32.34 | <0.01 |
| 3176V | 6:39 | 3:14 | 20:16 | ||||||||||||
| 3177V | 7:58 | 2:28 | 20:34 | 4:43 | 9.30 | 0.37 | 2:55 | 25.80 | 0.01 | 1:43 | 30.95 | 0.02 | 1:39 | 36.72 | 0.03 |
| 3187V | 8:00 | 3:58 | 21:18 | 3:17 | 18.34 | 0.06 | 3:58 | 24.40 | 0.04 | 15:24 | 33.83 | 0.32 | 7:40 | 13.63 | 0.16 |
| 3188V | 6:04 | 3:48 | 22:13 | 0:17 | 14.99 | 0.01 | 6:02 | 14.19 | 0.14 | 5:34 | 28.61 | 0.01 | |||
| 3197V | 5:52 | 2:16 | 19:59 | 13:59 | 15.80 | 0.07 | 22:12 | 17.67 | 0.03 | 8:16 | 51.15 | 0.03 | 8:56 | 46.02 | 0.04 |
| 3198V | 8:05 | 4:42 | 22:28 | 3:56 | 11.53 | <0.01 | 3:09 | 21.82 | 0.24 | 15:18 | 20.98 | 0.19 | |||
| 31A1V | 7:39 | 4:39 | 21:59 | 3:50 | 11.14 | <0.01 | 22:37 | 14.68 | 0.01 | 12:20 | 18.07 | 0.01 | 12:51 | 15.93 | 0.05 |
| 31A2V | 8:02 | 4:50 | 22:12 | 1:48 | 9.03 | <0.01 | 9:27 | 26.58 | 0.13 | 4:31 | 26.93 | 0.09 | |||
| 31A8V | 6:55 | 5:30 | 22:39 | 9:01 | 14.08 | 0.09 | 20:04 | 32.56 | <0.01 | 19:27 | 49.72 | 0.01 | |||
|
GROUP MEAN ± SEM |
7:27 ± 0:15 |
4:18 ± 0:19 |
22:02 ± 0:21 |
4:11 ± 0:18 |
13.74 ± 1.51 |
0:46 ± 0:14 |
20.80 ± 3.33 |
6:49 ± 0:32 |
30.48 ± 3.47 |
7:11 ± 0:24 |
30.71 ± 3.41 |
||||
|
GROUP FITTED ± SEM |
2:07 ± 0:18 |
6.56 ± 0.81 |
<0.01 | 23:26 ± 1:15 |
14.51 ± 5.38 |
0.02 | 9:55 ± 0:52 |
4.53 ± 1.87 |
0.04 | 9:25 ± 1:26 |
4.77 ± 3.59 |
0.21 | |||
Group mean values are the average acrophase and amplitude values of each immune factor across the number of participants studied. Group fitted values are the estimated acrophase and amplitude values of each immune factor derived from the cosinor analysis of the group data. All times are reported in clock time referenced from each individual’s scheduled wake time. PH = phase; AMP = amplitude; CBT = core body temperature; MEL = melatonin. No samples were collected from 3176V and 3174V under baseline conditions due to IV failure.
Table 2.
Acrophase times and amplitudes of the individual and population fitted curves of immune parameters under constant routine conditions*.
| MCP-1 | GM-CSF | IL-8 | TNF-α | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | WAKE TIME (hh:mm) |
CBT PH (hh:mm) |
MEL PH (hh:mm) |
PH (hh: mm) |
AMP (%) |
p | PH (hh: mm) |
AMP (%) |
p | PH (hh: mm) |
AMP (%) |
p | PH (hh: mm) |
AMP (%) |
p |
| 3090V | 8:59 | 6:26 | 23:50 | 3:46 | 5.68 | 0.29 | 5:01 | 75.75 | 0.01 | 2:27 | 62.14 | 0.1 | |||
| 30H6V | 6:30 | 4:39 | 21:28 | 2:05 | 22.19 | <0.01 | 5:09 | 32.58 | 0.01 | 0:25 | 31.46 | 0. 0 7 | 5:01 | 18.58 | 0.0 |
| 3174V | 8:00 | 4:40 | 23:57 | 0:50 | 17.84 | <0.01 | 23:59 | 11.98 | 0.13 | 10:45 | 23.31 | 0.05 | 10:08 | 27.94 | 0.0 |
| 3175V | 8:09 | 4:50 | 23:29 | 5:57 | 6.94 | <0.01 | 2:00 | 29.64 | 0.02 | 10:44 | 31.11 | 0.14 | 10:11 | 38.67 | 0.0 |
| 3176V | 6:39 | 3:14 | 20:16 | 0:17 | 14.70 | 0.02 | 9:41 | 30.15 | 0.01 | 11:29 | 45.74 | <0.01 | 11:28 | 35.45 | 0.0 |
| 3177V | 7:58 | 2:28 | 20:34 | 22:22 | 10.94 | 0.01 | 22:37 | 16.96 | 0.03 | 11:42 | 25.54 | 0.18 | 10:48 | 22.64 | 0.2 |
| 3187V | 8:00 | 3:58 | 21:18 | 5:01 | 16.17 | <0.01 | 3:59 | 17.40 | 0.02 | 11:10 | 129.62 | 0.02 | 12:30 | 45.60 | 0.0 |
| 3188V | 6:04 | 3:48 | 22:13 | 5:11 | 13.97 | 0.06 | 17:06 | 61.81 | 0.10 | 11:23 | 29.11 | 0.1 | |||
| 3197V | 5:52 | 2:16 | 19:59 | 23:53 | 16.94 | 0.02 | 23:17 | 22.42 | 0.03 | 21:40 | 36.86 | 0.03 | 11:47 | 10.44 | 0.3 |
| 3198V | 8:05 | 4:42 | 22:28 | 4:11 | 13.88 | 0.04 | 7:58 | 16.96 | 0.15 | 8:29 | 7.30 | 0.2 | |||
| 31A1V | 7:39 | 4:39 | 21:59 | 5:03 | 6.03 | 0.03 | 0:04 | 14.64 | 0.02 | 0:25 | 26.39 | <0.01 | 0:57 | 27.38 | <0.0 |
| 31A2V | 8:02 | 4:50 | 22:12 | 1:40 | 8.65 | 0.19 | 12:02 | 39.41 | 0.05 | 11:38 | 26.40 | 0.0 | |||
| 31A8V | 6:55 | 5:30 | 22:39 | 2:56 | 3.64 | 0.69 | 16:21 | 9.20 | 0.23 | 20:22 | 21.06 | 0.1 | |||
|
GROUP MEAN ± SEM |
7:27 ± 0:15 |
4:18 ± 0:19 |
22:02 ± 0:21 |
2:45 ± 0:10 |
12.12 ± 1.56 |
1:47 ± 0:19 |
21.97 ± 2.80 |
11:21 ± 0:25 |
42.55 ± 8.79 |
9:57 ± 0:19 |
28.67 ± 4.04 |
||||
|
GROUP FITTED ± SEM |
1:58 ± 0:45 |
11.44 ± 2.22 |
<0.01 | 0:19 ± 0:59 |
12.93 ± 3.62 |
<0.01 | 9:48 ± 0:46 |
9.41 ± 3.99 |
0.04 | 9:54 ± 0:56 |
6.16 ± 2.68 |
0.0 | |||
Group mean values are the average acrophase and amplitude values of each immune factor across the number of participants studied. Group fitted values are the estimated acrophase and amplitude values of each immune factor derived from the cosinor analysis of the group data. All times are reported in clock time referenced from each individual’s scheduled wake time. PH = phase; AMP = amplitude; CBT = core body temperature; MEL = melatonin.
3.2 Daily Rhythms in LPS induced cytokine and chemokine production under Baseline Conditions with Standard Sleep-Wake Schedules
Under baseline conditions with standard sleep-wake schedules there were significant 24-h rhythms observed in group fitted data in MCP-1 (p<0.01), GM-CSF and IL-8 (p<0.05) but not in TNF-α (Figure 2G–J; Table 1). Although the individual rhythms were best modeled with dual harmonic regression, the group fitted data for MCP-1 and GM-CSF were best modeled using single harmonic cosinor regression (Figure 2G and H) whereas IL-8 was best modeled using a dual harmonic cosinor regression with two distinct peaks (Figure 2I). MCP-1 and GM-CSF peaked at night within a few hours after bedtime at ~0200 h and ~2330 h respectively (Figure 2G and H) whereas IL-8, peaked twice with similar amplitudes, the first one a few hours after wake at ~1000 h and a second peak a few hours before bedtime at ~2100 h (Figure 2I). The nighttime acrophases of MCP-1 and GM-CSF were significantly different than the daytime acrophase of IL-8 (p<0.01) but MCP-1 and GM-CSF acrophases were not significantly different between each other (Figure 3A and Table 1). The rhythm amplitudes were not different between the different immune factors (p>0.05) (Table 1). Regression analysis restricted to samples stimulated with the same dose of 100 µg/ml (n = 8) showed similar rhythm characteristics (Supplemental Figure 3) as the rhythm profiles generated using all available data.
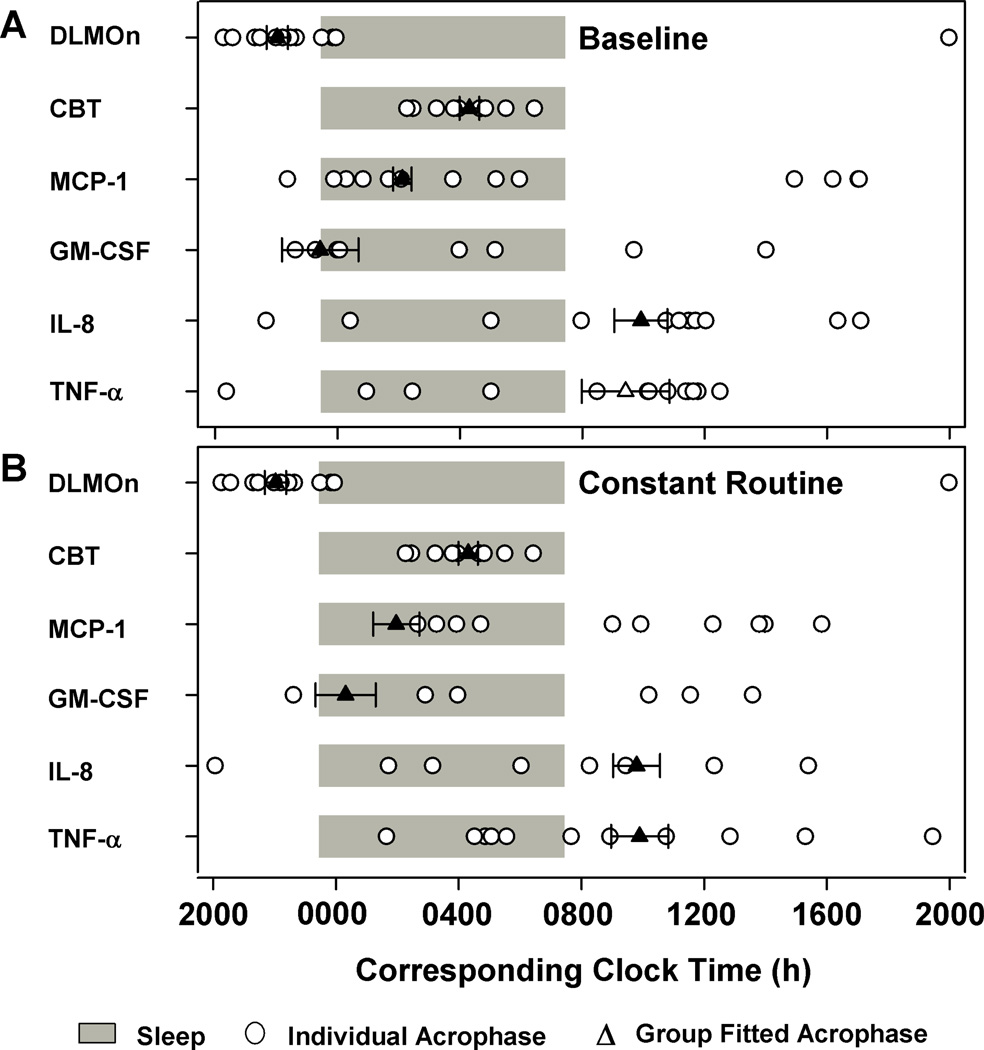
Figure 3. Relative phase distribution of endogenous circadian phase markers and immune parameters.
Transformed individual and group-mean data were fitted with single or dual harmonic cosinor regression to compute the acrophase of the rhythms under baseline standard sleep-wake (baseline; A) and constant routine conditions (B). Group mean (± SEM) sleep-wake times are represented by the gray bars. Group-fitted immune parameter acrophases, DLMOn and CBTmin from group-mean data are shown as solid symbols for significant results (▲) or empty symbols for non significant results (Δ) ± SEM. Individual immune rhythm acrophases are shown as filled white-circles (○). Individual values were assigned a time relative to scheduled wake time for each individual. Clock times are reported relative to scheduled wake. Time = 0 relative to scheduled wake was defined as 0730 h based on the group mean (mean ± SD: 0727 ± 0056 h).
At the individual level, the proportion of participants who exhibited significant 24-h rhythms under baseline standard sleep-wake conditions were 45% for MCP-1, 100% for GM-CSF, 36% for IL-8 and 27% for TNF-α (Table 1). Although most individuals had nocturnal peaks during sleep some individuals exhibited peaks a few hours before and after the sleep episode (Figure 3A and Table 1). In contrast to group fitted results, individual acrophase times and amplitudes revealed significant differences in amplitudes (p<0.01) but not in acrophase times (p>0.05) between immune parameters on average (Table 1). MCP-1 amplitude was significantly lower than IL-8 and TNF-α but not different as compared to GM-CSF (Table 1).
3.3 Circadian Rhythm in LPS Induced Cytokine and Chemokine Production
Under CR conditions there were significant 24-h nocturnal rhythms observed in group-fitted data in MCP-1, GM-CSF (p <0.01), IL-8 and TNF-α (p<0.05) (Figure 2G–J). MCP-1 and GM-CSF were best modeled with a single harmonic cosinor regression (Figure 2G and H) and IL-8 and TNF-α were best modeled using a dual harmonic cosinor regression (Figure 2I and J). Similar to the baseline condition, both MCP-1 and GM-CSF peaked during the biological night at ~0200 h and ~0000 h respectively, but IL-8 and TNF-α both peaked during the biological day, both at ~1000 h (Figure 3B and Table 2). The two nighttime acrophases were significantly different than the two daytime acrophases (p<0.01) but were not within each other (Figure 3B and Table 2). The amplitude of the cytokine rhythms were not significantly different. Similar to baseline conditions, regression analysis restricted to samples stimulated with the same dose of 100 µg/ml (n = 8) showed similar rhythm characteristics (Supplemental Figure 3) as the rhythm profiles generated using all available data.
At the individual level, the proportion of participants who exhibited significant 24-h rhythms under constant routine conditions were 69% for MCP-1, 87% for GM-CSF, 46% for IL-8 and 31% for TNF-α (Table 2). Similar to under baseline conditions, most individuals under CR conditions also had nocturnal peaks in LPS induced cytokine and chemokine production generally around the time of habitual sleep, although for IL-8 and TNF-α more individuals had daytime peaks occurring several hours after wake time (Figure 3B and Table 2). There were no significant differences in amplitudes or in acrophase times (p>0.05) between immune parameters (Table 2).
3.4 Effects of Sleep and Extended Wake on LPS induced cytokine and chemokine production
The prolonged wakefulness of the CR was not associated with a consistent linear change in LPS induced cytokine and chemokine production (Figure 2A–D). There was a significant main effect of time on MCP-1 (p<0.01) and GM-CSF (p<0.05), under both CR and standard sleep-wake conditions (Figure 2A and B), showing a decreasing trend with time under baseline conditions but an increasing trend under CR conditions. In addition, there was a significant main effect of behavioral state (baseline standard sleep-wake as compared to CR and extended wake) on MCP-1 and GM-CSF (p<0.01) with significantly lower levels overall during CR (Figure 2A).
4. DISCUSSION
In the current study we used an ex vivo assay to assess the potential for cytokine and chemokine production in the presence of the bacterial endotoxin LPS. The results demonstrate that the endogenous circadian pacemaker regulates LPS-induced expression of the pro-inflammatory cytokines TNF-α and GM-CSF and pro-inflammatory chemokines IL-8 and MCP-1 in humans. These rhythms are also apparent under normal sleep-wake conditions but behavioral and environmental cycles modulate the expression of the intrinsic circadian regulation.
By using the CR protocol, which is designed to remove or uniformly distribute external and behavioral influences that may affect the expression of endogenous rhythms (Czeisler and Klerman, 1999; Duffy and Dijk, 2002), we demonstrated that LPS induced cytokine and chemokine production rhythms are endogenously regulated. These rhythms are therefore not merely passive responses to changes in external or behavioral factors such as sleep-wake state, feeding/fasting, posture, activity and light but are internally generated by the circadian clock.
The extended CR procedure that we used also permits a comparison of rhythms under constant versus baseline conditions with standard sleep-wake schedules and identification of factors that may have a direct influence of immune rhythm expression. For example, MCP-1 induction appears suppressed under CR conditions as compared to standard sleep-wake conditions, and exhibits a different time course with time awake under the two conditions. While MCP-1 induction decreased with time under baseline conditions, MCP-1 induction increased with time under CR conditions. It is possible that the difference in time-dependent change in MCP-1 induction between CR and baseline conditions may be a time-into-study effect instead of time-awake, nonetheless the change was induced by transition from baseline to CR conditions, suggesting that behavioral factors including sleep deprivation can modulate the rhythms of LPS induced cytokine and chemokine production.
The effects of sleep on the immune system have been examined in several studies although the results are not always in agreement. Compared to extended wake, sleep has been associated with reduced counts of all lymphocyte subsets at specific times of the day in one study (Born et al., 1997), whereas another study reported increased monocyte count (Dinges et al., 1994) and a third found no differences in lymphocyte subsets studied under sleep and extended wake conditions (Palmblad et al., 1976). Moreover, the effects of sleep deprivation are not the same on all cytokines and chemokines (Born et al., 1997).
In addition to the adverse effects of sleep loss on immune function, recurrent circadian disruption with minimal sleep loss is associated with markedly altered immune response in mice (Brager et al., 2013; Castanon-Cervantes et al., 2010). Animals maintained under recurrent circadian disruption have heightened release of pro-inflammatory cytokines and four times greater mortality induced by endotoxic LPS challenge. Since the cytokines and chemokines studied in the present study have diverse roles in the inflammatory response cascade, their temporal relationship -insuring that internal events are timed appropriately relatively to each other - is likely functionally important. Internal circadian disruption, therefore, may alter this relative coordination and understanding the impact on health will be important in future studies.
Under constant conditions, the majority of the individual profiles were significantly modeled using a single-harmonic regression analysis rather than a dual-harmonic model, whereas under standard sleep-wake conditions majority of the individual profiles were significantly rhythmic using a dual-harmonic model with a 12-h component. These results support the hypothesis that as yet unidentified external factors induce direct effects on the timing of LPS induced cytokine and chemokine production in addition to the endogenous circadian regulation, as commonly observed for core body temperature, cortisol and other circadian rhythms.
Previous studies suggest that rhythmic changes in cell number may be one of the mechanisms controlling the circadian oscillations in cytokine and chemokine rhythms (Born et al., 1997). Endogenous glucocorticoid levels may also modulate the daily oscillations in chemokine and cytokine expression since glucocorticoid administration attenuates proinflammatory cytokine and chemokine expression (Cechin and Buchwald, 2014). The aim of the present study was to investigate whether daily rhythms in cytokine and chemokine expression are under endogenous regulation. Additional studies are required, however, to investigate a causative and mechanistic relationship between glucocorticoids, lymphocyte counts and other endocrine and/or molecular signals and daily rhythms in immune response.
Studies suggest that indwelling catheters can confound cytokine secretion, but it appears that the results vary depending on the type of cytokine studied. For example, IL-6 levels may be affected by the use of an indwelling catheter (Haack et al., 2002) whereas TNF-α may not (Haack et al., 2000). In our study, none of the parameters showed a sustained linear increase in levels as observed for IL-6 (Haack et al., 2002), even though the same catheter was used throughout the sampling interval. Therefore, it is unlikely that the temporal changes seen in the current study were substantially affected by the use of indwelling catheters. Cytokine secretion may be affected by heparin (Engstad et al., 1997; Patil et al., 2013), but only at supra-therapeutic doses (>10 IU/ml) (Hochart et al., 2008) whereas the heparin dose used in the present study was 5 IU/ml therefore, it is unlikely that the very low dose of heparin used in the current study may have substantially affected the results.
Previous work (De Groote et al., 1992) shows that IL-1β and IL-2, production is generally higher if isolated PBMC in vitro LPS stimulation assays are used, whereas IFN-γ and TNF-α is higher when whole blood stimulation assays are used. Importantly, the change in cytokine/chemokine production was in the same direction, with only the absolute levels differing, between in vivo and ex vivo assays. The ex vivo approach, however, preserves the cell-to-cell interactions and the ratio between concentrations of circulating stimulatory and inhibitory mediators, representing the natural in vivo state more closely, unlike the in vitro stimulation of isolated PBMCs. Therefore, the cytokine and chemokine production observed in the current study is likely a composite of the circadian changes in the production capacity of the effector cells, as has been shown previously (Gibbs et al., 2012; Hayashi et al., 2007; Keller et al., 2009), and the circadian changes in modulatory influences by other cells and cytokines. While the production levels may have been different if isolated effector cells were studied, the modulatory influences present in the ex vivo system reflect in vivo conditions more closely, however.
In addition, future studies are necessary to investigate the endogenous circadian rhythms in basal cytokine and chemokine expression and the putative role of the underlying basal rhythms on stimulated response rhythms. Daily oscillations in basal cytokine levels have been reported (Born et al., 1997; Young et al., 1995), but elicited responses as studied here may be more indicative of disease risk. The current results form the foundation of ongoing studies that seek to address whether shift work disease may be predicted and ameliorated by surveillance of these responses in peripheral blood, since such responses are dramatically enhanced following simulated shift work in animal studies (Castanon-Cervantes et al., 2010). Thus our measurement of LPS-elicited responses, rather than basal variation, represent a strength of our study.
Under standard sleep-wake conditions, significant daily rhythms have been reported for IL-8 (Hermann et al., 2006) and TNF-α (Straub and Cutolo, 2007) in response to LPS stimulation. Significant daily rhythms in basal non-stimulated levels have been reported also for TNF-α, and IL-6 (Vgontzas et al., 2003) and GM-CSF (Young et al., 1995). Significant daily oscillations have been detected in mice in response to LPS stimulation using an ex vivo protocol similar to the one used in the present study in IL-6, MCP-1 and TNF-α but not GM-CSF (Gibbs et al., 2012). In contrast to previous reports of daily oscillations in TNF-α, we were unable to detect a significant 24-h rhythm in TNF-α under standard sleep-wake conditions but did observe significant circadian oscillations in TNF-α under constant conditions. Differences in sampling frequency, method of LPS stimulation, and rhythmometric analysis may have contributed to this difference. Moreover, TNF-α was best modeled using dual-harmonic regression even under constant conditions suggesting that, besides the circadian rhythm, additional factors such as sleep-wake patterns, meal timing and activity levels may influence TNF-α, which can vary significantly between studies.
Our study shows that human immune function is regulated, at least in part, by the endogenous circadian system and that there is considerable inter-individual variation in rhythm characteristics and internal phase relationships. The inter-individual variations likely account for the large differences between the group-mean acrophase and amplitude values and the acrophase and amplitude values derived from the group fitted profile, under both the CR and baseline conditions with standard sleep-wake schedules. These variations have potentially important consequences for therapeutic interventions that may be optimized by incorporating an individuals’ circadian phase into treatment timing.
The large inter-individual differences observed in the current study is in agreement with a previous report of large inter-individual differences in rhythm characteristics in lipid profiles (Chua et al., 2013).Using a constant routine protocol similar to the one used in the current study, they showed that 13% of the 263 lipids assayed had circadian variation, but only ~20% of those rhythmic lipids were also rhythmic amongst all individuals, and the timing of lipid rhythms ranged up to 12 h apart between individuals. We see similarly large distribution in acrophases and amplitudes of immune parameters and extend the findings to differences within the same parameter between standard sleep-wake and constant routine conditions. As was suggested in the case of the lipid profile variances (Chua et al., 2013) it is possible that individuals have different circadian metabolic and immunologic phenotypes, and as our results would suggest the impact of external influences such as sleep-wake and feeding behavior is likely highly variable between individuals. These differences may explain, in part, the differences in metabolic and immunologic outcomes between individuals when exposed to the same sleep and circadian challenges such as shiftwork.
Additional studies are warranted to examine whether other cytokines and chemokines are also under circadian regulation, and whether disruption of these rhythms functionally impairs immune and inflammatory responses. Functional genomics and proteomics studies conducted under similar conditions could also investigate the temporal regulation of innate immune response at the molecular level and whether the underlying temporal orchestration is perturbed in pathological states strongly associated with inflammation such as asthma and cardiovascular disease, and in turn may help identify potential therapeutic targets, including appropriately timed chronotherapy, for these chronic conditions.
Supplementary Material
HIGHLIGHTS.
We assessed the endogenous circadian control and environmental and behavioral influences on ex-vivo LPS stimulation of human whole blood.
Significant 24-hour rhythms were observed under standard sleep-wake conditions in MCP-1, GM-CSF and IL-8 but not TNF-α
Significant 24-hour rhythms were observed in all four immune factors under constant routine conditions.
The rhythm amplitudes, expressed as a percentage of mean, were comparable between immune factors and across conditions.
The acrophase time (time of the fitted peak) was different between immune factors, and included daytime and nighttime peaks and changes across behavioral conditions.
ACKNOWLEDGEMENTS
We thank Elizabeth B. Klerman M.D. Ph.D., Peter Dearborn, research staff, and research participants at the Division of Sleep Medicine, BWH; the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at the Brigham and Women’s Hospital, Jonathan Williams M.D. for medical supervision; Core Laboratory staff (BWH) for melatonin assays. This work was supported by the National Space Biomedical Research Institute: HPF01301; National Heart, Lung, and Blood Institute: 1RC2HL101340-01; National Institute for Neurological Disorders and Stroke: U54NS060659; Atlanta Clinical and Translational Science Institute Pilot grant supported in part by PHS Grants: UL1 RR025008, KL2 RR025009 or TL1 RR025010 from the Clinical and Translational Science Award program. SAR, SWL and CAC were supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. SAS is supported by NASA Grant NASANNX1 OAR 1 OG and NHLBI grant K24-HL076446. FAS was supported by NHLBI Grant R01 HL094806. The project described was supported by Brigham and Women’s Hospital General Clinical Research Center grant M01 RR02635. The project described was supported by Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
SAR has received research funding from Government of Ontario/Pharmacia Canada Inc./Genesis Research Foundation/OBGYN Graduate Scholarship in Science and Technology at the University of Toronto, Faculty of Medicine and the Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award from Canadian Institutes of Health Research, Standard Life (Canada) Inc.; SAR holds a patent for Prevention of Circadian Rhythm Disruption by Using Optical Filters and Improving sleep performance in subject exposed to light at night; SAR owns equity in Circadian ZircLight, Inc.; SAR is a co-investigator on studies sponsored by Biological Illuminations, LLC; Vanda Pharmaceuticals Inc. CAC (Disclosures for past 12 months) CAC has received consulting fees from or served as a paid member of scientific advisory boards for: Bombardier, Inc.; Boston Red Sox; Boston Celtics; Cephalon, Inc. (acquired by Teva Pharmaceutical Industries Ltd. October 2011); Michael Jackson’s mother and children; Koninklijke Philips Electronics, N.V.; Novartis; United Parcel Services (UPS); and Vanda Pharmaceuticals, Inc.; and Zeo Inc. CAC owns an equity interest in Apple; Lifetrac, Inc.; Microsoft; Somnus Therapeutics, Inc.; and Vanda Pharmaceuticals, Inc., and received royalties from McGraw Hill, Penguin Press/ Houghton Mifflin Harcourt, and Philips Respironics, Inc. Dr. Czeisler has also received research support from Cephalon, National Football League Charities, ResMed and Philips Respironics. CAC has received lecture fees from APSS (Associated Professional Sleep Societies); Harvard School of Public Health; Japan Society for Occupational Health; University of Washington. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which CAC directs, has received unrestricted research and educational gifts and endowment funds from: Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., Gerald McGinnis, Jazz Pharmaceuticals, Merck & Co., Inc., Peter C. Farrell, Ph.D., ResMed, and Respironics, Inc. The HMS/DSM has received gifts from many outside organizations and individuals including: Committee for Interns and Residents, Gerald McGinnis, Jazz Pharmaceuticals, Jordan’s Furniture, NeuroScience, Novartis Consumer Health, Purdue Pharma, ResMed Foundation, Safeway, Transcept Pharmaceuticals, United Healthcare, Vanda Pharmaceuticals, Inc., Weight Watchers International and YMCA of the USA. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc. CAC is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, CAC has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. SWL has received consulting fees from Naturebright, Sound Oasis, Thomas Jefferson University and Wyle Integrated Science and Engineering (NASA); unrestricted lighting equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and Philips Lighting; unrestricted monetary gift to support research from Swinburne University of Technology, Australia; fellowship gift from Optalert, Pty, Melbourne, Australia; SWL holds equity in iSLEEP, Pty, Melbourne, Australia; advance author payment and royalties from Oxford University Press and payment for editing a textbook section from Elsevier; honoraria and/or travel and accommodation support for invited seminars, conference presentations or teaching from 8th International Conference on Managing Fatigue; Harvard University; Lighting Science Group Corp; Ontario Association of Fire Chiefs; Rio Tinto; Woolcock Institute of Medical Research; Wyle Integrated Science and Engineering; SWL has received investigator-initiated research grants from Biological Illuminations LLC, Philips Lighting and Philips-Respironics Inc.; sponsor-initiated research contracts with Vanda Pharmaceuticals; SWL holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women’s Hospital per Hospital policy. No income has been received. SWL also served as a paid expert on behalf of four public bodies in arbitration hearings related to sleep, circadian rhythms and work hours.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors or their family members have a consulting relationship with a sponsor of the research reported in this manuscript. Possible conflicts of Interest for all authors are disclosed below. OCC, FAJLS, SAS and AJD have no conflicts of interest to disclose related to this work.
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version
REFERENCES
- Abo T, Kawate T, Itoh K, Kumagai K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol. 1981;126:1360–1363. [PubMed] [Google Scholar]
- Adams KL, Castanon-Cervantes O, Evans JA, Davidson AJ. Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J Biol Rhythms. 2013;28:272–277. doi: 10.1177/0748730413494561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Brager AJ, Ehlen JC, Castanon-Cervantes O, Natarajan D, Delisser P, Davidson AJ, Paul KN. Sleep loss and the inflammatory response in mice under chronic environmental circadian disruption. PLoS One. 2013;8:e63752. doi: 10.1371/journal.pone.0063752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechin SR, Buchwald P. Effects of representative glucocorticoids on TNFalpha- and CD40L–induced NF-kappaB activation in sensor cells. Steroids. 2014;85:36–43. doi: 10.1016/j.steroids.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EC, Shui G, Lee IT, Lau P, Tan LC, Yeo SC, Lam BD, Bulchand S, Summers SA, Puvanendran K, Rozen SG, Wenk MR, Gooley JJ. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci U S A. 2013;110:14468–14473. doi: 10.1073/pnas.1222647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:312–318. doi: 10.1097/BOR.0b013e3283521c78. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–132. [PubMed] [Google Scholar]
- De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–248. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD, Yang H, Sayeed KS, Stote KS, Rumpler WV, Baer DJ, Longo DL, Mattson MP, Taub DD. Controlled meal frequency without caloric restriction alters peripheral blood mononuclear cell cytokine production. J Inflamm (Lond) 2011;8:6. doi: 10.1186/1476-9255-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Engstad CS, Gutteberg TJ, Osterud B. Modulation of blood cell activation by four commonly used anticoagulants. Thromb Haemost. 1997;77:690–696. [PubMed] [Google Scholar]
- Esquifino AI, Selgas L, Arce A, Maggiore VD, Cardinali DP. Twenty-four-hour rhythms in immune responses in rat submaxillary lymph nodes and spleen: effect of cyclosporine. Brain Behav Immun. 1996;10:92–102. doi: 10.1006/brbi.1996.0010. [DOI] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000741. 31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Haack M, Reichenberg A, Kraus T, Schuld A, Yirmiya R, Pollmacher T. Effects of an intravenous catheter on the local production of cytokines and soluble cytokine receptors in healthy men. Cytokine. 2000;12:694–698. doi: 10.1006/cyto.1999.0665. [DOI] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hermann C, von Aulock S, Dehus O, Keller M, Okigami H, Gantner F, Wendel A, Hartung T. Endogenous cortisol determines the circadian rhythm of lipopolysaccharide-- but not lipoteichoic acid--inducible cytokine release. Eur J Immunol. 2006;36:371–379. doi: 10.1002/eji.200535470. [DOI] [PubMed] [Google Scholar]
- Hochart H, Jenkins PV, Preston RJ, Smith OP, White B, O’Donnell J. Concentration-dependent roles for heparin in modifying lipopolysaccharide-induced activation of mononuclear cells in whole blood. Thromb Haemost. 2008;99:570–575. doi: 10.1160/TH07-06-0424. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- James FO, Cermakian N, Boivin DB. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. 2007;30:1427–1436. doi: 10.1093/sleep/30.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Lee MH, Song HK, Ko GJ, Kwon OS, Lim TK, Kim SH, Han SY, Han KH, Lee JE, Han JY, Kim HK, Cha DR. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 2010;78:883–894. doi: 10.1038/ki.2010.263. [DOI] [PubMed] [Google Scholar]
- Kawate T, Abo T, Hinuma S, Kumagai K. Studies of the bioperiodicity of the immune response. II. Co-variations of murine T and B cells and a role of corticosteroid. J Immunol. 1981;126:1364–1367. [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinski M, Scheer FA, Shea SA. Influence of the Circadian System on Disease Severity. Sleep Med Clin. 2009;4:143–163. doi: 10.1016/j.jsmc.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Manning PJ, Sutherland WH, McGrath MM, de Jong SA, Walker RJ, Williams MJ. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity (Silver Spring) 2008;16:2046–2052. doi: 10.1038/oby.2008.334. [DOI] [PubMed] [Google Scholar]
- Mills JN. Human circadian rhythms. Physiological Reviews. 1966;46:128–171. doi: 10.1152/physrev.1966.46.1.128. [DOI] [PubMed] [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. J Physiol (London) 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009;117:95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- Palmblad J, Cantell K, Strander H, Froberg J, Karlsson CG, Levi L, Granstrom M, Unger P. Stressor exposure and immunological response in man: interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20:193–199. doi: 10.1016/0022-3999(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Patil R, Shukre S, Paranjape R, Thakar M. Heparin and EDTA anticoagulants differentially affect the plasma cytokine levels in humans. Scand J Clin Lab Invest. 2013;73:452–455. doi: 10.3109/00365513.2013.798869. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Puttonen S, Viitasalo K, Harma M. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. 2011;28:528–535. doi: 10.3109/07420528.2011.580869. [DOI] [PubMed] [Google Scholar]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijkens T, Hoeksema MA, Beckers L, Smeets E, Meiler S, Levels J, Tjwa M, de Winther MP, Lutgens E. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. Faseb J. 2014;28:2202–2213. doi: 10.1096/fj.13-243105. [DOI] [PubMed] [Google Scholar]
- Seok SJ, Lee ES, Kim GT, Hyun M, Lee JH, Chen S, Choi R, Kim HM, Lee EY, Chung CH. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant. 2013;28:1700–1710. doi: 10.1093/ndt/gfs555. [DOI] [PubMed] [Google Scholar]
- Shackelford PG, Feigin RD. Periodicity of susceptibility to pneumococcal infection: influence of light and adrenocortical secretions. Science. 1973;182:285–287. doi: 10.1126/science.182.4109.285. [DOI] [PubMed] [Google Scholar]
- Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007;56:399–408. doi: 10.1002/art.22368. [DOI] [PubMed] [Google Scholar]
- Vaughan GM. New sensitive serum melatonin radioimmunoassay employing the Kennaway G280 antibody: Syrian hamster morning adrenergic response. J of Pineal Res. 1993;15:88–103. doi: 10.1111/j.1600-079x.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, Kales A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- Wang W, Barnaby JY, Tada Y, Li H, Tor M, Caldelari D, Lee DU, Fu XD, Dong X. Timing of plant immune responses by a central circadian regulator. Nature. 2011a;470:110–114. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med. 2011b;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MR, Matthews JP, Kanabrocki EL, Sothern RB, Roitman-Johnson B, Scheving LE. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol Int. 1995;12:19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.