Abstract
Nonsense-mediated mRNA decay (NMD) is one of three regulatory mechanisms that monitor the cytoplasm for aberrant mRNAs. NMD is usually triggered by premature translation termination codons that arise from mutations, transcription errors, or inefficient splicing, but which also occur in transcripts with alternately spliced isoforms or upstream open reading frames, or in the context of long 3′-UTRs. This surveillance pathway requires detection of the nonsense codon by the eukaryotic release factors (eRF1 and eRF3) and the activities of the Upf proteins, but the exact mechanism by which a nonsense codon is recognized as premature, and the individual roles of the Upf proteins, are poorly understood. In this review, we highlight important differences between premature and normal termination. Based on our current understanding of normal termination and ribosome recycling, we propose a similar mechanism for premature termination events that includes a role for the Upf proteins. In this model, the Upf proteins not only target the mRNA and nascent peptide for degradation, but also assume the role of recycling factors and rescue a ribosome stalled at a premature nonsense codon. The ATPase and helicase activities of Upf1, with the help of Upf2 and Upf3, are thus thought to be the catalytic force in ribosome subunit dissociation and ribosome recycling at an otherwise poorly dissociable termination event. While this model is somewhat speculative, it provides a unified vision for current data and a direction for future research.
Keywords: Nonsense mediated mRNA decay, Premature translational termination, Ribosome recycling
Three cytoplasmic surveillance pathways monitor the integrity of the translation process in eukaryotes and target aberrant transcripts that affect ribosomal progression. These pathways, No-Go Decay (NGD) [1, 2], Non-Stop Decay (NSD) [3, 4], and Nonsense-mediated mRNA Decay (NMD) [5, 6], cope with ribosomes respectively stalled on mRNA by secondary structures or sequence elements, the lack of a stop codon, or premature termination. Although elimination of the targeted mRNAs is the most well characterized aspect of each pathway, major ancillary functions include resolution of the stalled mRNPs, rescue of ribosomes and other protein synthesis components for further rounds of translation, and degradation of the nascent polypeptide and the aberrant mRNA. NGD and NSD, and the roles of their associated factors in promoting dissociation of stalled elongation complexes and release of intact peptidyl-tRNA, have been extensively reviewed [2, 4]. For NMD, most of the attention has converged on mechanisms underlying the activation of mRNA decay. At its core however, NMD is an anomalous translation termination event. Here we consider the interplay between translation termination and NMD substrate recognition, and highlight the ways that this pathway, like NGD and NSD, also appears to promote disassembly and reutilization of translation machinery that is otherwise bogged down at a dead end.
Substrates of the NMD pathway
NMD targets polysome-associated mRNAs [7–9] that account for as much as 15% of the complexity of an organism’s transcriptome [10–18]. The premature termination codons (PTCs) that are characteristic of NMD-targeted mRNAs can arise from genetic “errors” such as mutations in genomic DNA, alternative pre-mRNA processing, or non-productive DNA rearrangements, but they are also inherent to a subset of normal transcripts. For example, some mRNAs with upstream open reading frames (uORFs) are degraded by the NMD pathway [10, 12, 19–21], with the distinction between those mRNAs that do or do not trigger NMD reflecting the extent of uORF nonsense codon occupancy by the ribosome [19, 22] or the presence of downstream sequences that inactivate NMD [23, 24]. Notably, termination codons that trigger NMD do not need to be premature. Termination codons associated with atypically long 3′-UTRs are also sufficient to elicit NMD [25–29], an observation which led to the notion that termination context dictates an mRNA’s susceptibility to NMD [30]. The broad range of transcripts encompassed as NMD substrates has substantial biological impact: not only does NMD ensure removal of “junk” (e.g., some byproducts of alternate splicing, intron-containing transcripts that enter the cytoplasm, or pseudogene mRNAs [10]), but it also effectively renders most nonsense alleles as null alleles [31, 32] and has been co-opted to regulate the levels and locations of specific proteins [33, 34].
Regulation of NMD by the UPF genes
The proteins encoded by the UPF1 (SMG2), UPF2 (NMD2/SMG3), and UPF3 (SMG4) genes are the key factors controlling NMD [35–41]. Inactivation of their function by mutation, complete deletion, or down regulation stabilizes and increases accumulation of nonsense-containing mRNAs while having no significant effects on the abundance and stability of most wild-type transcripts [10, 41, 42]. These three proteins are mostly cytoplasmic (Upf3 shuttles between the nucleus and the cytoplasm) [43, 44] and interact with each other, the ribosome, and multiple mRNA decay and translation factors (including the Dcp2 subunit of the decapping enzyme and the eukaryotic release factors (eRFs) [37, 45–48]). In yeast, overexpression of Upf1 can compensate for mutations in Upf2 and Upf3, but not vice versa [49], indicating that Upf1 is NMD’s key regulator. Upf1 is a superfamily I RNA helicase with two major domains: a cysteine- and histidine-rich (CH) domain at its N-terminus and a helicase domain towards its C-terminus [50–54]. The CH domain encompasses two zinc-knuckle modules similar to those of ubiquitin ligases [55] and the RNA helicase domain includes two core helicase segments (RecA1 and RecA2) and two regulatory segments (1B and 1C) [53, 54, 56]. The Upf1 helicase, RNA binding, and ATP hydrolysis activities are critical for several steps in NMD, i.e., association with the 40S ribosomal subunit, disassembly of a terminating mRNP, and recycling of components of the protein synthetic and NMD machineries. These activities are regulated by Upf2 and Upf3 by interactions between the Upf1 CH-domain and the C-terminus of Upf2 [40, 54, 55, 57]. In the absence of Upf2, the Upf1 CH domain interacts with the helicase domain, yielding a closed conformation [53, 58] that increases RNA binding by the helicase domain and decreases ATPase and helicase activities. Upf2 binding opens the structure of Upf1, moving the CH domain away from the helicase domain, reducing RNA binding, and switching Upf1 from RNA clamping to RNA unwinding capability [53, 59]. In addition to manifesting intramolecular interactions, in yeast the CH domain of Upf1 is also involved in interaction with the ribosomal protein Rps26 [60], self-association [61], and binding to the decapping enzyme [37, 62], and possibly to eRF3 in human cells [46], suggesting that it may play a role in sequential molecular interactions during execution of NMD.
Upf2 contains three mIF4G domains (structures comparable to the middle domain of eukaryotic initiation factor eIF4G) in its N-terminal two-thirds [63–66], the most C-terminal of which (mIF4G-3) interacts with the central RRM (RNA Recognition Motif) in Upf3 [40, 64]. In addition to its roles as a scaffolding protein that bridges Upf1 and Upf3 [40, 43, 59, 67] and a switch for the Upf1 RNA helicase activity [53], Upf2 also recruits the Smg-1 kinase that phosphorylates Upf1 in metazoans [66]. In yeast, the Upf2 mIF4G-1 domain binds RNA and at least one other protein (Dbp6, a DEAD-box helicase) that may have a role in NMD [68]. Upf3 is a basic protein with an RNP-type RNA-binding domain (RBD) [40, 64]. The nonspecific RNA-binding activity of Upf2-Upf3 is thought to be a property of a conserved basic region of Upf2 [64]. However, in vitro studies challenge the capacity of the Upf2:Upf3 complex to bind RNA [59], thus leaving open the possibility that the Upf1 interaction domain on Upf2 is the principal contributor to the assembly of the trimeric Upf1:Upf2:Upf3 surveillance complex.
Other regulators of NMD
Upf proteins interact with the release factors. Upf1 interacts with both eRF1 and eRF3 [26, 45, 46, 69], and in human cells this leads to formation of the SURF (Smg-1-Upf1-Release Factors) complex [69, 70]. In addition, in yeast, Upf2 and Upf3 interact with eRF3 and could compete with eRF1 for a specific eRF3 interaction domain [71]. Importantly, Upf1 binding to eRF1 and eRF3 inhibits Upf1’s ATPase activity [45], a result supporting the notion that Upf1 is inactive when it is first recruited to a prematurely terminating ribosome and that subsequent steps in NMD require Upf1 activation by interaction with a Upf2:Upf3 complex, as seen with the human factors [59].
In many eukaryotes other than yeast the Smg-1 kinase and the Smg-8 and Smg-9 proteins, as well as the PP2A phosphatase, regulate NMD by controlling Upf1’s phosphorylation status [69, 70, 72, 73], while Smg-5 and Smg-7 form a complex that promotes mRNA deadenylation and decapping, and Smg-6 is an endonuclease thought to cleave NMD substrates [74–77]. Smg-8 and -9 repress the Smg-1 kinase activity, maintaining Upf1 in a dephosphorylated state in the SURF complex until it has interacted with Upf2 and Upf3. The latter interaction promotes Smg-1-mediated phosphorylation of Upf1, an apparent requirement for subsequent Upf1 interaction with the Smg-6 endonuclease and the Smg-5/7 complex. Upf1 and Upf2 are also phosphorylated in yeast, but there are no significant yeast homolog to any of the Smg proteins that regulate phosphorylation, nor is there good evidence that the yeast phosphorylation events regulate NMD [78, 79]. In mammalian cells, and to some extent in other eukaryotes, NMD is often enhanced by an exon-exon junction at least 50–55 nt downstream of a nonsense codon, a phenomenon reflecting the involvement of the exon-junction complex (EJC), a dynamic structure deposited during splicing whose core proteins include eIF4AIII, Y14, Magoh, and MLN51 [80, 81]. Upf3 has been shown to associate with the EJC core [67, 81–85], and this observation led to a popular model for EJC regulation of NMD [86] (see below).
Normal translation termination
NMD and translation termination appear to be linked because nonsense codon recognition by the translation apparatus is a requirement for NMD [8, 19, 87–90]. In eukaryotes, termination at in-frame UAA, UAG, or UGA codons requires the release factors eRF1 and eRF3 (Sup45 and Sup35 in yeast) (Figure 1). eRF1 recognizes nonsense codons in the ribosomal A site, after which a conformational change in eRF1 allows its conserved GGQ motif to activate the peptidyltransferase center of the ribosome and mediate peptide release [91–93]. eRF3 is a GTPase that stimulates eRF1 activity on the ribosome, conferring GTP dependency upon the termination process [94–97]. Subsequent to peptide hydrolysis, the termination complex is dissociated and recycled by the combined activities of the ABCE1 (Rli1 in yeast) ATPase, the essential initiation factors eIF3, eIF1, eIF1A, and the non-essential eIF3 subunit eIF3j [93, 98–100]. ABCE1 interacts with eRF1 and also with yeast eIF3j (Hcr1p) [101]. Cryo-EM reconstitution of the ribosome-eRF1-Rli1 complex shows that Rli1 is bound to the complex at the same position as eRF3, and the presence of non-hydrolyzable GTP analogs inhibits the subunit splitting activity of Rli1 [99], suggesting that ABCE1 and eRF3 cannot bind the ribosome-eRF1 complex simultaneously [93].
Figure 1. Translation termination at a normal stop codon.
The figure depicts a current understanding of the different steps occurring between recognition of a stop codon by eRF1 and eRF3 and complete dissociation of the ribosome. Some steps remain to be clarified, such the possible stimulatory role of Pab1 on the efficiency of translation termination and the dissociation of eRF3 after GTP hydrolysis.
In addition to eRF1 and eRF3 several other proteins have been shown to influence translation termination, including poly(A)-binding protein (Pab1 in yeast; PABPC1 in humans). Poly(A)-binding protein interacts with eRF3 and overexpressing or deleting its gene respectively leads to enhanced or reduced termination efficiency in yeast or human cells [46, 102–104]. eRF3-Pab1 interaction has been proposed to play a role in the formation of an mRNP complex favorable to normal translation termination [102, 103, 105, 106]. Purification of full-length human and yeast eRF3 has proved challenging, thus limiting in vitro translation termination systems to the use of a truncated version of eRF3 lacking its Pab1 interaction domain [92, 107]. Thus, the formal role of eRF3-Pab1 interaction in translation termination remains an open question [108–111]. It is possible that Pab1 is not directly involved in mediating efficient termination, but promotes appropriate post-termination events to ensure recycling of the ribosome and the termination factors.
Mutations in the NLP3, DBP5, and GLE1 genes, all of which encode mRNA export factors, have been shown to promote nonsense codon readthrough and to alter cellular sensitivity to compounds that affect translation [112–114]. While these results suggest that the respective export factors may regulate the termination process, it’s more likely that the observed effects on readthrough are indirect consequences of their effects on mRNP structure.
Premature translation termination is aberrant
Although NMD typically begins when a nonsense codon occupies the ribosomal A site, the ensuing events appear to be mechanistically different from normal translation termination. In addition to the fact that most normal termination events do not trigger NMD two key lines of evidence support this contention. First, toeprint analyses in yeast cell-free translation systems fail to yield any toeprinting signals from ribosomes at normal termination codons (NTCs) unless eRF1 is inactivated by a temperature-sensitive lesion [30]. In contrast, ribosomes at premature termination codons (PTCs) yield toeprint signals consistent with A-site occupancy even without eRF1 inactivation [30]. Similar results have been obtained with the NTC and a codon 39 PTC of human β-globin mRNA [115]. Second, although nonsense codon readthrough can occur at some NTCs [116, 117], direct comparisons demonstrate that PTCs are considerably more amenable to readthrough than NTCs. This conclusion is most clearly drawn from experiments with ataluren (PTC124), a small molecule drug developed to promote therapeutic nonsense suppression [118, 119]. Ataluren promotes readthrough at PTCs present in both reporter mRNAs and mRNAs derived from bona fide PTC-containing alleles, but fails to yield detectable readthrough at the NTCs of an equally diverse set of transcripts [118–126]. These observations indicate that at least one step in termination is proceeding considerably slower at PTCs than at NTCs, a difference that may well be attributable to termination-enhancing factors associated with a normal 3′-UTR that are absent from the “faux” 3′-UTR created by a PTC.
Consistent with the notion that Upf proteins play a role in recognition of such inefficiency in termination, deletion of any of the UPF genes further increases PTC readthrough [33, 49, 71, 127]. However, such nonsense suppression can be attributed at least in part to an increase in intracellular Mg2+ concentration caused by stabilization of the uORF-containing ALR1 and ALR2 mRNAs that encode yeast’s principal Mg2+ transporters [33]. Adding complexity to the issue, the ATPase and subunit splitting function of ABCE1 is inhibited when Mg2+ concentration is increased [100, 128]. Since the same ATPase domain is also responsible for interacting with eRF1 [101], it is possible that the increase in intracellular Mg2+ levels and the increase in readthrough at a PTC when NMD is inhibited could also reflect a decrease in ABCE1 function.
Further indications of the aberrant nature of premature termination are observations that reinitiation of translation after premature termination is markedly reduced in yeast cells or extracts when any one of the three Upf proteins is absent or inactive [30, 129], and that yeast cell-free extracts derived from a upf1Δ mutant manifest a defect in ribosome recycling that can be complemented by purified Upf1 [129]. Similarly, in human cells, ATPase- or helicase-deficient Upf1 leads to the accumulation of mRNPs containing endonucleolytically cleaved β-globin mRNA decay intermediates that are blocked in subsequent exonucleolytic degradation, and the latter mRNPs may also include the terminating ribosome [130]. All these observations suggest that, like the NGD and NSD pathways [1, 2, 4], one ancillary role for NMD and its Upf factors is the dissociation of an otherwise poorly dissociable ribosome:mRNP complex [5, 106, 129], a function that could require Pab1 and other associated factors at a normal termination codon [30, 131–133].
mRNA targeting by Upf1
All NMD studies in yeast, worms, flies, and mammals point to a central role of Upf1 in targeting an mRNA for degradation after premature termination, promoting the recruitment of the decay enzymes, and recycling of the premature termination complex. An important question, then, is how Upf1 is recruited to a premature termination event. mRNP immunoprecipitation experiments show that Upf1 preferentially associates with NMD substrate mRNAs in yeast and worms [11, 134], a non-trivial observation in light of the fact that NMD substrates comprise no more than 5% of the total mRNA mass [10]. Two possibilities for the selective association of Upf1 with NMD substrates are likely. In the first, Upf1 routinely associates with mRNA or ribosomes and a ribosomal encounter with a PTC stabilizes this association, activates Upf1, and launches the NMD process. In essence, this is the gist of the pioneer round/EJC model, where Upf1 is thought to associate with any ribosome that has been pre-bound by the eRF1 and eRF3 release factors, only to be activated if a downstream EJC can contribute Upf2 and Upf3 to the formation of a complex that will then incorporate the Smgs, phosphorylate Upf1, and recruit decapping and/or endonucleolytic enzymes [86, 135, 136]. However, the proposed role for a retained EJC in Upf1 activation clearly does not explain how mRNAs derived from intron-less precursors, e.g., most yeast mRNAs or those metazoan mRNAs that are subject to NMD without prior splicing, proper EJC spacing, or EJC components [137–141]. Recent studies in human cells suggested a variation of this model. Instead of binding directly to the ribosome Upf1 might occupy all translationally active mRNAs, presumably poised to be activated when a ribosome encounters a PTC and otherwise removed by elongating ribosomes and thus restricted to 3′-UTRs [142–146]. This model could explain how mRNAs without EJCs might be targeted by NMD.
The second possibility is that Upf1 only associates with ribosomes when termination is aberrant or inefficient (Figure 2). The faux-UTR model posits that efficient translation termination and proper recycling of the termination complex depends on interactions between the release factors and proteins bound to a normal 3′-UTR and that the mRNP structure associated with the 3′-UTR created by a PTC must lack at least one critical factor that normally enhances termination [5, 30, 106]. Accordingly, Upf1 association with the prematurely terminating ribosome could be favored by the novel mRNP context of a faux 3′-UTR, the loss of effective interaction between one of the release factors and a protein normally associated with the 3′-UTR, or the slow dissociation of the termination complex after peptide hydrolysis [5]. Three observations favor the second model: a) mRNA marking models like the pioneer round/EJC model need to invoke immunity to NMD for any mRNA not targeted for degradation in its initial round of translation, but experiments exploiting inducible NMD in yeast [19, 147], and demonstrating NMD susceptibility of eIF4E-associated mRNAs in mammalian cells [148, 149], argue that NMD immunity is unlikely; b) EJC enhancement of NMD may be indirect [80, 150–152]; and c) in yeast, the number of Upf1 (and Upf2 and Upf3) molecules per cell is approximately 1/30th to 1/100th the number of ribosomes [49, 153], thus precluding stoichiometric association with every mRNA or ribosome. However this is not the case in mammalian cells where the numbers are more comparable [154, 155]. These lines of evidence suggest that the promotion of normal termination might rely more on an mRNP structure organized by multiple factors that include eRF3 and Pab1, and Upf proteins can recognize the absence of these factors marked by an inefficient termination event.
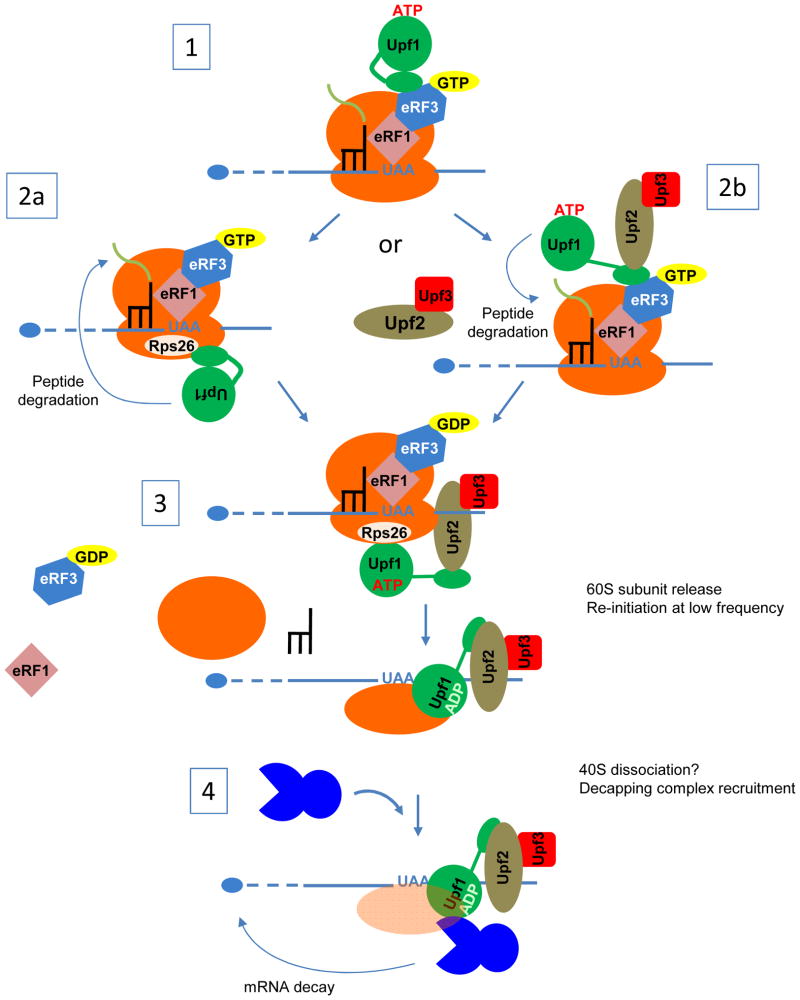
Figure 2. Proposed mechanism for NMD in yeast.
This model is speculative and aims to account for multiple observations on NMD in a uniform scenario. 1. eRF1 and eRF3 are recruited to a ribosome paused at a stop codon in an inappropriate 3′ mRNP context. This leads to improper termination, possibly linked to the inability of eRF3 to leave the complex, and could be favored by the absence of Pab1. Upf1 in an inactive form is recruited to eRF3-eRF1. Two alternative outcomes are then considered: 2a. After its recruitment to the termination complex, Upf1 binds to Rps26 and is joined by Upf2 and Upf3 and “opened” for activation. This step promotes peptide degradation (as indicated by the arrow). 2b. Upf2-Upf3 binds to Upf1 on the termination complex and activates Upf1. The complex then associates with Rps26 through the Upf1 CH domain, and promotes peptide degradation (as indicated by the arrow). 3. Upf1 is active, associated with Upf2-Upf3, and bound to the ribosome via Rsp26. These components promote dissociation of the termination complex, a step that requires the Upf1 helicase activity and leaves the 40S subunit still bound to the mRNA (the events leading to 40S removal are unknown). This step is different from the “normal” recycling event involving Rli1 (see Figure 1). 4. The presence of Upf1 promotes mRNA decapping by Dcp1-Dcp2 (as indicated by the arrow), via Upf1 interaction with Dcp2. Destabilization of the mRNA closed-loop structure, subsequent to improper recycling, could also promote mRNA decay.
Conclusions
The NMD pathway, one of several cytoplasmic quality control mechanisms ensuring the fidelity of gene expression, minimizes the accumulation of potentially toxic polypeptides that arise as products of premature translation termination. NMD is regulated in all eukaryotes by the three Upf proteins, Upf1, Upf2, and Upf3. Of these, Upf1, a superfamily I RNA helicase, is the central regulator whose activation depends on Upf2 and Upf3 binding. NMD is triggered by premature translation termination, an event mechanistically different from normal termination. Premature termination appears to be an inefficient termination event, and this may reflect different mRNP complexes at PTCs and NTCs. Since eRF1 and eRF3 are required for activation of NMD events after stop codon recognition by eRF1 and GTP hydrolysis by eRF3 may encompass the differences between premature and normal termination. Normal termination and ribosome recycling appear to depend on interactions between the release factors and proteins associated with a normal 3′-UTR. We propose that the inefficiency of premature termination is triggered by the mRNP structure downstream of a PTC which, in turn, may lead to poor release factor binding at the A-site or to slow dissociation of the release factor(s) after peptide hydrolysis (Figure 2). These shortcomings are thought to be resolved by the recruitment of Upf1 to the premature termination complex, activation of its ATPase and helicase to promote ribosome reutilization, and interaction with the decapping enzyme and the proteasome to initiate NMD and nascent polypeptide degradation [156]. Premature termination could lead to a unique mRNP structure that might provide sufficient specificity for Upf1 interaction with eRF3 or the small ribosomal subunit, and Upf1 activation might dissociate the 60S subunit, similar to ATPase activity of Rli1. However the mechanism of Upf2 and Upf3 recruitment, the timing of peptide hydrolysis (before or after Upf association), and Upf1 association with the 40S ribosomal subunit in yeast [60, 129] remain to be understood.
In short, two ribosome recycling pathways could exist. One promoted by termination at a normal stop codon, which requires a “proper” mRNP structure that includes Pab1, eRF1-eRF3, and ABCE1/Rli1 among other factors, and another at a premature termination codon, in which the ribosome is stalled, possibly because eRF3 is not able to leave the complex, and which leads to recruitment of the Upf proteins and the onset of the events of NMD. Both pathways would thus rely on ATP hydrolysis to promote subunit splitting. Clearly, this model is highly speculative and will require experimental validation.
Nonsense-mediated mRNA decay (NMD) targets anomalous translation termination events
Premature termination differs mechanistically from normal termination
Premature termination leads to a poorly dissociable mRNP complex
The UPF proteins recruit mRNA decay factors
The UPF factors may also promote ribosome dissociation and recycling
Acknowledgments
A.C. and A.J. were supported by a grant (R37 GM27757) from the U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harigaya Y, Parker R. No-go decay: a quality control mechanism for RNA in translation. Wiley Interdiscip Rev RNA. 2010;1:132–141. doi: 10.1002/wrna.17. [DOI] [PubMed] [Google Scholar]
- 2.Shoemaker CJ, Green R. Translation drives mRNA quality control. Nature Struct Mol Biol. 2012;19:594–601. doi: 10.1038/nsmb.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasudevan S, Peltz SW, Wilusz CJ. Non-stop decay--a new mRNA surveillance pathway. Bioessays. 2002;24:785–788. doi: 10.1002/bies.10153. [DOI] [PubMed] [Google Scholar]
- 4.Graille M, Seraphin B. Surveillance pathways rescuing eukaryotic ribosomes lost in translation. Nat Rev Mol Cell Biol. 2012;13:727–735. doi: 10.1038/nrm3457. [DOI] [PubMed] [Google Scholar]
- 5.Kervestin S, Jacobson A. NMD: a multifaceted response to premature translational termination. Nat Rev Mol Cell Biol. 2012;13:700–12. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popp MW, Maquat LE. Organizing principles of mammalian nonsense-mediated mRNA decay. Ann Rev Genet. 2013;47:139–165. doi: 10.1146/annurev-genet-111212-133424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkin AL, et al. Relationship between yeast polyribosomes and Upf proteins required for nonsense mRNA decay. J Biol Chem. 1997;272:22163–22172. doi: 10.1074/jbc.272.35.22163. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, et al. Polysome-associated mRNAs are substrates for the nonsense-mediated mRNA decay pathway in Saccharomyces cerevisiae. RNA. 1997;3:234–244. [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W, et al. Nonsense-mediated mRNA decapping occurs on polyribosomes in Saccharomyces cerevisiae. Nat Struct Mol Biol. 2010;17:244–247. doi: 10.1038/nsmb.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F, et al. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol Cell. 2003;12:1439–1452. doi: 10.1016/s1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 11.Johansson MJ, et al. Association of yeast Upf1p with direct substrates of the NMD pathway. Proc Natl Acad Sci U S A. 2007;104:20872–20877. doi: 10.1073/pnas.0709257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehwinkel J, et al. Nonsense-mediated mRNA decay factors act in concert to regulate common mRNA targets. RNA. 2005;11:1530–1544. doi: 10.1261/rna.2160905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendell JT, et al. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nature Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 14.Weischenfeldt J, et al. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. 2008;22:1381–1396. doi: 10.1101/gad.468808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramani AK, et al. High resolution transcriptome maps for wild-type and nonsense-mediated decay-defective Caenorhabditis elegans. Genome Biol. 2009;10:R101. doi: 10.1186/gb-2009-10-9-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayson S, et al. A role for nonsense-mediated mRNA decay in plants: pathogen responses are induced in Arabidopsis thaliana NMD mutants. PLoS One. 2012;7:e31917. doi: 10.1371/journal.pone.0031917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapin A, et al. In vivo determination of direct targets of the nonsense-mediated decay pathway in Drosophila. G3. 2014;4:485–496. doi: 10.1534/g3.113.009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann J, Hol EM, Jack HM. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol Cell Biol. 2006;26:1272–1287. doi: 10.1128/MCB.26.4.1272-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaba A, Jacobson A, Sachs MS. Ribosome occupancy of the yeast CPA1 upstream open reading frame termination codon modulates nonsense-mediated mRNA decay. Mol Cell. 2005;20:449–460. doi: 10.1016/j.molcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Nyiko T, et al. Plant upstream ORFs can trigger nonsense-mediated mRNA decay in a size-dependent manner. Plant Mol Biol. 2009;71:367–378. doi: 10.1007/s11103-009-9528-4. [DOI] [PubMed] [Google Scholar]
- 21.Decourty L, et al. Long open reading frame transcripts escape nonsense-mediated mRNA decay in yeast. Cell Rep. 2014;6:593–8. doi: 10.1016/j.celrep.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama-Kadokura N, et al. Polyamine-responsive ribosomal arrest at the stop codon of an upstream open reading frame of the AdoMetDC1 gene triggers nonsense-mediated mRNA decay in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:1556–1567. doi: 10.1093/pcp/pcu086. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Echevarria MJ, Peltz SW. Utilizing the GCN4 leader region to investigate the role of the sequence determinants in nonsense-mediated mRNA decay. EMBO J. 1996;15:2810–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Echevarria MJ, Peltz SW. The RNA binding protein Pub1 modulates the stability of transcripts containing upstream open reading frames. Cell. 2000;101:741–751. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 25.Kebaara BW, Atkin AL. Long 3′-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:2771–2778. doi: 10.1093/nar/gkp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G, Rebbapragada I, Lykke-Andersen J. A competition between stimulators and antagonists of Upf complex recruitment governs human nonsense-mediated mRNA decay. PLoS Biol. 2008;6:e111. doi: 10.1371/journal.pbio.0060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kertesz S, et al. Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 2006;34:6147–6157. doi: 10.1093/nar/gkl737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaborske JM, Zeitler B, Culbertson MR. Multiple transcripts from a 3′-UTR reporter vary in sensitivity to nonsense-mediated mRNA decay in Saccharomyces cerevisiae. PLoS One. 2013;8:e80981. doi: 10.1371/journal.pone.0080981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yepiskoposyan H, et al. Autoregulation of the nonsense-mediated mRNA decay pathway in human cells. RNA. 2011;17:2108–2118. doi: 10.1261/rna.030247.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amrani N, et al. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- 31.Culbertson MR. RNA surveillance. Unforeseen consequences for gene expression inherited genetic disorders and cancer. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 32.Miller JN, Chan CH, Pearce DA. The role of nonsense-mediated decay in neuronal ceroid lipofuscinosis. Hum Mol Genet. 2013;22:2723–2734. doi: 10.1093/hmg/ddt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson MJ, Jacobson A. Nonsense-mediated mRNA decay maintains translational fidelity by limiting magnesium uptake. Genes Dev. 2010;24:1491–1495. doi: 10.1101/gad.1930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MH, Schedl T. Translation repression by GLD-1 protects its mRNA targets from nonsense-mediated mRNA decay in C. elegans. Genes Dev. 2004;18:1047–1059. doi: 10.1101/gad.1188404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leeds P, et al. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991;5:2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- 36.Pulak R, Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993;7:1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- 37.He F, Jacobson A. Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes Dev. 1995;9:437–454. doi: 10.1101/gad.9.4.437. [DOI] [PubMed] [Google Scholar]
- 38.Cui Y, et al. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995;9:423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- 39.Perlick HA, et al. Mammalian orthologues of a yeast regulator of nonsense transcript stability. Proc Natl Acad Sci USA. 1996;93:10928–32. doi: 10.1073/pnas.93.20.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He F, Brown AH, Jacobson A. Upf1p, Nmd2p and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol Cell Biol. 1997;17:1580–1594. doi: 10.1128/mcb.17.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatfield D, et al. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 2003;22:3960–3970. doi: 10.1093/emboj/cdg371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lykke-Andersen J, Shu MD, Steitz JA. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell. 2000;103:1121–31. doi: 10.1016/s0092-8674(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 43.Serin G, et al. Identification and characterization of human orthologues to Saccharomyces cerevisiae Upf2 protein and Upf3 protein (Caenorhabditis elegans SMG-4) Mol Cell Biol. 2001;21:209–23. doi: 10.1128/MCB.21.1.209-223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirley RL, et al. A factor required for nonsense-mediated mRNA decay in yeast is exported from the nucleus to the cytoplasm by a nuclear export signal sequence. J Cell Sci. 1998;111(Pt 21):3129–3143. doi: 10.1242/jcs.111.21.3129. [DOI] [PubMed] [Google Scholar]
- 45.Czaplinski K, et al. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 1998;12:1665–1677. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanov PV, et al. Interactions between UPF1, eRFs PABP and the exon junction complex suggest an integrated model for mammalian NMD pathways. EMBO J. 2008;27:736–747. doi: 10.1038/emboj.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol Cell Biol. 2002;22:8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lejeune F, Li X, Maquat LE. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating and exonucleolytic activities. Mol Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 49.Maderazo AB, et al. Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p. Mol Cell Biol. 2000;20:4591–4603. doi: 10.1128/mcb.20.13.4591-4603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leeds P, et al. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altamura N, et al. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J Mol Biol. 1992;224:575–587. doi: 10.1016/0022-2836(92)90545-u. [DOI] [PubMed] [Google Scholar]
- 52.Koonin E. A new group of putative RNA helicases. Trends Biochem Sci. 1992;17:495–497. doi: 10.1016/0968-0004(92)90338-a. [DOI] [PubMed] [Google Scholar]
- 53.Chakrabarti S, et al. Molecular mechanisms for the RNA-dependent ATPase activity of Upf1 and its regulation by Upf2. Mol Cell. 2011;41:693–703. doi: 10.1016/j.molcel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Clerici M, et al. Unusual bipartite mode of interaction between the nonsense-mediated decay factors UPF1 and UPF2. EMBO J. 2009;28:2293–2306. doi: 10.1038/emboj.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadlec J, et al. Crystal structure of the UPF2-interacting domain of nonsense-mediated mRNA decay factor UPF1. RNA. 2006;12:1817–1824. doi: 10.1261/rna.177606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Z, et al. Structural and functional insights into the human Upf1 helicase core. EMBO J. 2007;26:253–264. doi: 10.1038/sj.emboj.7601464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He F, Brown AH, Jacobson A. Interaction between Nmd2p and Upf1p is required for activity but not for dominant-negative inhibition of the nonsense-mediated mRNA decay pathway in yeast. RNA. 1996;2:153–170. [PMC free article] [PubMed] [Google Scholar]
- 58.Fiorini F, Boudvillain M, Le Hir H. Tight intramolecular regulation of the human Upf1 helicase by its N- and C-terminal domains. Nuc Acids Res. 2013;41:2404–2415. doi: 10.1093/nar/gks1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamieh H, et al. NMD factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and stimulate its RNA helicase activity. Nat Struct Mol Biol. 2008;15:85–93. doi: 10.1038/nsmb1330. [DOI] [PubMed] [Google Scholar]
- 60.Min EE, et al. Yeast Upf1 CH domain interacts with Rps26 of the 40S ribosomal subunit. RNA. 2013;19:1105–1115. doi: 10.1261/rna.039396.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He F, Ganesan R, Jacobson A. Intra- and intermolecular regulatory interactions in Upf1 the RNA helicase central to nonsense-mediated mRNA decay in yeast. Mol Cell Biol. 2013;33:4672–4684. doi: 10.1128/MCB.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peltz SW, et al. Nonsense-mediated mRNA decay in yeast. Prog Nucleic Acid Res Mol Biol. 1994;47:271–298. doi: 10.1016/s0079-6603(08)60254-8. [DOI] [PubMed] [Google Scholar]
- 63.Aravind L, Koonin EV. Eukaryote-specific domains in translation initiation factors: implications for translation regulation and evolution of the translation system. Genome Res. 2000;10:1172–84. doi: 10.1101/gr.10.8.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadlec J, Izaurralde E, Cusack S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nature Struct Mol Biol. 2004;11:330–337. doi: 10.1038/nsmb741. [DOI] [PubMed] [Google Scholar]
- 65.Ponting CP. Novel eIF4G domain homologues linking mRNA translation with nonsense-mediated mRNA decay. Trends Biochem Sci. 2000;25:423–6. doi: 10.1016/s0968-0004(00)01628-5. [DOI] [PubMed] [Google Scholar]
- 66.Clerici M, et al. Structural and functional analysis of the three MIF4G domains of nonsense-mediated decay factor UPF2. Nucleic Acids Res. 2014;42:2673–2686. doi: 10.1093/nar/gkt1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melero R, et al. The cryo-EM structure of the UPF-EJC complex shows UPF1 poised toward the RNA 3′ end. Nat Struct Mol Biol. 2012;19:498–505. S1–2. doi: 10.1038/nsmb.2287. [DOI] [PubMed] [Google Scholar]
- 68.Fourati Z, et al. A highly conserved region essential for NMD in the Upf2 N-terminal domain. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.09.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashima I, et al. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006;20:355–367. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita A, et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 2009;23:1091–1105. doi: 10.1101/gad.1767209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W, et al. The role of Upf proteins in modulating the translation read-through of nonsense-containing transcripts. EMBO J. 2001;20:880–890. doi: 10.1093/emboj/20.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grimson A, et al. SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA Decay in Caenorhabditis elegans. Mol Cell Biol. 2004;24:7483–7490. doi: 10.1128/MCB.24.17.7483-7490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohnishi T, et al. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol Cell. 2003;12:1187–1200. doi: 10.1016/s1097-2765(03)00443-x. [DOI] [PubMed] [Google Scholar]
- 74.Eberle AB, et al. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat Struct Mol Biol. 2009;16:49–55. doi: 10.1038/nsmb.1530. [DOI] [PubMed] [Google Scholar]
- 75.Huntzinger E, et al. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA. 2008;14:2609–2617. doi: 10.1261/rna.1386208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fukuhara N, et al. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol Cell. 2005;17:537–547. doi: 10.1016/j.molcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Unterholzner L, Izaurralde E. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol Cell. 2004;16:587–596. doi: 10.1016/j.molcel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Lasalde C, et al. Identification and functional analysis of novel phosphorylation sites in the RNA surveillance protein Upf1. Nucleic Acids Res. 2014;42:1916–1929. doi: 10.1093/nar/gkt1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W, et al. A role for Upf2p phosphorylation in Saccharomyces cerevisiae nonsense-mediated mRNA decay. Mol Cell Biol. 2006;26:3390–4000. doi: 10.1128/MCB.26.9.3390-3400.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh G, et al. The cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. Cell. 2012;151:750–764. doi: 10.1016/j.cell.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lykke-Andersen J, Shu MD, Steitz JA. Communication of the position of exon-exon junctions to the mRNA surveillance machinery by the protein RNPS1. Science. 2001;293:1836–1839. doi: 10.1126/science.1062786. [DOI] [PubMed] [Google Scholar]
- 82.Le Hir H, Moore MJ, Maquat LE. Pre-mRNA splicing alters mRNP composition: evidence for stable association of proteins at exon-exon junctions. Genes Dev. 2000;14:1098–1108. [PMC free article] [PubMed] [Google Scholar]
- 83.Sun X, Maquat LE. mRNA surveillance in mammalian cells: the relationship between introns and translation termination. RNA. 2000;6:1–8. doi: 10.1017/s1355838200991660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Hir H, et al. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001;20:4987–4997. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lejeune F, et al. The exon junction complex is detected on CBP80-bound but not eIF4E-bound mRNA in mammalian cells: dynamics of mRNP remodeling. EMBO J. 2002;21:3536–3545. doi: 10.1093/emboj/cdf345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishigaki Y, et al. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–17. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 87.Welch EM, Jacobson A. An internal open reading frame triggers nonsense-mediated decay of the yeast SPT10 mRNA. EMBO J. 1999;18:6134–6145. doi: 10.1093/emboj/18.21.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gozalbo D, Hohmann S. Nonsense suppressors partially revert the decrease of the mRNA level of a nonsense mutant allele in yeast. Curr Genet. 1990;17:77–79. doi: 10.1007/BF00313252. [DOI] [PubMed] [Google Scholar]
- 89.Losson R, Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci USA. 1979;76:5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belgrader P, Cheng J, Maquat LE. Evidence to implicate translation by ribosomes in the mechanism by which nonsense codons reduce the nuclear level of human triosephosphate isomerase mRNA. Proc Natl Acad Sci USA. 1993;90:482–486. doi: 10.1073/pnas.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng Z, et al. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev. 2009;23:1106–1118. doi: 10.1101/gad.1770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alkalaeva EZ, et al. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. doi: 10.1016/j.cell.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 93.Becker T, et al. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature. 2012;482:501–506. doi: 10.1038/nature10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson RJ, Hellen CU, Pestova TV. Termination and post-termination events in eukaryotic translation. Adv Protein Chem Struct Biol. 2012;86:45–93. doi: 10.1016/B978-0-12-386497-0.00002-5. [DOI] [PubMed] [Google Scholar]
- 95.Salas-Marco J, Bedwell DM. GTP hydrolysis by eRF3 facilitates stop codon decoding during eukaryotic translation termination. Mol Cell Biol. 2004;24:7769–7778. doi: 10.1128/MCB.24.17.7769-7778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kononenko AV, et al. Role of the individual domains of translation termination factor eRF1 in GTP binding to eRF3. Proteins. 2008;70:388–393. doi: 10.1002/prot.21544. [DOI] [PubMed] [Google Scholar]
- 97.Frolova L, et al. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA. 1996;2:334–341. [PMC free article] [PubMed] [Google Scholar]
- 98.Pisarev AV, Hellen CU, Pestova TV. Recycling of eukaryotic posttermination ribosomal complexes. Cell. 2007;131:286–299. doi: 10.1016/j.cell.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pisarev AV, et al. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol Cell. 2010;37:196–210. doi: 10.1016/j.molcel.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barthelme D, et al. Ribosome recycling depends on a mechanistic link between the FeS cluster domain and a conformational switch of the twin-ATPase ABCE1. Proc Natl Acad Sci USA. 2011;108:3228–3233. doi: 10.1073/pnas.1015953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khoshnevis S, et al. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep. 2010;11:214–219. doi: 10.1038/embor.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoshino S, et al. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J Biol Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- 103.Cosson B, et al. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence PSI(+) propagation. Mol Cell Biol. 2002;22:3301–3315. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kononenko AV, et al. GTP-dependent structural rearrangement of the eRF1:eRF3 complex and eRF3 sequence motifs essential for PABP binding. Nucleic Acids Res. 2010;38:548–558. doi: 10.1093/nar/gkp908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hilleren P, Parker R. mRNA surveillance in eukaryotes: kinetic proofreading of proper translation termination as assessed by mRNP domain organization? RNA. 1999;5:711–719. doi: 10.1017/s1355838299990519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amrani N, Sachs MS, Jacobson A. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol. 2006;7:415–425. doi: 10.1038/nrm1942. [DOI] [PubMed] [Google Scholar]
- 107.Shoemaker CJ, Green R. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc Natl Acad Sci USA. 2011;108:E1392–8. doi: 10.1073/pnas.1113956108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roque S, et al. Interaction between the poly(A)-binding protein Pab1 and the eukaryotic release factor eRF3 regulates translation termination but not mRNA decay in Saccharomyces cerevisiae. doi: 10.1261/rna.047282.114. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kervestin S, et al. Testing the faux-UTR model for NMD: analysis of Upf1p and Pab1p competition for binding to eRF3/Sup35p. Biochimie. 2012;94:1560–1571. doi: 10.1016/j.biochi.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cosson B, et al. Characterization of the poly(A) binding proteins expressed during oogenesis and early development of Xenopus laevis. Biol Cell. 2002;94:217–231. doi: 10.1016/s0248-4900(02)01195-4. [DOI] [PubMed] [Google Scholar]
- 111.Gray NK, et al. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 2000;19:4723–4733. doi: 10.1093/emboj/19.17.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Estrella LA, Wilkinson MF, Gonzalez CI. The shuttling protein Npl3 promotes translation termination accuracy in Saccharomyces cerevisiae. J Mol Biol. 2009;394:410–422. doi: 10.1016/j.jmb.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gross T, et al. The DEAD-box RNA helicase Dbp5 functions in translation termination. Science. 2007;315:646–649. doi: 10.1126/science.1134641. [DOI] [PubMed] [Google Scholar]
- 114.Bolger TA, et al. The mRNA export factor Gle1 and inositol hexakisphosphate regulate distinct stages of translation. Cell. 2008;134:624–633. doi: 10.1016/j.cell.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peixeiro I, et al. Interaction of PABPC1 with the translation initiation complex is critical to the NMD resistance of AUG-proximal nonsense mutations. Nucleic Acids Res. 2012;40:1160–1173. doi: 10.1093/nar/gkr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodenough E, et al. Cryptic MHC class I-binding peptides are revealed by aminoglycoside-induced stop codon read-through into the 3′ UTR. Proc Natl Acad Sci USA. 2014;111:5670–5675. doi: 10.1073/pnas.1402670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dunn JG, et al. Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. eLife. 2013;2:e01179. doi: 10.7554/eLife.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 119.Peltz SW, et al. Ataluren as an agent for therapeutic nonsense suppression. Ann Rev Med. 2013;64:407–25. doi: 10.1146/annurev-med-120611-144851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li M, et al. Muscle dysfunction and structural defects of dystrophin-null sapje mutant zebrafish larvae are rescued by ataluren treatment. FASEB J. 2014;28:1593–1599. doi: 10.1096/fj.13-240044. [DOI] [PubMed] [Google Scholar]
- 121.Gregory-Evans CY, et al. Postnatal manipulation of Pax6 dosage reverses congenital tissue malformation defects. J Clin Invest. 2014;124:111–116. doi: 10.1172/JCI70462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lentini L, et al. Toward a Rationale for the PTC124 (Ataluren) Promoted Readthrough of Premature Stop Codons: A Computational Approach and GFP-Reporter Cell-Based Assay. Mol Pharm. 2014;11:653–664. doi: 10.1021/mp400230s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drake KM, et al. Correction of nonsense BMPR2 and SMAD9 mutations by Ataluren in Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol. 2013;49:403–409. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y, et al. Premature termination codon read-through in the ABCC6 gene: potential treatment for pseudoxanthoma elasticum. J Invest Dermatol. 2013;133:2672–2677. doi: 10.1038/jid.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuschal C, et al. Repair of UV photolesions in xeroderma pigmentosum group C cells induced by translational readthrough of premature termination codons. Proc Natl Acad Sci USA. 2013;110:19483–19488. doi: 10.1073/pnas.1312088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goldmann T, et al. PTC124-mediated translational readthrough of a nonsense mutation causing Usher syndrome type 1C. Hum Gene Ther. 2011;22:537–547. doi: 10.1089/hum.2010.067. [DOI] [PubMed] [Google Scholar]
- 127.Kvas S, Gloor GB, Brandl CJ. Loss of nonsense mediated decay suppresses mutations in Saccharomyces cerevisiae TRA1. BMC Genet. 2012;13:19. doi: 10.1186/1471-2156-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sims LM, Igarashi RY. Regulation of the ATPase activity of ABCE1 from Pyrococcus abyssi by Fe-S cluster status and Mg(2)(+): implication for ribosomal function. Arch Biochem Biophys. 2012;524:114–122. doi: 10.1016/j.abb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 129.Ghosh S, et al. Translational competence of ribosomes released from a premature termination codon is modulated by NMD factors. RNA. 2010;16:1832–1847. doi: 10.1261/rna.1987710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Franks TM, Singh G, Lykke-Andersen J. Upf1 ATPase-dependent mRNP disassembly is required for completion of nonsense- mediated mRNA decay. Cell. 2010;143:938–950. doi: 10.1016/j.cell.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eberle AB, et al. Posttranscriptional gene regulation by spatial rearrangement of the 3′ untranslated region. PLoS Biol. 2008;6:e92. doi: 10.1371/journal.pbio.0060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silva AL, et al. Proximity of the poly(A)-binding protein to a premature termination codon inhibits mammalian nonsense-mediated mRNA decay. RNA. 2008;14:563–576. doi: 10.1261/rna.815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Behm-Ansmant I, et al. A conserved role for cytoplasmic poly(A)-binding protein 1 (PABPC1) in nonsense-mediated mRNA decay. EMBO J. 2007;26:1591–1601. doi: 10.1038/sj.emboj.7601588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johns L, et al. Caenorhabditis elegans SMG-2 selectively marks mRNAs containing premature translation termination codons. Mol Cell Biol. 2007;27:5630–5638. doi: 10.1128/MCB.00410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 136.Schweingruber C, et al. Nonsense-mediated mRNA decay - mechanisms of substrate mRNA recognition and degradation in mammalian cells. Biochim Biophys Acta. 2013;1829:612–623. doi: 10.1016/j.bbagrm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Wen J, Brogna S. Splicing-dependent NMD does not require the EJC in Schizosaccharomyces pombe. EMBO J. 2010;29:1537–1551. doi: 10.1038/emboj.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J, et al. Intron function in the nonsense-mediated decay of beta-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA. 1998;4:801–15. doi: 10.1017/s1355838298971849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rajavel KS, Neufeld EF. Nonsense-mediated decay of human HEXA mRNA. Mol Cell Biol. 2001;21:5512–5519. doi: 10.1128/MCB.21.16.5512-5519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.LeBlanc JJ, Beemon KL. Unspliced Rous sarcoma virus genomic RNAs are translated and subjected to nonsense-mediated mRNA decay before packaging. J Virol. 2004;78:5139–5146. doi: 10.1128/JVI.78.10.5139-5146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang J, et al. Boundary-independent polar nonsense-mediated decay. EMBO Rep. 2002;3:274–279. doi: 10.1093/embo-reports/kvf036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hurt JA, Robertson AD, Burge CB. Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Res. 2013;23:1636–1650. doi: 10.1101/gr.157354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kurosaki T, Maquat LE. Rules that govern UPF1 binding to mRNA 3′ UTRs. Proc Natl Acad Sci USA. 2013;110:3357–3362. doi: 10.1073/pnas.1219908110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hogg JR, Goff SP. Upf1 senses 3′UTR length to potentiate mRNA decay. Cell. 2010;143:379–389. doi: 10.1016/j.cell.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shigeoka T, et al. Evidence that the Upf1-related molecular motor scans the 3′-UTR to ensure mRNA integrity. Nucleic Acids Res. 2012;40:6887–6897. doi: 10.1093/nar/gks344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zund D, et al. Translation-dependent displacement of UPF1 from coding sequences causes its enrichment in 3′ UTRs. Nature Struct Mol Biol. 2013;20:936–943. doi: 10.1038/nsmb.2635. [DOI] [PubMed] [Google Scholar]
- 147.Maderazo AB, et al. Nonsense-containing mRNAs that accumulate in the absence of a functional nonsense-mediated mRNA decay pathway are destabilized rapidly upon its restitution. Mol Cell Biol. 2003;23:842–851. doi: 10.1128/MCB.23.3.842-851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rufener SC, Muhlemann O. eIF4E-bound mRNPs are substrates for nonsense-mediated mRNA decay in mammalian cells. Nat Struct Molec Biol. 2013;20:710–717. doi: 10.1038/nsmb.2576. [DOI] [PubMed] [Google Scholar]
- 149.Durand S, Lykke-Andersen J. Nonsense-mediated mRNA decay occurs during eIF4F-dependent translation in human cells. Nat Struct Mol Biol. 2013;20:702–709. doi: 10.1038/nsmb.2575. [DOI] [PubMed] [Google Scholar]
- 150.Wiegand HL, Lu S, Cullen BR. Exon junction complexes mediate the enhancing effect of splicing on mRNA expression. Proc Natl Acad Sci USA. 2003;100:11327–11332. doi: 10.1073/pnas.1934877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gudikote JP, et al. RNA splicing promotes translation and RNA surveillance. Nature Struct Mol Biol. 2005;12:801–819. doi: 10.1038/nsmb980. [DOI] [PubMed] [Google Scholar]
- 152.Nott A, Le Hir H, Moore MJ. Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev. 2004;18:210–222. doi: 10.1101/gad.1163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 154.Gorlach M, Burd CG, Dreyfuss G. The mRNA poly(A)-binding protein: localization, abundance and RNA-binding specificity. Exp Cell Res. 1994;211:400–407. doi: 10.1006/excr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 155.Pal M, et al. Evidence that phosphorylation of human Upfl protein varies with intracellular location and is mediated by a wortmannin-sensitive and rapamycin-sensitive PI 3-kinase-related kinase signaling pathway. RNA. 2001;7:5–15. doi: 10.1017/s1355838201000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuroha K, Tatematsu T, Inada T. Upf1 stimulates degradation of the product derived from aberrant messenger RNA containing a specific nonsense mutation by the proteasome. EMBO Rep. 2009;10:1265–1271. doi: 10.1038/embor.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]